纳米羟基磷灰石的精氨酸表面修饰与铕掺杂及其细胞活性
来源期刊:中国有色金属学报(英文版)2011年第8期
论文作者:赵颜忠 朱军 朱晒红 黄艳艳 李志友 周科朝
文章页码:1773 - 1778
关键词:羟基磷灰石;精氨酸;铕;掺杂;细胞活性
Key words:hydroxyapatite; arginine; europium; hydrothermal synthesis; cell viability
摘 要:采用水热合成法制备经精氨酸表面修饰和铕掺杂的羟基磷灰石纳米颗粒(HAP-Eu)。利用透射电镜(TEM)、Zeta 电位分析仪、傅立叶红外光谱仪和X 射线衍射(XRD)对HAP-Eu的成分、形貌、结构、晶粒粒径和Zeta 电位进行表征,并通过成像流式细胞仪研究细胞活性。结果表明:制备的HAP-Eu 粒径较均匀,约为100 nm,特征峰明显;在pH=7.5 时,颗粒的表面净电荷均值约为30.10 mV;在人上皮细胞和内皮细胞中无毒性。
Abstract:
The arginine-modified and europium-doped hydroxyapatite nanoparticles (HAP-Eu) were synthesized by hydrothermal synthesis. The prepared nanoparticles were characterized by transmission electron microscopy (TEM), X-ray diffractometry (XRD), Fourier transform infrared (FTIR) and zeta potential analyzer. The cell viability of HAP-Eu was tested by image flow cytometry. The results indicated that HAP-Eu is short column shapes and its size is approximately 100 nm, its zeta potential is about 30.10 mV at pH of 7.5, and shows no cytotoxicity in human epithelial cells and endothelial cells.

ZHAO Yan-zhong1, 2, 3, ZHU Jun2, ZHU Shai-hong1, 3, HUANG Yan-yan1, LI Zhi-you2, ZHOU Ke-chao2, 3
1. Medical Experiment Center in the 3rd Xiangya Hospital, Central South University, Changsha 410013, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China;
3. Research Center for Medical Material and Instruments, Central South University, Changsha 410013, China
Received 7 June 2010; accepted 2 August 2011
Abstract: The arginine-modified and europium-doped hydroxyapatite nanoparticles (HAP-Eu) were synthesized by hydrothermal synthesis. The prepared nanoparticles were characterized by transmission electron microscopy (TEM), X-ray diffractometry (XRD), Fourier transform infrared (FTIR) and zeta potential analyzer. The cell viability of HAP-Eu was tested by image flow cytometry. The results indicated that HAP-Eu is short column shapes and its size is approximately 100 nm, its zeta potential is about 30.10 mV at pH of 7.5, and shows no cytotoxicity in human epithelial cells and endothelial cells.
Key words: hydroxyapatite; arginine; europium; hydrothermal synthesis; cell viability
1 Introduction
Hydroxyapatite (HA) is the principal mineral constituent of natural bones and teeth. As the main inorganic component of biological bone and tooth enamel, it shows excellent biocompatibility, bioactivity osteoconductivity and af?nity [1-4]. It has been widely used as an implant biomedical material in orthopedic and dental treatments [5-6]. Especially, recent progress in the preparation of HA nanoparticle helped it exploit a great deal of new uses. It is low crystalline with highly active surfaces and used as carrier in drug delivery systems as well as for protein separation as an absorptive material [7-8]. Interestingly, HA nanoparticles can retard the multiplication of cancer cells [9]. Our previous research [10] reported that HA nanoparticles incorporating pEGFP-N1 are able to deliver DNA into gastric cancer cells without any significant cytotoxicity, whose transfer efficiency is equal to 50% of liposome’s under the equivalent conditions. FRAYSSINET [11] used coprecipitates of HA nanoparticles and DNA to successfully transfect the LacZ reporter gene into bone cells, which further proved the gene transfer ability of HA nanoparticles. TAN et al [12] discovered that after being modified by protamine, the gene transfer efficiency of HA nanoparticles can be enhanced more times. SUN et al [13] successfully used HA nanoparticles to delivery NT-3 gene into the Cochlear neurons of guinea pig both in vitro and in vivo. It could be concluded that all above researches demonstrated HA nanoparticles may be a potential highly effective and safe material as a gene carrier with possible clinical applications.
HA nanoparticles with distinctive inner and outer surfaces can be differentially functionalized for targeting and biocompatibility. To optimize the efficacy in gene delivery, the hydrophilic arginine was conjugated with a guanidyl group onto the surface of HA nanoparticles in a previous study. Arginine has a guanidyl group —(CH2)3NHC(NH2)+ and its isoelectric point is 10.76. Under the experimental conditions, the pH value of the reaction fluid will be consistently lower than the isoelectric point of arginine, and the arginine will have positive zeta potentials during the whole process. They can interact electrostatically with cell membranes. On the other hand, some research revealed that arginine with guanidyl group can facilitate the cellular uptake of covalently attached particles [14], but the mechanism of their uptake is disputed [15]. These physicochemical properties of HA nanoparticles that provide for intracellular penetration of drug molecules have great importance for gene delivery.
In this study, we focus on functionalizing the surfaces of HA nanoparticles using arginine in order to improve the cellular uptake and transfection efficiency. At the same time, almost nontoxic and more stable inorganic europium was selected as fluorescent bioimaging probes and doped with HA nanoparticles. To improve the therapeutic potential of the nanoparticle-based carriers for the intracellular delivery, it is important to understand the physicochemical properties of nanoparticles affecting the cellular uptake mechanism and the intracellular trafficking. Here we present a facile synthesis process to produce ultrafine arginine-modified and europium-doped HA nanoparticles (HAP-Eu) with controlled size and shape. Using the obtained HAP-Eu, their characterization, physicochemical and cell viabilities, were examined to aim at effective and safe gene transfection.
2 Experimental
2.1 Materials
The materials used in this research included calcium nitrate (Ca(NO3)2·4H2O), ammonium phosphate ((NH4)3PO4·3H2O) (AR, China National Pharmaceutical Group Shanghai Chemical Agent Corporation), arginine (Sigma Corporation). All chemicals and solvents were of the highest analytical grade available. Cell culture media, fetal bovine serum was purchased from American Type Culture Collection (ATCC). Ham’s F-12 medium with L-glutamine was purchased from Fisher Scientific (Logan, UT, USA). Phosphate buffer salt solution (PBS) and penicillin-streptomycin were obtained from Sigma-Aldrich (Logan, UT, USA).
2.2 Hydrothermal synthesis
Europium-doped hydroxyapatite nanoparticles were synthesized by hydrothermal method. Aqueous solution with calcium nitrate (Ca(NO3)2·4H2O) and europium nitrate (Eu(NO3)3) was added dropwisely into ammonium dibasic phosphate ((NH4)2HPO4)) and arginine solution, and then were completely stirring. The mole ratio of Ca/P should be 1.67. The reaction temperature should be 60℃. During the reaction, the solution pH was maintained at 9.5 by using ammonia solution or urea. After the calcium and phosphate solution was stirred even, the solution was transferred into an autoclave. The reaction was continued under the set solution temperature until completion. At the end of the experiment, the solids were collected by centrifugation (10 000 r/min) and filtration and then were washed thoroughly using ethanol and deionized water. The product was dried overnight at the vacuum condition.
2.3 Characterization
The solids were characterized by transmission electron microscope (TEM, JEOL Japan) to analyze the nanoparticle crystalline appearance and the particle size. X-ray diffractometry (XRD) was used to analyze phase of HAP-Eu (Rigaku D-Max/2550VB+, Tokyo, Japan, Cu Kα radiation, λ=1.541 78 ?, 40 kV, 30 mA), where the scanning angle and speed were 25°-55°, 2.4 (°)/min, or 5°-75°, 5 (°)/min. The Fourier transform infrared spectrometer was Nicolet Nexus 470, KBr flaking. The excitation and emission spectra of the modified HA nanoparticles were determined by a RF-5301pc spectrofluorometer (Shimadzu, Japan).
2.4 Preparation of nanoparticles Suspension
0.5 g of HAP-Eu powder after being modified by argininc was added into a centrifuge tube and 20 mL of deionized water was added to make its concentration as 25 mg/mL. It was dispersed by ultrasonic wave for 60 min (Ultrasonic Hemogenizer 24710, USA), and was stood for 2 h. The hydroxyapatite nanoparticles suspension had no delamination and appears milky. The nanoparticles were added into a 50 mL glass flask and were sterilized under high pressure for later use. 1 mL nanoparticles suspension sample was taken and ultrasonic treated for 8 min, and was observed with transmission electron microscope (TEM, Tecnai G2 20ST, FEI Co.) for the nano suspension particles sizes and dispersion status.
2.5 Measurement of zeta potential
Under pH value of 7.4, using Zetasizer 3000 HS nano size and potential analyzer (British Malvern Instrument Corporation), the electrophoretic mobility (EPM) of HAP-Eu nanoparticles was measured, and the zeta potential was obtained. Respectively, taking 8 samples, each sample was measured repeatedly for 3 times and their mean value was obtained.
2.6 Preparation of human epithelial cells and endothelial cells and culture
Both human lung epithelial (A549) cells and human aortic endothelial cells (HAECs) were purchased from ATCC. A549 cells were cultured in F-12k medium containing 10% fetal bovine serum. HAECs were cultured in complete media consisting of culture media 199 (M199) supplemented with 20% FBS, 5% low serum growth supplement (LSGS), ~20 ng/mL endothelial growth factor (EGF), and 1% penicillin–streptomycin (all from Invitrogen). Both cells were incubated in a humid environment at 37°C and 5% CO2 in a humidified atmosphere. Upon 80%-90% confluency, the cells were passaged or used for experiments.
2.7 Cell viability
The effect of varying concentrations of HAP-Eu on cell viability in human lung epithelial (A549) cells and human aortic endothelial cells (HAECs) was assessed respectively using fluorescence microscopy and image analysis. In brief, both cell lines were cultured in 6-well tissue culture plates (Fishtech, Logan, USA) at a density of 105 cell/well. Three different concentrations of samples (20, 100, 200 μg/mL) were added to the wells. After the cells were exposed to nanoparticles for 4, 8, 24, or 48 h, the experiments were terminated by imaging flow cytometry (ChemoMetec, Denmark), and the number of cells in each flask was determined using NC-3000? Viability and Cell Count Assay. The protocol of this assay followed manufacturer’s instructions. It is a quick and easy two-color assay, use the cell stain Acridine Orange for cell detection, and the nucleic stain DAPI for detecting non-viable cells, to determine viability of cells in a population based on plasma membrane integrity and esterase activity.
2.8 Statistics
All experiments were repeated at least three times, and the values are expressed as means ± standard deviations. Statistical analysis was performed using student’s t-test, with the significant level with p value of 0.05.
3 Results and discussion
3.1 Features
Under the TEM examination, the hydrothermal synthesized HAP-Eu is short column shapes and its size is approximately 100 nm (Fig. 1(a)). The result of the dynamic light scattering also confirmed that the size distribution of these nanoparticles is relatively homogeneous (Fig. 1(b)). During the process of synthesizing nanoparticles under the hydrothermal equilibrium conditions, Arginine’s absorption of the seeded out HA crystal face selectively affects particles growth. The positive electron guanidyl group —(CH2)3NHC(NH2)+ of arginine is able to have static effect with the negative electron hydroxyl (—OH) exposed on the HA (001) face, resulting in intendancy of arginine to be absorbed on the (001) face of HA nanoparticles.
3.2 Characterization
Figure 2 shows the XRD patterns of HA samples. It can be seen that the prepared nanoparticles show similar XRD graphs. Their characteristic peaks are sharp and apparent, confirming that the resulting Eu-doped HA had the typical pattern of the pure HA nanoparticles. All diffraction peaks could be assigned to the standard one (JCPDs 9-432).

Fig. 1 TEM image (a) and dynamic light scattering result (b) of HAP-Eu crystal synthesized by hydrothermal method

Fig. 2 XRD patterns of HAP-Eu nanoparticles with different dopant of Eu: (a) 0 ; (b) 1% ; (c) 3%; (d) 5%; (e) 7.5%
Surface functionality could be a key factor in cellular response. Therefore, aminated HA nanoparticles with diameters of 100 nm were prepared by arginine. The successful introduction of surface functionality was proved by FT-IR (Fig. 3), which shows the infrared spectrometric waveforms of two sample groups are similar and the main peak positions are identical. The stronger peak lines occur at positions of 565.25, 604.21, 1035.78 and 3441.75 cm-1, and the weaker or broader position peak lines occur at positions of 1 106.57, 1 420.30, 1 631.24 and 3 570.12 cm-1. The four vibration patterns corresponding to peak positions of phosphate radicals in theory respectively are: ν1 peak around 960 cm-1, ν2 peak in 470-440 cm-1 region, ν3 peak in 1 190- 976 cm-1 region, ν4 peak in 600-560 cm-1 region. Therefore, the strong peaks at 565.25, 604.21 and 1 035.78 cm-1 and the weak peaks of 1 106.57 cm-1 are generated by the phosphate radicals of HA naoparticles. The water molecule characteristic peaks in crystal lattice occur in 3 550-3 200 cm-1 region, thus the peaks at 3441.75 and 3570.12 cm-1 positions are the reflection of lattice water and hydroxy group (OH-). The characteristic peak at 1 631.24 cm-1 is the vibration peak of H2O, indicating the surface of the solid samples absorbs a small amount of steam. The characteristic peak of amino group (—NH2) occurs in the 1 400-1 420 cm-1 region and the 1420.30 cm-1 peak is perhaps the reflection of the absorption on HA nanoparticles of the ammonium radical (NH4+) and amino acid residue derived from the raw material ammonium dibasic phosphate. For the added arginine sample, the intensity of this peak is somewhat strengthened, illustrating actual existence of amino acid residue.

Fig. 3 FTIR spectra of arginine-modified nanoparticles: (a) Without amino acid; (b) With arginine; (c) With arginine unwashed
Europium ion (Eu3+) could be used as a luminescent probe in the bimolecular system. And Ca ions on the HA surface could be replaced by the other metal cations with similar ionic radii, especially lanthanide ions. The formation of Eu-doped HA nanoparticles could be confirmed by the luminescence study. The luminescence spectrum of europium-doped HA nanoparticles is shown in Fig. 4. The emission spectrum with the excitation of 394.4 nm (Fig. 4(a)) shows the luminescence at the wavelengths of 588.8 and 612.6 nm, which can be ascribed to 5D0-7F1, and 5D0-7F2 transitions of Eu, respectively. These emission effects could not be observed in the pure HA crystallites due to the absence of the featured Eu element. Thus, the presence of Eu in the HA nanoparticles was confirmed. In addition, the more efficient emission with a maximum intensity at 612.6 nm is in the range of the emission filter chosen for the confocal microscopy. An excitation at 394.4 nm with the highest intensity is close to the visible range. However, another excitation peak was recorded at 464.8 nm, close to the available excitation wavelength in the confocal microscope. Observations on living cells are possible as this excitation wavelength is in the visible region.
3.3 Zeta potential
Figure 5 shows the zeta potential of HAP-Eu at the pH value of 7.5. The results suggested that under the weak alkalescent condition (pH=7.5), the zeta potential of HAP-Eu is (30.1±6.3) mV and that of unmodified HA is (-10.6±4.2) mV (The figure isn’t shown). This illustrates that arginine surface functionalization of HA nanoparticles and cationic aminated functional groups increased its zeta potential value. This change comes from the absorption of amino acids of amino acid residue on the HAP-Eu nanopartilces surface. In the later researches, this substance is designed to be extracted from the aqueous solution medium synthesized from HAP-Eu and titrated to further discuss the hydrothermal crystalline behavior of HA nanoparticles affected by arginine and the hidden mechanism of the surface electronic charge status.
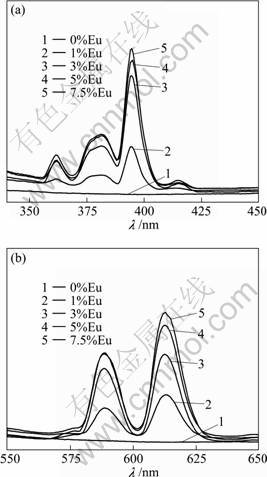
Fig. 4 Luminescence excitation (a) and emission (b) spectra of europium-doped HA nanoparticles

Fig. 5 Zeta potential curve of HAP-Eu nanoparticles at pH value of 7.5
3.4 Cell viability
To ensure that arginine functionalization and europium doped HA nanoparticles do not exert any toxicity on the cells during biological examination, we first analyzed the effects of HAP-Eu on cell viability. The effect of varying concentrations and exposure time of the nanoparticles on cell viability was evaluated against two different human cell models: an epithelial lung cancer cell line (A549) and a normal nonmalignant aortic endothelial cells line (HAECs). The two cell lines were chosen as representative models of the various cellular environments that HAP-Eu are likely to interact with in vivo. The results showed that the studied HAP-Eu did not affect the cells survival in a concentration and time dependent manner. Both the cells exposed to nanoparticles survived well similar to those of the controls (Fig. 6). In addition, compared to lung cancer cells, cell viability of aortic endothelial cells seemed to be some different. Reports in the literature [16] on similar studies with silica nanoparticles suggested that cancer cells are more resistant to nanoparticle-mediated toxicity than normal nonmalignant primary cells of the body. Such resistance is attributed to the fact that normal primary cells of the body are more susceptible to nanoparticle-mediated cellular membrane injury, leading to more rapid onset of apoptotic or necrotic cell death. In conclusion, our data indicated that HAP-Eu is a potential gene carrier in vitro, and further preclinical and clinical development of this carrier for cancer gene therapy is warranted.

Fig. 6 Cell viability assay showing effect of varying concentrations of nanoparticles on growth inhibition: (a) Human lung epithelial (A549) cancer cells; (b) Human aortic endothelial cells (HAECs) cultured in vitro
4 Conclusions
1) During the hydrothermal synthesis process, adding arginine and europium nitrate is advantageous to prepare even-sized and particle-tendency hydroxyapatite nanoparticles.
2) As arginine surface functionalization changes HA nanoparticles surface electron, its zeta potential is changed from (-10.6±4.2) mV of the unmodified into (30.1±6.3) mV of the functionalized.
3) Cell viability test demonstrated that HAP-Eu had a good biocompatibility to human cancer cells and non-cancer cells in a concentration and time dependent manner.
References
[1] WEI G, MA P X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering [J]. Biomaterials, 2004, 25: 4749-4757.
[2] QIU H J, YANG J, KODALI P, KOH J, AMEER G A. A citric acid-based hydroxyapatite composite for orthopedic implants [J]. Biomaterials, 2006, 27: 5845-5854.
[3] LEGEROS R Z. Properties of osteoconductive biomaterials: calciumphosphates [J]. Clinical Orthopaedics and Related Research, 2002, 395: 81-98.
[4] AOKI H, AOKI H, KUTSUNO T. An in vivo study on the reaction of hydroxyapatite-sol injected into blood [J]. Journal of Materials Science: Materials in Medicine,2000, 11: 67-72.
[5] JIANG W, CHENG J, DINESH K. Improved mechanical properties of nanocrystalline hydroxyapatite coating for dental and orthopedic implants [C]//Mater Res Soc Symp Proc. USA: Materials Research Society, 2009: 1140-HH03-03.
[6] ROYA M, AMIT B, SUSMITA B. Induction plasma sprayed nano hydroxyapatite coatings on titanium for orthopaedic and dental implants [J]. Surface and Coatings Technology, 2011, 205(1): 2785-2792.
[7] MATSUMOTO T, OKAZAKI M, INOUE M. Hydroxyapatite particles as a controlled release carrier of protein [J]. Biomateials, 2004, 25(17): 3807-3812.
[8] BOONSONGRIT Y, ABE H, SATO K, NAITO M, YOSHIMURA M, ICHIKAWA H, FUKUMORI Y. Controlled release of bovine serum albumin from hydroxyapatite microspheres for protein delivery system [J]. Materials Science and Engineering B, 2008, 148: 162-165.
[9] LIU Z S, TANG S L, AI Z L. Effects of hydroxyapatite nanoparticles on proliferation and apoptosis of human hepatoma BEL-7402 cells [J]. World Journal of Gastroenterology, 2003, 9(9): 1968-1971.
[10] ZHU Shai-hong, HUANG Bo-yun, ZHOU Ke-chao, HUANG Su-ping,LIU Fang, LI Yi-ming,XUE Zhi-gang, LONG Zhi-gao. Hydroxyapatite nanoparticles as a novel gene carrier [J]. Journal of Nanoparticle Research, 2004, 6(2): 307-311.
[11] FRAYSSINET P. Bone cell transfection in tissue culture using hydroxyapatite microparticles [J]. Journal of Biomedical Materials Research, Part A, 2006, 79(2): 225-228.
[12] TAN K,CHEANG P, IAWH, PYPL K. Nanosized bioceramic particles could function as efficient gene delivery vehicles with target specificity for the spleen [J]. Gene Therapy, 2007, 14: 828-835.
[13] SUN Hong, JIANG Ming, ZHU Shai-hong. In vitro and in vivo studies on hydroxyapatite nanoparticles as a novel vector for inner ear gene therapy [J]. Chinese Journal of Otorhinolaryngology Head and Neck Surgery, 2008, 43(1): 51-57.
[14] BROOKS H, LEBLEU B, VIVES E. Tat peptide-mediated cellular delivery: Back to basics [J]. Advanced Drug Delivery Reviews, 2005, 57(4): 559-577.
[15] UMEZAWA N, GELMAN M A, HAIGIS M C, RAINES R T, GELLMAN S H. Translocation of a beta-peptide across cell membranes [J]. Journal of the American Chemical Society, 2002, 124(3): 368-369.
[16] NAN A, BAI X, SON S J, LEE S B, GHANDEHARI H. Cellular uptake and cytotoxicity of silica nanotubes [J]. Nano letters, 2008, 8(8): 2150-2154.
[17] JIANG X, WEISE S, HAFNER M, REOCKER C. Quantitative analysis of the protein corona on fept nanoparticles formed by transferrin binding [J]. Journal of the Royal Society Interface, 2010, 7(Suppl 1): S5-S13.
[18] OLEG L, TATIANA S, CORNELIA L, BEIL J. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line [J]. ACS Nano, 2011, 5(3): 1657-1669.
赵颜忠1, 2, 3, 朱 军2, 朱晒红2, 3, 黄艳艳1, 李志友2, 周科朝2, 3
1. 中南大学 湘雅三医院医学实验中心,长沙 410013;
2. 中南大学 粉末冶金国家重点实验室,长沙 410083;
3. 中南大学 医用材料与器械研究中心,长沙 410013
摘 要:采用水热合成法制备经精氨酸表面修饰和铕掺杂的羟基磷灰石纳米颗粒(HAP-Eu)。利用透射电镜(TEM)、Zeta 电位分析仪、傅立叶红外光谱仪和X 射线衍射(XRD)对HAP-Eu的成分、形貌、结构、晶粒粒径和Zeta 电位进行表征,并通过成像流式细胞仪研究细胞活性。结果表明:制备的HAP-Eu 粒径较均匀,约为100 nm,特征峰明显;在pH=7.5 时,颗粒的表面净电荷均值约为30.10 mV;在人上皮细胞和内皮细胞中无毒性。
关键词:羟基磷灰石;精氨酸;铕;掺杂;细胞活性
(Edited by YUAN Sai-qian)
Foundation item: Project (81071869) supported by the National Natural Science Foundation of China; Project (2009637526) supported by China Scholarship Council (CSC Program); Project (2010QZZD006) supported by the Key Program of Central South University Advancing Front Foundation
Corresponding author: ZHU Shai-hong; Tel: +86-731-88618958; E-mail: zhushaihong@medmail.com.cn; ZHOU Ke-chao; Tel: +86-731-88836418; E-mail: zhoukechao@csu.edu.cn
DOI: 10.1016/S1003-6326(11)60929-1

