
Preparation of carbon nanotubes by ethanol catalytic combustion technique using nickel salt as catalyst precursor
LI Fei(李 飞) 1,2 , ZOU Xiao-ping(邹小平) 1,2, CHENG Jin(程 进)1,2, ZHANG Hong-dan(张红丹)1,2, REN Peng-fei(任鹏飞) 1,2
1. Research Center for Sensor Technology, Beijing Information Technology Institute, Beijing 100101, China;
2. Beijing Key Laboratory for Sensor, Beijing 100101, China
Received 10 April 2006; accepted 25 April 2006
Abstract: A simple growth technique of carbon nanotubes (CNTs) by combustion of ethanol was developed. In the experiment, copper plate was employed as substrate, nickel nitrate (Ni(NO3)2) and nickel chloride (NiCl2) as catalyst precursor, and ethanol as carbon source. The cleaned copper substrate was dipped into catalyst precursor solution for mounting catalyst precursor particles. The dip-coated substrate was then placed into ethanol flame for about 10 min after drying. The black wool-like production grown on copper plate was obtained. This route is called an ethanol catalytic combustion(ECC) process. The black powders were characterized by means of scanning electron microscopy(SEM), transmission electron microscopy(TEM), energy dispersive X-ray spectrometer(EDS) and Raman spectroscopy. The results show that the techique is much simpler and more economical to meet the future broader applications.
Key words: carbon nanotubes; ethanol catalytic combustion; nickel nitrate; nicket chloride; precursor
1 Introduction
There has been much interest in the production and processing of carbon nanotubes (CNTs) since the first observation of carbon nanotubes in 1991 by Iijima[1]. Many researches have been made on carbon nanotubes because of their novel mechanical, electrical and physical properties[2-4], such as theoretical analysis, preparation methods and devices based on carbon nanotubes[5]. Carbon nanotubes are grown by the diffusion of carbon through a metal catalyst and its subsequent precipitation as graphitic filaments[6]. In the last decade, many preparation methods of carbon nanotubes were developed, such as arc discharge[7], plasma-enhanced chemical vapor deposition[8], pyrolysis[9], solar method[10], and simple thermal chemical vapor deposition by pyrolysis of ethyl alcohol[11]. However, the method above exhibited some drawbacks, such as high cost, low production and complex reaction condition, etc.
Here, we present a simple growth technique of carbon nanotubes by combustion of ethyl alcohol, that is, ethanol catalytic combustion (ECC) technique. Flame synthesis of other novel carbon nanostructures such as fullerenes was reported[12] and flame synthesis of pure CNTs has also met with a little success, so an ethanol burner was used as the source of the flame in our experiment.
Compared to the above methods, ECC technique tenders several inherent advantages: 1) the process of the synthesis can be performed at the common laboratory; 2) ethanol flame synthesis is proven to be an economical method for large areas with multiple flames; 3) liquid ethanol, the very common laboratory fuel, can quite naturally offer both elevated temperature and the hydrocarbon reactant for CNTs synthesis at atmospheric pressure; 4) flame synthesis allows a controlled residence time within a desirable region.
2 Experimental
The salient feature of the proposed method is the use of the very common laboratory ethanol burners. The fuel is pure ethanol. The first step was to prepare the catalyst particles which approximately determine the final diameter of the nanotube (in some cases also whether a single wall or multi-wall nanotube is formed). Hence, it is necessary to control this parameter. Here, the wet catalyst method in which the solution contains catalyst in the form of nickel salt form was applied to the substrate. Nickel nitrate was adopted as the catalyst for the formation of the CNTs. Some quantities of nickel nitrate powers were dissolved in the pure ethanol. And then the solution of catalyst precursor was sonicated for tens of minutes to form a suspension of catalyst. In the experiment, the cleaned copper plate was employed as the substrate. One drop of the saturated catalyst solution was applied with a dip-pen to the copper substrate, which was then placed in a flame without introducing any other gas for the nanotubes growth. The surface of the substrate was faced down against the flame when it was inserted into the central core of the flame. The substrate with catalyst precursor was combusted for several minutes and then carbon deposits accumulated on the copper plate. After a desired time, the sample was cooled to room temperature and dried in air at room temperature. These deposits were removed and examined by means of transmission electron microscopy (TEM) with a JEOL 2010 microscope and scanning electron microscopy (SEM) with a JEOL 6500F microscope. The process of using nickel chloride as the catalyst precursor for synthesizing CNTs was the same as that of using nickel nitrate. TEM was employed to confirm the structure of the nanotubes.
3 Results and discussion
Theoretically, CNTs are considered to be rolled from two-dimensional graphite sheets, that is, a single-walled carbon nanotube (SWCNT) is formed from only one graphite sheet, and a multi-walled carbon nanotube (MWCNT) is formed from several graphite sheets. SEM and TEM images of the carbon nanotubes are shown in Figs.1 and 2. Fig.1 shows SEM image of a typical sample of carbon nanotubes grown on copper substrate using nickel chloride as catalyst precursor for about 10 min synthesis duration. These CNTs have diameters ranging from several tens of nanometers to several hundreds of nanometers. Fig.2(a) shows a TEM image of the deposit material from ethanol. A structure of the CNTs can be seen clearly, which consists of the graphene cylinder. Careful observation of this TEM image reveals that the nanotube has one end connected to a particle. This suggests that the spherical particles drive the growth of the nanotube. The phenomenon is possibly illustrated by a tip-growth mechanism, that is, the catalyst precursor particle moves with the growing carbon nanotube tip. However, the length of the CNTs is small. Truly, the carbon nanotube length is affected by several parameters including the concentration of catalyst solution and growth temperature[13]. Besides, it is also related to the reactivity of the catalyst precursor[14]. Furthermore, the CNTs are not good in morphology. The growth direction was possibly affected by the flame perturbation during the growth of CNTs at the atmosphere. Fig.2(b) shows curly CNTs. Under ideal growth conditions, well-crystallized and straight CNTs could be obtained[15]. And the nanotube should contain graphene walls parallel to the tube axis without any defects. The occurrence of defects (e.g. pentagons or heptagons) would cause the nanotube to bend during growth. Moreover, flame perturbation could be another factor. During the growth of CNTs, flame perturbation drives the catalyst precursor particle to move with a departure from the axis of CNTs.

Fig.1 Low magnification SEM image of deposit material using nickel chloride as catalyst precursor
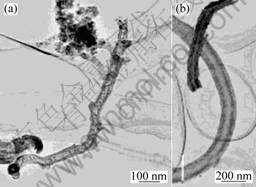
Fig.2 TEM images of carbon nanotubes: (a) Using nickel nitrate as catalyst precursor; (b) Using nickel chloride as catalyst precursor
Fig.3 shows a section image of a carbon nanotube. It can be clearly seen that there is a hollow core in the CNT, indicating that the CNT contains several concentric, coaxial graphene cylinders possibly.

Fig.3 High magnification TEM image of CNT with nickel nitrate catalyst precursor
Fig.4 shows the energy dispersive X-ray spectrometer (EDS) of the sample, showing that the sample consists of Cu and Ni. The existence of Cu is probably from the substrate. The reason for the existence of Ni is as follows: during growth of the CNTs, the salt solution is often reduced to oxide nanoparticles by calcination[15]. Metal oxides are stable and improve the catalyst-support interaction at growth temperature in some cases, metal oxides are used directly as the catalyst[16]. During growth, these oxides are reduced to metal nanoparticles which can catalyze the subsequent growth of carbon nanotubes.
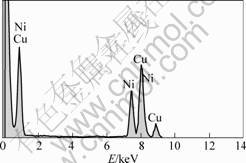
Fig.4 Energy dispersive X-ray spectrometer (EDS) of catalyst from nickel chloride catalyst precursor
Raman spectroscopy is a simple and good tool for analyzing the structure of the CNTs. Raman spectra of CNTs were excited with the 514.5 nm line of a laser by a spectrometer at room temperature. Two peaks (1 345.5 cm-1 and 1 583.4 cm-1 in Fig.5; 1 341.2 cm-1 and 1 589.1 cm-1 in Fig.6) can be observed in the range of 1 200-
1 700 cm-1 in a typical Raman spectrum. 1 250-1 450 cm-1 is the disorder-induced phonon mode (D-band), which is caused by the disordered components[17]. It has a high sensitivity to the disordered structures in carbon materials. 1 550-1 600 cm-1 is the graphite band (G-band)
which is produced from the high degree of symmetry and order of carbon materials, and generally used to identify well-ordered CNTs[17]. In Figs.5 and 6, the peak centered at 1 583.4 cm-1 and 1 589.1 cm-1 indicate a good arrangement of the hexagonal lattice of graphite. Figs.5 and 6 show that the G-band (1 345.5 cm-1) is higher than its D-band (1 583.4 cm-1) in Fig.5, but the G-band (1 341.2 cm-1) is less than its D-band (1589.1 cm-1) in Fig.6. Based on these data, it can be concluded that the sample using NiCl2 as the catalyst precursor has a higher graphitization degree than that using Ni(NO3)2 as the catalyst precursor.
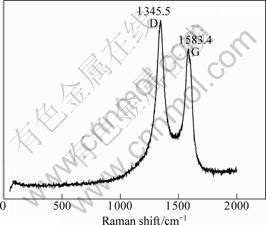
Fig.5 Raman spectrum of sample using Ni(NO3)2 as catalyst precursor

Fig.6 Raman spectrum of sample using NiCl2 as catalyst precursor
4 Conclusions
Carbon nanotubes are synthesized by the ECC technique using NiCl2 or Ni(NO3)2 as catalyst precursor. In addition, liquid ethanol can be successful used as a kind of carbon source for CNTs synthesis. Compared to CVD, pyrolysis and other methods, the catalyst does not need to be exteriorly added when using ethanol flame. The present method has the advantage of being much simpler and more economical. Further extensive research will be on the growth of well-aligned CNTs and explore the possibility for mass-production.
References
[1] IIJIMA S. Helical microtubules of graphitic carbon[J]. Nature, 1991, 354: 56-58.
[2] DRESSELHAUS M S, DRESSELHAUS G, SAITO R. Physics of carbon nanotubes[J]. Carbon, 1995, 33: 883-891.
[3] YAKOBSON B I, BRABEC C J, BERNHOLC J. Nanomechanics of carbon tubes: instabilities beyond linear response[J]. Phys Rev Lett, 1996, 76: 2411-2514.
[4] RUOFF R S, LORENTS D C. Mechanical and thermal properties of carbon nanotubes[J]. Carbon, 1995, 33: 925-930.
[5] MODI A, KORATKAR N, LASS E, WEI B Q, AJAYAN P M. Miniaturized gas ionization sensors using carbon nanotubes[J]. Nature, 2003, 424: 171-174.
[6] GAVILLET J, LOISEAU A, JOURNET C, WILLAIME F, DUCASTELLE F, CHARLIER J C. Root-growth mechanism for single-wall carbon nanotubes[J]. Phys Rev Lett, 2001, 87(27): 27550401-27550404.
[7] BETHUNE D S, KIANG C H, DE VRIES M S, GORMAN G, SAVOY R, VAZQUEZ J, BEYERS R. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls[J]. Nature, 1993, 363: 605-607.
[8] REN Z F, HUANG Z P, XU J W, WANG J H, BUSH P, SIEGAL M P, PROVENCIO P N. Synthesis of large arrays of well-aligned carbon nanotubes on glass[J]. Science, 1998, 282: 1105-1107.
[9] CHEN P, ZHANG H B, LIN G D, HONG Q, TSAI K P. Growth of carbon nanotubes by catalytic decomposition of CH4 or Co on a Ni-MgO catalyst[J]. Carbon, 1997, 35: 1495-1501.
[10] PAN C, XU X R. Synthesis of carbon nanotubes from ethanol flame[J]. J Mater Sci Lett, 2002, 21: 1027-1029.
[11] ZOU X P, ABE H, SHIMIZN T, ANDO A, NAKAYAMA H, ZHU S M, ZHOU H S. Simple thermal ehemical vapor deposition sythesis and electrical property of multi-walled carbon nanotubes[J]. Physica E, 2004, 24: 14-18.
[12] HOWARD J B, THOMAS MCKINNON J, MAKAROVSKY Y, LAFLEUR A L, ELAINE JOHNSON M. Fullerenes C60 and C70 in flames[J]. Nature, 1991, 352: 139-141.
[13] ZHENG L X, O’CONNELL M J, DOORN S K, LIAO X Z, ZHAO Y H, AKHADOV E A, HOFFBAUER M A, ROOP B J, JIA Q X, DYE R C, PETERSON D E, HUANG S M, LIU J, ZHU Y T. Ultralong single-wall carbon nanotubes[J]. Nature, 2004, 3: 673-675.
[14] HEYNINGB O T, BERNIER P, GLERUP M. A low cost method for the direct synthesis of high Y-branched nanotubes[J]. Chem Phys Lett, 2005, 409: 43-47.
[15] TEO K B K, SINGH C, CHHOWALLA M, MILNE W I. Catalytic synthesis of carbon nanotubes and nanofibers[J]. Encyclopedia of Nanoscience and Nanotechnology, 2003, 10: 1-22.
[16] KONG J, CASSEL A M, DAI H. Chemical vapor deposition of methane for single-walled carbon nanotubes[J]. Chem Phys Lett, 1998, 292: 567-574.
[17] LIU Y, PAN C X, WANG J. Raman spectra of carbon nanotubes and nanofibers prepared by ethanol flames[J]. J Mater Sci, 2004, 39: 1091-1094.
(Edited by CHEN Wei-ping)
Foundation item: Project (KM200510772013) supported by the Science and Technology Development Program of Education Committee of Beijing City; Project (2005-2007) supported by Academic Innovative Team Program (Novel Sensor and Materials: Nanodevice and Nanomaterials) of Education Committee of Beijing City
Corresponding author: ZOU Xiao-ping; Tel: +86-10-64884673-812; Fax: +86-10-64879486; E-mail: xpzou2005@gmail.com