低品位石煤矿中钒的浮选回收
来源期刊:中国有色金属学报(英文版)2014年第4期
论文作者:王 丽 孙 伟 刘润清 辜小川
文章页码:1145 - 1151
关键词:钒;石煤;浮选;预富集
Key words:vanadium; stone coal; flotation; pre-concentration
摘 要:用脱泥-浮选方法对低品位石煤矿进行钒的预富集。通过X荧光光谱分析(XRF)、X射线衍射分析(XRD)和扫描电镜(SEM-EDS)方法对该矿进行矿物组成及微观结构分析。结果表明,在酸性条件下,使用醚胺(EA)作为捕收剂可以将石煤中含钒矿物与脉石矿物进行分离。通过脱泥-浮选流程,最终得到的钒精矿中五氧化二钒的品位为1.88%,回收率为76.58%,并且抛除了72.51%的尾矿。对低品位石煤矿进行钒的预富集,可以提高五氧化二钒的品位,降低耗酸物质的含量及冶炼成本。
Abstract: Pre-concentration of vanadium from low-grade stone coal by the method of desliming–flotation was investigated. The mineral composition and microstructure of stone coal were studied systematically by means of X-ray fluorescence spectrometry (XRF), X-ray diffraction (XRD) and scanning electron microscopy (SEM). The results show that selective separation of vanadium-bearing minerals can be achieved by flotation in acidic solution using melamine (EA). The final vanadium concentrate with V2O5 grade of 1.88% and recovery rate of 76.58% is obtained by desliming–flotation process and 72.51% of the raw ore is rejected as tailings. The pre-concentration of vanadium from low-grade stone coal can increase V2O5 grade and decrease the content of acid consuming minerals, which would enable economical utilization of metallurgical vanadium extraction technology.
Trans. Nonferrous Met. Soc. China 24(2014) 1145-1151
Li WANG, Wei SUN, Run-qing LIU, Xiao-chuan GU
School of Mineral Processing and Bioengineering, Central South University, Changsha 410083, China
Received 20 March 2013; accepted 22 September 2013
Abstract: Pre-concentration of vanadium from low-grade stone coal by the method of desliming–flotation was investigated. The mineral composition and microstructure of stone coal were studied systematically by means of X-ray fluorescence spectrometry (XRF), X-ray diffraction (XRD) and scanning electron microscopy (SEM). The results show that selective separation of vanadium-bearing minerals can be achieved by flotation in acidic solution using melamine (EA). The final vanadium concentrate with V2O5 grade of 1.88% and recovery rate of 76.58% is obtained by desliming–flotation process and 72.51% of the raw ore is rejected as tailings. The pre-concentration of vanadium from low-grade stone coal can increase V2O5 grade and decrease the content of acid consuming minerals, which would enable economical utilization of metallurgical vanadium extraction technology.
Key words: vanadium; stone coal; flotation; pre-concentration
1 Introduction
Vanadium is an essential microelement and widely distributed in nature. As an important strategic material, it has extensive applications in various fields, including steel industry and alloy materials [1,2]. In China, vanadium mainly exists in vanadic titanomagnetite and stone coal. Stone coal is a unique vanadium resource with huge reserve [3,4]. It was reported that the gross reserve of V2O5 in stone coal was 118 million tons, which was 2.7 times that in vanadic titanomagnetite and exceeded the total reserve in other countries [5]. The grade of V2O5 in stone coal is generally 0.13%-1.2%.
As a conventional extracting process of vanadium from stone coal, salt roasting-water leaching is being eliminated due to the consequent problems, such as serious environment pollution and low recovery in production [6]. In recent decades, many novel technologies have been proposed and developed to improve the extracting process, including blank roasting-acid leaching, direct acid leaching and oxygen pressure acid leaching [7]. Unfortunately, these researches only focused on the recovery rate of vanadium and pollution problem, instead of the production cost. In 2012, the market price of V2O5 decreased to about $10000 per ton [8], close to the production cost. As a result, only relatively high-grade ore (>0.5% V2O5) has economic value and is worth smelting, which only accounts for 40% of the total ore [9,10]. Considering the low economic benefit and heavy demand for vanadium resources, it is becoming an urgent task to develop novel process for the effective utilization of low-grade stone coals [11].
Beneficiation of low-grade ores before metal extracting process is an effective method to raise the grade of metal and further reduce production cost. ZHAO et al [12] used gravity separation to pre-concentrate vanadium from stone coal. Even though only 9.7% vanadium was lost by this method, the yield of tailing was low and only accounted for 28.9% of the feed ore. Among various kinds of beneficiation methods, flotation has been widely used in the treatment of sulfide and oxide ores due to its high throughput and selectivity [13,14]. In stone coal, vanadium mainly exists in illite, muscovite, roscoelite, and kaolin in the form of isomorphism. It also appears in tantalite and garnet in the form of absorption [15]. Besides, quartz, calcite and carbonaceous are found to be the main gangues in stone coal [16]. Although many studies have been reported on the flotation process of these minerals in the past decades, there is less information on pre-concentration of vanadium in low-grade stone coal by the method.
This work focused on the desliming process and flotation technology of low-grade stone coal in Shanxi province on the basis of its mineralogy. Recovery of slime concentrate was carried out in order to avoid its adverse effect on flotation. Then, flotation condition tests were conducted to study the effects of grinding fineness, pulp pH value and kinds and dosages of reagents on the recovery and grade of V2O5. Closed cycle tests were subsequently carried out based on the optimum parameters of condition tests.
2 Experimental
2.1 Material and characterization
The stone coal in our experiments was obtained from Shanxi Province, China. Chemical composition was characterized by X-ray fluorescence spectrometry (XRF) using a Philips spectrometer. Phase composition and microstructure of the stone coal were investigated by powder X-ray diffraction (XRD, D8-ADVANCE, Bruker Co. Ltd., Germany), chemical phase analysis and scanning electron microscopy with an energy dispersive spectrometer (SEM-EDS, JSM-6490LV, JEOL Co. Japan).
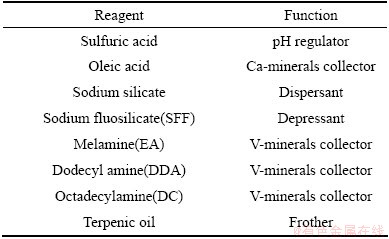
Moreover, particle size distribution of the stone coal (<3 mm) was measured using a wet sieve analysis. The sample was subjected to successive sieving starting from large to small sieves with 1.5, 0.6, 0.154, 0.074, 0.05, and 0.038 mm. The stone coal was gently sieved into a series of fractions (<0.038, 0.038-0.05, 0.05-0.074, 0.074-0.154, 0.154-0.60, 0.6-1.5 and >1.5 mm). Each fraction was filtered, dried, weighed, and analyzed by XRF. The analytical grade reagents used in this study and their abbreviations are listed in Table 1.
Table 1 Reagents used in flotation test
2.2 Flotation
After being crushed to less than 2 mm and deslimed, the ore (500 g) was ground using a XMQ ball mill. Flotation was carried out in a 1.5 L flotation cell at an agitation speed of 2045 r/min, in which pH regulator, depressant and collector were added at a pulp density of 30% (mass fraction). Two-stage flotation experiments were carried out using combination of reverse and direct flotation. Calcite was primarily floated to avoid negative effect on the subsequent flotation of vanadium-bearing minerals, and then the tailing from calcite flotation was further subjected to direct flotation. The detailed process flowsheet is given in Fig. 1. Flotation products such as slime, concentrate and tailing were filtered, dried, weighed, and analyzed by XRF.

Fig. 1 Process flowsheet of stone coal
3 Results and discussion
3.1 Mineralogical characteristics
The mineralogical assembly consisted of quartz as the ore mineral and calcite, barite, dolomite, feldspar, muscovite, montmorillonite as the accessory minerals based on the XRD results. The chemical composition of stone coal is listed in Table 2, which shows relatively low vanadium (V2O5) content of 0.68% and high calcium oxide content of 2.98%. Vanadium phase analysis was carried out according to the sequential extraction procedures. The results are shown in Table 3. 68.80% of vanadium was found to exist in muscovite, while the others were in iron-aluminium oxides.
Table 2 Chemical composition of stone coal

Microstructure and phase structure of the stone coal (<74 μm) were analyzed by SEM (Fig. 2) and EDS (Table 4). Vanadium in the stone coal existed in the form of muscovite and iron-aluminium oxides, which was consistent with the result of phase composition of vanadium. The major gangue minerals were quartz, calcite and barite. The desired mineral (V-muscovite) appeared either needle-like as isolated particles (Area 3) or flake-like (Area 3) closely associated with quartz. Iron-aluminium oxides (Area 6) containing vanadium exhibited morphology of ultrafine particles dispersed in the ore. Calcite (Area 1) and barite (Area 2) appeared as isolated particles with relatively big size than quartz. Moreover, the coarse quartz particles were found to be coated by many V-muscovite fines in the size range of 0-5 μm (Fig. 2(d)). This phenomenon indicated a tenacious slime coating on the primary ore.
Table 3 Phase composition of vanadium in stone coal

3.2 Particle size analysis of raw ore
The stone coal was gently sieved into a series of fractions (<0.038, 0.038-0.05, 0.05-0.074, 0.074-0.154, 0.154-0.60, 0.6-1.5 and >1.5 mm). The particle size distribution is shown in Fig. 3. It can be seen that the yields of fine particles (<0.038 mm) and relatively coarse particles (>0.154 mm) were 18.46% and 71.76%, which were larger than that of particles (0.038-0.154 mm). Interestingly, the grade of V2O5 decreased with the increase of particle size. The grade of V2O5 in the fine particles (<0.038 mm) was 1.86%, about three times that in raw ore.
3.3 Flotation
3.3.1 Effect of slime
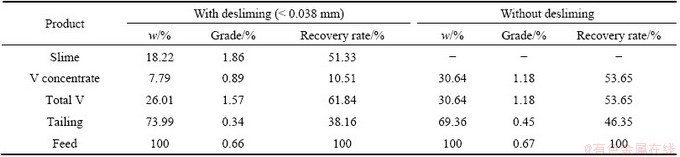
Table 5 shows the flotation results of stone coal with and without desliming at the same dosage of reagents. Concentrate products containing slime and flotation concentrate were obtained with a V2O5 grade of 1.57% and recovery rate of 61.84%, which were much higher than those without desliming. The results show that the slime in the stone coal might have an adverse impact on the flotation performance due to severe mechanical entrainment of valuable minerals in laboratory bench scale. Thus, vanadium recovery from the slime was investigated with different dispersants by settlement. The slime with a V2O5 grade of 2.30% and recovery rate of 45.90% was obtained after a two-stage desliming process. In the first stage, sodium silicate (2000 g/t) was firstly added in the pulp (solid to liquid ratio: 1/3), followed by agitation for 15 min at a speed of 500 r/min. The slime was pumped out after letting stand for 5 min. In the second stage, sodium silicate (1000 g/t) was added into the pulp as other parameters remained unchanged.

Fig. 2 SEM images of stone coal
Table 4 EDS analysis including elemental composition and mineral identification of stone coal
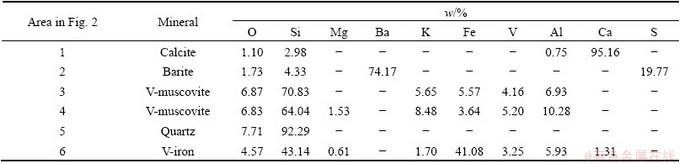
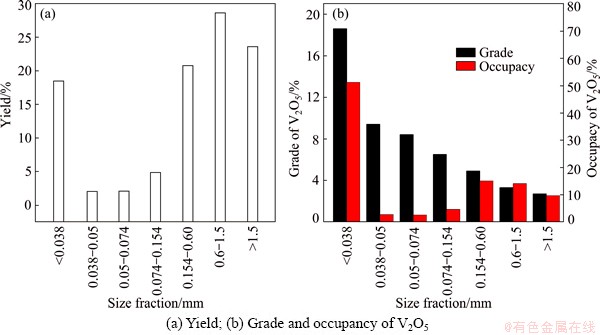
Fig. 3 Particle size distribution of raw ore (<2 mm)
Table 5 Effect of desliming on vanadium-bearing minerals flotation
3.3.2 Effect of grinding fineness
Flotation tests were carried out in the pH range of 4-5 using EA (100 g/t) as collector and SFF (200 g/t) as depressant. The effects of grinding fineness on the grade and recovery rate of V2O5 were investigated as shown in Fig. 4(a). It was indicated that the grade of V2O5 in flotation concentrate decreased slightly with the increase of grinding fineness. There was a sharp increase in the recovery rate of V2O5 from 15.15% to 22.89% with an increase in grinding fineness from 50.12% to 76.56%, while the recovery rate turned to decrease with the further increase of grinding fineness. Therefore, the optimum grinding fineness was determined as 76.56%. The optimum flotation parameters of calcite were obtained by condition experiments as follows: pH (7-8), water glass as depressant (2000 g/t) and oleic acid as collector (200 g/t). All further flotation experiments of vanadium minerals were carried out based on the optimum grinding fineness and flotation conditions of calcite.
3.3.3 Effect of pulp pH
The effects of pulp pH on the recovery rate and grade of V2O5 in vanadium concentrate are shown in Fig. 4(b). It was indicated that the grade of V2O5 decreased sharply while the recovery rate remained unchanged with the increase of pulp pH. The optimum flotation results were achieved around pH 2. It was known that strong acidity would result in high cost of reagents and corrosion to equipments. Therefore, the pulp pH was recommended as 3, with which the grade and recovery rate of V2O5 in vanadium concentrate were obtained as 1.42% and 22.50%, respectively.

Fig. 4 Effects of grinding fineness (a), pulp pH (b) and sodium fluosilicate dosage (c) on grade and recovery rate
3.3.4 Effect of sodium fluosilicate dosage
A reagent dosage of sodium fluosilicate from 50 to 400 g/t was selected and its effects on the recovery rate and grade of V2O5 in the vanadium concentrate are illustrated in Fig. 4(c). The results showed that the recovery rate of V2O5 decreased from 26.91% to 22.89% and the grade increased from 1.23% to 1.38% with the increase of sodium fluosilicate dosage. Nevertheless, the grade and recovery rate of V2O5 slowed down when sodium fluosilicate dosage exceeded 150 g/t. Thus, sodium fluosilicate with a dosage of 150 g/t was adopted in the following experiments.
3.3.5 Effects of flotation collectors and EA dosage
Three kinds of cationic collectors (EA, DDA and DC) were used in the flotation of vanadium-bearing minerals and the flotation results are presented in Fig. 5(a). It was demonstrated that EA had the best flotation selectivity while DC and DDA showed poor selectivity for vanadium minerals in stone coal. Therefore, EA was determined as the collector to separate vanadium-bearing minerals from the gangue.
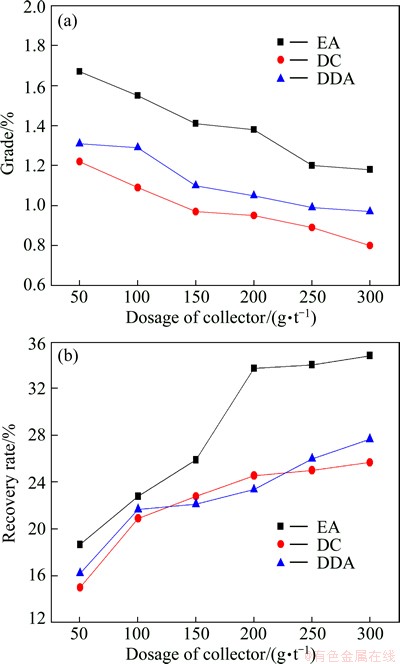
Fig. 5 Effects of collector type and EA dosage on grade (a) and recovery rate (b)
Under the flotation condition of pulp pH 3 and sodium fluosilicate 150 g/t, the effects of EA dosage on the recovery rate and grade of V2O5 in vanadium concentrate are shown in Fig. 6(b). There was a rapid increase of V2O5 recovery rate when the initial EA dosage increased from 50 to 200 g/t, whereas a gentle increase of V2O5 recovery rate was observed when the initial EA dosage exceeded 200 g/t. The grade of V2O5 in concentrate decreased slightly with the increase of EA dosage. Thus, the optimum EA dosage was determined as 200 g/t for further flotation tests, with which a concentrate with V2O5 recovery rate of 33.70% and grade of 1.38% can be obtained.
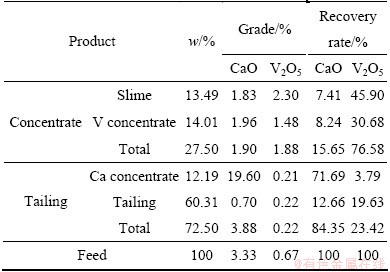
3.4 Locked cycle test
The experimental results of closed cycle test are shown in Table 6. A total vanadium concentrate (slime and V concentrate) can be obtained with V2O5 grade of 1.88% and recovery rate of 76.58%. Besides, the grade and recovery rate of CaO in calcium concentration were 19.60% and 71.69%. Most of CaO in the raw ore was taken off while there was only 3.79% loss rate of V2O5 in calcium concentrate. Therefore, the flotation process can not only improve the grade of V2O5, but also decrease the content of high acid consumption minerals.
In order to investigate the effects of flotation reagents and other factors on follow-up metallurgy process, direct acid leaching of flotation products was conducted using calcium fluoride and sodium chlorate as leaching agents. The leaching efficiencies of vanadium concentrate and tailing were found to be 86.25% and 95.77%, while the leaching efficiency of raw ore was 85.41%. The results indicated no adverse effect of flotation process on the leaching efficiency of stone coal. This may be attributed to the lower reagent dosages in desliming and flotation compared with leach technology of vanadium.
Table 6 Closed cycle results of stone coal
3.5 Economic evaluation
The grade of V2O5 can be improved from 0.67% to 1.88% by desliming–flotation process. As a result, the raw ore needed for extracting per ton of V2O5 can be lowered to 53.48 t from the initial 149.25 t, which largely reduces the investment cost of metallurgy. 72.50% of the feed ore, including most of the acid consuming minerals, can be rejected, which reduces the follow-up metallurgical production cost in terms of thermal energy and leaching reagent dosages. The pulp concentration in desliming process is close to that in flotation process, while less amount of sulfuric acid is needed for high sedimentation rate of desliming. Besides, only conventional reagents were used in this study, resulting in relatively low costs of desliming and flotation.
4 Conclusions
1) Desliming–flotation technique was applied in the pre-concentration of vanadium from low-grade stone coal based on its mineralogical characters. The grade of V2O5 decreases with the increase of particle size in raw ore (<3 mm).
2) Desliming was found to be indispensible before flotation to recover ultrafine particles and improve the flotation performance. The slime concentrate with V2O5 grade of 2.30% and recovery rate of 45.90% is obtained through a two-stage desliming process.
3) Systematic evaluation of flotation parameters on V2O5 grade and recovery rate was conducted, including grinding fineness, pulp pH, kinds and dosages of reagents. EA is proven to be an effective cationic collector for vanadium-bearing minerals. Closed cycle test was carried out based on the flotation conditions. A total vanadium concentrate (slime and flotation concentrate) can be achieved with V2O5 grade of 1.88% and recovery rate of 76.58%.
References
[1] CHEN Xiang-yang, LAN Xin-zhe, ZHANG Qiu-li, MA Hong-zhou, ZHOU Jun. Leaching vanadium by high concentration sulfuric acid from stone coal [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S1): s123-s126.
[2] ZHANG Yi-min, BAO Shen-xu, LIU Tao, CHEN Tie-jun, HUANG Jing. The technology of extracting vanadium from stone coal in China: History, current status and future prospects [J]. Hydrometallurgy, 2011, 109(1): 116-124.
[3] HE Dong-sheng, FENG Qi-ming, ZHANG Guo-fang, OU Le-ming, LU Yi-ping. An environmentally-friendly technology of vanadium extraction from stone coal [J]. Minerals Engineering, 2007, 20(12): 1184-1186.
[4] CAI Zhen-lei, FENG Ya-li, LI Hao-ran, DU Zhu-wei, LIU Xin-wei. Co-recovery of manganese from low-grade pyrolusite and vanadium from stone coal using fluidized roasting coupling technology [J]. Hydrometallurgy, 2013, 131-132: 40-45.
[5] YE Pu-hong, WANG Xue-wen, WANG Ming-yu, FAN Ye-ye, XIANG Xiao-yan. Recovery of vanadium from stone coal acid leaching solution by coprecipitation, alkaline roasting and water leaching [J]. Hydrometallurgy, 2012, 117: 108-115.
[6] LIU Hui-bin, DU Hao, WANG Da-wei, WANG Shao-na, ZHENG Shi-li, ZHANG Yi. A kinetics analysis of decomposition of vanadium slag by KOH sub-molten salt method [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1489-1500.
[7] WANG Ming-yu, XIANG Xiao-yan, ZHANG Li-ping, XIAO Lian-sheng. Effect of vanadium occurrence state on the choice of extracting vanadium technology from stone coal [J]. Rare Metals, 2008, 27(2): 112-115.
[8] RUBIO J, SOUZA M L, SMITH R W. Overview of flotation as a wastewater treatment technique [J]. Minerals Engineering, 2002, 15(3): 139-155.
[9] DENG Zhi-gan, WEI Chang, FAN Gang, LI Min-ting, LI Cun-xiong, LI Xin-bin. Extracting vanadium from stone-coal by oxygen pressure acid leaching and solvent extraction [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S1): s118-s122.
[10] ZHU Yang-ge, ZHANG Guo-fan, FENG Qi-ming, LU Yi-ping, OU Le-ming, HUANG Si-jie. Acid leaching of vanadium from roasted residue of stone coal [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S1): s107-s111.
[11] MOSKALYK R R, ALFANTAZI A M. Processing of vanadium: A review [J]. Minerals Engineering, 2003, 16(9): 793-805.
[12] ZHAO Yun-liang, ZHANG Yi-min, LIU Tao, CHEN Tie-jun, BIAN Ying, BAO Shen-xu. Pre-concentration of vanadium from stone coal by gravity separation [J]. International Journal of Mineral Processing, 2013, 121: 1-5.
[13] RAHMAN R M, ATA S, JAMESON G J. The effect of flotation variables on the recovery of different particle size fractions in the froth and the pulp [J]. International Journal of Mineral Processing, 2012, 106: 70-77.
[14] LIU J J, MACGREGOR J F, DUCHESNE C, BARTOLACCI G. Flotation froth monitoring using multiresolutional multivariate image analysis [J]. Minerals Engineering, 2005, 18(1): 65-76.
[15] LI Cun-xiong, WEI Chang, DENG Zhi-gan, LI Ming-gan, LI Xin-bin, GAN Fan. Recovery of vanadium from black shale [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S1): s127-s131.
[16] ZHANG Yi-min, HU Yang-jia, BAO Shen-xu. Vanadium emission during roasting of vanadium-bearing stone coal in chlorine [J]. Minerals Engineering, 2012, 30: 95-98.
王 丽,孙 伟,刘润清,辜小川
中南大学 资源加工与生物工程学院,长沙 410083
摘 要:用脱泥-浮选方法对低品位石煤矿进行钒的预富集。通过X荧光光谱分析(XRF)、X射线衍射分析(XRD)和扫描电镜(SEM-EDS)方法对该矿进行矿物组成及微观结构分析。结果表明,在酸性条件下,使用醚胺(EA)作为捕收剂可以将石煤中含钒矿物与脉石矿物进行分离。通过脱泥-浮选流程,最终得到的钒精矿中五氧化二钒的品位为1.88%,回收率为76.58%,并且抛除了72.51%的尾矿。对低品位石煤矿进行钒的预富集,可以提高五氧化二钒的品位,降低耗酸物质的含量及冶炼成本。
关键词:钒;石煤;浮选;预富集
(Edited by Xiang-qun LI)
Foundation item: Project (2012BAB07B05) supported by the National Technology Support Project of China; Project (2013zzts066) supported by Independent Innovation Foundation of Central South University, China
Corresponding author: Wei SUN; Tel: +86-731-88830482; E-mail: sunmemghu@126.com
DOI: 10.1016/S1003-6326(14)63173-3

