
Synthesis technology of spinel AlON
WEI Chun-cheng(魏春城), TIAN Gui-shan(田贵山)
School of Material Science and Engineering, Shandong University of Technology, Zibo 255049, China
Received 15 July 2007; accepted 10 September 2007
Abstract: The high-purity AlON ceramics, AlON/Al2O3 and AlON/AlN multiphase ceramics were prepared by pressureless sintering under nitrogen-based atmosphere with raw materials of Al2O3, AlN and Al powders. The compositions of the phase were determined by XRD, the fracture appearance was analyzed by SEM. The results indicate: the aluminum oxynitride phase in the AlON ceramics and other multiphase ceramics exist in the form of Al5O6N, whose solid-solution zone is 33.4%(mole fraction) AlN belonging to spinel structures. The average grain size of high-purity AlON is about 3 ?m. The grain boundary is smooth without other phases. The density is 2.82 g/cm3, the microhardness (10 N) and about bending strength are 1 470 MPa and 94 MPa, respectively.
Key words: pressureless sintering; spinel; aluminum oxynitride; high-purity
1 Introduction
The spinel aluminum oxynitride(AlON) is an important single-phase, stable solid solution ceramic of A12O3-AlN system, and has become a quite potential new material with unique performances. There are some excellent optical, physical, mechanical and chemical properties of AlON ceramics, in particular it can be sintered into the transparent one for its isotropy, and it is the optimum material for the infrared windows and covers to resist high temperature[1]. In addition, AlON ceramics are provided with high-temperature-resistance and chemical corrosion-resistance, with little wettability to liquified glass and hot metal, and with high resistance to heat shocks. Therefore, it is an excellent refractory[2-3]. Its transparence range covers infrared region (0.2 ?m) to ultraviolet region (6.0 ?m), and its strength and microhardness (2 N) reach 380 MPa and 19 110 MPa, respectively[1].
So far, AlN-Al2O3 binary system[4] has been discovered to possess kinds of nitrogen aluminum oxynitride phases. According to the crystal structures, these are divided into two groups approximately: one is the wurtzite structure, the other is the spinel structure. The solid solution zone of the later is 27%-40%(mole fraction) AlN. AlON ceramics are usually prepared by carbothermal reduction method[5], solid phase reaction method[6] or self-propagating combustion method[7]. In carbothermal reduction method, AlON ceramics are difficult to densify, and CO will generate during the preparation. In solid phase reaction of AlN and Al2O3 powders, there are many problems, such as the high sintering temperature, the long reaction time and the multiphase AlON. The low-purity of AlON is the main disadvantage in self-propagating combustion method.
In this experiment, by choosing Al powders as sintering-aid, high-purity spinel AlON ceramics have been fabricated in pressureless sintering for nitrogen-based atmosphere with shorter reaction time and lower sintering temperature. It is discovered that AlON ceramics are composed of single-phase Al5O6N.
2 Experimental
2.1 Sample preparation
In this experiment, high purity Al2O3, AlN, and Al powders were selected as the raw materials with grain size of 2, 0.5 and 1 ?m, respectively. The raw materials were weighed and mixed according to Y1, Y2, Y3, Y4 (see Table 1). The mixtures were ground for 24 h with absolute alcohol as grind medium. After being discharged and dried, the mixtures were pressed by dry pressing method under 20 MPa. Finally the sintering
Table 1 Compositions of starting materials of various samples (mass fraction, %)
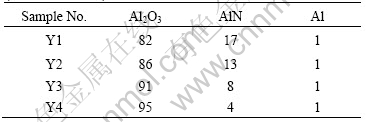
process was carried out in the multi-function sintering furnace. The vacuum sintering was performed under 1×10-3 Pa. The temperature was elevated with a heating rate of 6 ℃ /min. When the sintering temperature reached 1 000 ℃, nitrogen gas was inflated to form atmospheric pressure. After the temperature reached 1 750 ℃, the holding time was 1 h.
2.2 Properties testing
The density of samples was measured based on Archimedes drainage method. The phase structures were quantitatively analyzed by XRD (D/MAX-RB), which enabled to confirm the phase compositions during the reaction. The microstructure was observed and analyzed with scanning electron microscope (SEM Sirion200, Netherlands). The microhardness was determined with HV-1000 microhardness tester. The samples were machined into strips in dimension of 3 mm×4 mm×40 mm, and tested in three-point bend test with span of 30 mm and loading velocity of 0.5 mm/min.
3 Results and discussion
3.1 Phase analysis
Fig.1 shows the XRD patterns of different compositions after being sintered at 1 750 ℃. No impurity peaks exist in Y3, in contrast the diffraction peaks of AlON are very strong, which indicates the high purity in the sintering body. Table 2 lists the quantitative analysis of the XRD patterns. It is clear from the patterns that if the content of AlON changes, the compositions of different sintered bodies will change. Y1 and Y2 are AlON/AlN multiphase ceramics, Y3 is high-purity AlON, and Y4 is AlON/Al2O3 multiphase ceramic. The AlON phase exists in the form of Al5O6N with 66.7%Al2O3 and 33.3% AlN (mole fraction). Besides, the solid solution zone of spinal structure is 27%-40% (mole fraction) AlN. Therefore, the sample belongs to the spinel aluminum oxynitride. The solution reaction is as follows:
Al2O3(s)+AlN(s) AlON (s) (1)
AlON (s) (1)
According to calculation, 245 g of Al5O6N can be formed through the reaction of 204 g of Al2O3 and 41 g
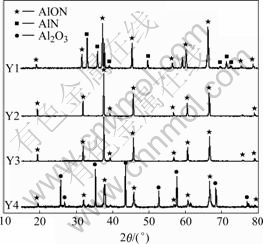
Fig.1 XRD patterns of AlON at 1 750 ℃
Table 2 Quantitative analyses of AlON (mass fraction, %)
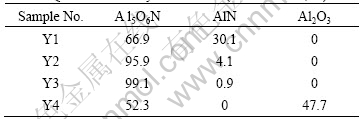
of AlN, which indicates the theoretical value of mass proportion between Al2O3 and AlN is 5?1. In contrast, the mass proportion of mixture Y3 that was prepared to AlON ceramics was 9?1, which suggested the dosage of AlN was shorter than the theoretical value. Thus, in the solid phase reaction of AlON, other reactions occurred at the same time. BACHELARD[8] has proposed that AlN produces Al2O3 at 300-700 ℃, so the reaction is as follows:
4AlN+3O2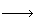 2Al2O3+2N2 (2)
2Al2O3+2N2 (2)
Along with temperature increasing, under the condition of nitrogen and high temperature, reversible reaction will occur that part of Al2O3 produces AlN, then AlN will produce AlON with the Al2O3 again, which affects the mixture ratio between Al2O3 and AlN. Obviously, at 1 750 ℃, if we prolong the reaction time of Y4, it is likely to obtain high-purity Al5O6N aluminum oxynitride ceramics.
3.2 Microtopographt analysis
Fig.2 shows the SEM micrographs of fractures of samples sintered, which shows great size differences between crystal grains.
Fig.2(a) shows that the average grain diameter is about 2 ?m, and the size distribution is non-uniform with massive tiny particles, which is AlN particles judged from the particle size and the appearance. Also, there are many pores. In Fig.2(b) the average grain diameter is

Fig.2 SEM photographs of AlON: (a) Sample Y1; (b) Sample Y2; (c) Sample Y3; (d) Sample Y4
about 3 ?m, and the grain-size distribution is uniform. The characteristics of grains in Fig.2(c) are as follows: the grains distribute uniformly without abnormal growth, which indicates the crystals grow perfectly; there are no glass phase or crystallization phase between the grains; the average size of the particle size is approximately 3 ?m, which is the smallest one in all the literatures that have been reported[9-10], and it is in favor of promoting the mechanical strength. The sample possesses compact structure, few pores, whose grains in Fig.2(d) are about 10 ?m. This suggests that the increasing of A12O3 leads to densification.
3.3 Properties of sintering body
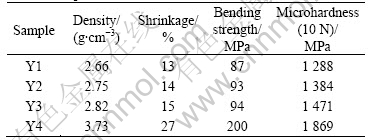
The density, bending strength and microhardness of all samples after sintering reaction are listed in Table 3. It is evident that the samples(Y4) with low content of AlN are easy to sinter, and the sintering behavior is similar to Al2O3. In contrast, when the content of AlN is higher (Y1, Y2, Y3), it is difficult to densify. The density of the high-purity AlON sample Y3 is 2.82 g/cm3, which is approximately 76% of the theoretical density of 3.71 g/cm3. Therefore, massive pores exist in the test sample. The density of the sample Y4 is 3.73 g/cm3, which is higher than the theoretical density of AlN of 3.26 g/cm3 and the theoretical density of AlON, but smaller than the theoretical density of Al2O3 of 3.83 g/cm3. So that, in the sample there is Al2O3, which is in agreement with the results of XRD. It is reported that the bending strength of AlON is higher than that of Al2O3[11-12]. However, in the experiment, the bending strength of Y3 is less than that of Y4 because of higher porosity. Further analysis indicates that increasing density will be able to enhance the bending strength and microhardness.
Table 3 Properties of AlON
4 Conclusions
1) High-purity AlON ceramics was prepared by nitrogen and pressureless sintering at 1 750 ℃ for 1 h, which exists in the form of Al5O6N. The solid-melting zone is 33.4%(mole fraction) AlN, which indicates spinel structure. In the high-purity AlON, no obvious impurity phase can be determined. The grains bond directly, so there is no remarkable glass phase along the grain boundaries. The average diameter is 3 ?m, the density, microhardness (10 N) and bending strength are 2.82 g/cm3, 1 470 MPa and 94 MPa, respectively.
2) The microhardness and bending strength of AlON/Al2O3 multiphase ceramics are higher than those of AlON/AlN multiphase ceramics and AlON ceramics.
References
[1] HARTNETT T M. Optical properties of AlON [J]. Infrared Physics & Technology, 1998, 39: 203- 205.
[2] ZHANG Zuo-tai, LI Wen-chao, SAIYIN Bater. Manufacture and properties of AlON-TiN particulate composites [J]. Materials and Design, 2005, 26: 363-368.
[3] WANG X, LI W, SEETHARAMAN S. Thermodynamic study and synthesis of γ-aluminum oxynitride [J]. Scand J Metall, 2002, 31: 1-6.
[4] MCCAULEY J W, CORBIN N D. Phase relations and reaction sintering of transparent cubic aluminum oxynitride spinel (AlON) [J]. J Am Ceram Soc, 1979, 62: 476-479.
[5] LI Ya-wei, LI Nan, YUAN Run-zhang. Effect of raw materials on carbo-thermal reduction synthesis of aluminum oxynitride spinel powder [J]. J Mater Sci Lett, 1999, 34: 2547-2552.
[6] GROMOV A, ILYIN A, DITTS A, VERESHCHAGIN V. Combustion of Al–Al2O3 mixtures in air [J]. J Eur Ceram Soc, 2005, 25: 1575-1579.
[7] ZIENTARA D, BUCKO M, LIS J. Alon-based materials prepared by SHS technique [J]. Journal of the European Ceramic Society, 2007, 27: 775-779.
[8] BACHELARD R. Aluminium nitride by carbothermal nitridation Joubert [J]. Mater Sci Eng, 1989, 9: 247-251.
[9] PEI Xin-mei. Fabrication and properties of AlON ceramics [J]. Journal of Wuhan University of Technology, 2001, 7(7): 8-10.
[10] WANG Xi-dong, WANG Fu-ming, LI Wen-chao. Synthesis, microstructures and properties of γ-aluminum oxynitride [J]. Materials Science and Engineering A, 2003, 342: 245-250.
[11] YUN Si-ning, JIANG Ming-xue, LI Yong, LI Jian-long, TANG Shi-yin, WANG Li. Structure, property and application of aluminum oxynitride ceramics [J]. Naihuo Cailiao, 2003, 37(3): 173. (in Chinese)
[12] LI Ya-wei, LI Nan, YUAN Run-zhang. State of the art: a review on aluminum oxynitride spinel [J]. Naihuo Cailiao, 2000, 34(2): 108-111. (in Chinese)
(Edited by PENG Chao-qun)
Foundation item: Project(2001AA333040) supported by the High-tech Research and Development Program of China
Corresponding author: TIAN Gui-shan; Tel: +86-533-2781321; E-mail: tgs@sdut.edu.cn