LiNi0.5Mn0.5O2电极在LiNO3电解液中的 电化学性能及循环衰减机理
来源期刊:中国有色金属学报(英文版)2014年第2期
论文作者:王海燕 何菡娜 周 南 金冠华 唐有根
文章页码:415 - 422
关键词:锂离子电池;LiNi0.5Mn0.5O2;循环衰减机制;循环伏安法;LiNO3溶液
Key words:lithium ion battery; LiNi0.5Mn0.5O2; cyclic fading mechanism; cyclic voltammetry; LiNO3 solution
摘 要:研究LiNi0.5Mn0.5O2电极在LiNO3水溶液中的电化学行为,同时分析该电极在不同pH值电解液中的循环衰减原因。循环伏安测试显示LiNi0.5Mn0.5O2在浓度为5 mol/L的LiNO3水溶液中具有较好的锂离子脱嵌能力。对比发现,LiNi0.5Mn0.5O2电极在浓度为5 mol/L,pH值为12的LiNO3水溶液中具有最好的循环稳定性能。通过交流阻抗法、X射线衍射分析及电极形貌的对比分析发现:电极在浓度为5 mol/L,pH值为12的LiNO3水溶液中循环时,电极的表面形貌和电极结构都能得到较好的保持,电极的电荷传递阻抗得到明显抑制,因此在该pH值电解液中的循环稳定性最好。
Abstract: Electrochemical behavior of layered LiNi0.5Mn0.5O2 in LiNO3 aqueous solution and its cyclic fading mechanism in electrolytes with different pH values were investigated. CV results show that LiNi0.5Mn0.5O2 has good electrochemical reversible behaviors in 5 mol/L LiNO3 solution. Meanwhile, the electrode in 5 mol/L LiNO3 with pH value of 12 demonstrates the best electrochemical stability. Based on the electrochemical impedance spectroscopy (EIS), X-ray diffraction (XRD) and scanning electron microscopy (SEM) results, it is proposed that suppressed charge-transfer resistance is the major reason, which is probably ascribed to the more stable electrode surface and less structure change.
Trans. Nonferrous Met. Soc. China 24(2014) 415-422
Hai-yan WANG, Han-na HE, Nan ZHOU, Guan-hua JIN, You-gen TANG
Key Laboratory of Resources Chemistry of Nonferrous Metals, Ministry of Education, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 31 December 2012; accepted 13 April 2013
Abstract: Electrochemical behavior of layered LiNi0.5Mn0.5O2 in LiNO3 aqueous solution and its cyclic fading mechanism in electrolytes with different pH values were investigated. CV results show that LiNi0.5Mn0.5O2 has good electrochemical reversible behaviors in 5 mol/L LiNO3 solution. Meanwhile, the electrode in 5 mol/L LiNO3 with pH value of 12 demonstrates the best electrochemical stability. Based on the electrochemical impedance spectroscopy (EIS), X-ray diffraction (XRD) and scanning electron microscopy (SEM) results, it is proposed that suppressed charge-transfer resistance is the major reason, which is probably ascribed to the more stable electrode surface and less structure change.
Key words: lithium ion battery; LiNi0.5Mn0.5O2; cyclic fading mechanism; cyclic voltammetry; LiNO3 solution
1 Introduction
Aqueous lithium ion battery (ALIB) first reported by LI et al [1] has received considerable attention for fundamental studies as well as probable extensive applications owing to its low cost, intrinsic safety, environmental friendliness and easy operation [1-13]. During the low voltage power supplies, it is well proved that energy density of ALIB is of competition with those of Pd-acid and Ni-Cd batteries. However, in comparison with the corresponding lithium ion battery with non-aqueous electrolyte, it is still much lower for the limited stable electrochemical window of aqueous solution. Moreover, cyclic life of ALIB is much poorer.
It is well known that selection of appropriate intercalation cathode and anode materials with proper Li-ion insertion/extraction potential and high discharge capacity as well as long cyclic performance is a key access to ALIB with high energy density and excellent cyclic stability. Up to now, electrochemical properties of LiMn2O4 [1,2,5,13], LiCoO2 [8,12], LiNi0.81Co0.19O2 [14], LiFePO4 [15,16], LiMn0.05Ni0.55Fe0.9PO4 [17], LiMnPO4 [18], LiNi1/3Co1/3Mn1/3O2 [7,10,11] as cathode materials and VO2(B) [1,2], LixV2O5 [9,11], LiV3O8 [8,10,12,14], PPy coated MoO3 [19], TiP2O7 [20], LiTi2(PO4)3 [17,20], Na2V6O16·xH2O [21], H2V3O8 [22] as anode materials in different lithium aqueous electrolytes have been reported. It has been found that electrochemical performance of such materials strongly depends on the pH value of the used aqueous electrolyte [7,9]. Unfortunately, electrochemical stable window of the electrolyte reduces with increasing the content of OH- in electrolyte, which is not conducive to enough release of lithium ion from bulk material. Thus, detailed studies of electrochemical behavior of electrode materials in aqueous electrolytes, especially the effects of pH value on electrochemical stability are essential. Our group [11] studied the effect of pH value on electrochemical stability of LiNi1/3Co1/3Mn1/3O2 in LiNO3 solution and chose 5 mol/L LiNO3 with pH 11 as electrolyte for aqueous lithium ion battery. WANG et al [7] investigated the capacity fade mechanism of LiNi1/3Co1/3Mn1/3O2 at Li2SO4 solution and found that the electrode was more stability in electrolyte with pH 13 than that with pH 11. Meantime, they considered that capacity fading of the electrode was attributed to H+ intercalation when cycling in neutral electrolyte. On the basis of previous reports, it is still very necessary to find proper aqueous electrolyte with the best pH value for various cathode materials and further investigate the cycling fading mechanism, which would favor the best electrochemical behavior of electrode in aqueous solution.
Layered LiNi0.5Mn0.5O2 is successively proposed by SPAHR et al [23], and MAKIMURA and OHZUKU [24] as a promising positive material for lithium ion battery. It has a reversible capacity as high as 200 mA·h/g in the voltage range of 2.5-4.5 V, good cyclic ability and excellent thermal stability. However, there is no study involving its electrochemical properties in aqueous electrolyte. In this work, layered LiNi0.5Mn0.5O2 was prepared and proposed to be cathode material for aqueous lithium ion battery, and the cyclic fading mechanism of electrode in LiNO3 solutions with different pH values was further discussed.
2 Experimental
Precursor [Ni0.5Mn0.5](OH)2 was synthesized by surfactant (sodium dodecyl sulfonate, SDS) assisted co-precipitation method. The appropriate amounts of NiSO4 and MnSO4 (cationic ratio of Ni:Mn=1:1) and surfactant SDS (the mass concentration of 0.056 g/L) were dissolved and then poured into a round-bottomed flask with long neck. The solution (about 150 mL) was stirred for 2 h by magnetic stirring apparatus, with the reaction temperature of 45 °C. After that, the appropriate alkaline solution consisted of NaOH and ammonia (molar ratio=4:1) was added drop-wise into round-bottomed flask under vigorous stirring. The hydroxide precipitation was then filtered, washed, and dried overnight at 110 °C. After being simply ground by hand, the powder was mixed with required amount of LiOH (cationic ratio of Li:Ni=2.1:1). Then the mixed powder was ground for 5 h by ball milling and calcined at 800 °C for 12 h.
Crystal structure was identified by X-ray diffractometer ( Rigaku D/MAX2500, Japan) with a Cu Kα radiation in the angular range of 10°-80° (2θ). The surface morphology was observed by scanning electron microscope (SEM, JSM6430F).
A three-electrode electrochemical cell was employed for the measurement of cyclic voltammetry (CV) in LiNO3 aqueous electrolyte. Saturated calomel electrode (SCE) and platinum (Pt) electrode were used as the reference and the counter electrodes, respectively. The positive electrode was constituted 85% active material as cathode material, 10% black as conducting additive, and 5% polytetrafluoroethylene (PTFE) as binder. It was pressed onto a 100 mm2 stainless steel mesh used as the current collector at 300 kg/cm2 and then dried at 110 °C for 8 h in a vacuum oven in order to remove moisture. CV test was carried out at different scanning rates in electrochemical station (Shanghai Chenhua, China) at room temperature. Electrochemical impedance spectroscopy (EIS) was also performed in the same electrochemical station. The frequency range was from 100 kHz to 10 mHz and the amplitude voltage was 5 mV. Before the EIS test, each cell was charged to 0.5 V and then the voltage was kept stable for 2 h.
3 Results and discussion
Figure 1 shows the X-ray diffraction pattern of as-prepared LiNi0.5Mn0.5O2 powder. Obviously, all the peaks can be well indexed to LiNi0.5Mn0.5O2 phase with the space group of 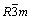 and there is no impurity phase. As mentioned, it is hard to synthesize the pure phase of LiNi0.5Mn0.5O2 by solid state method [24]. However, the XRD pattern indicates that well crystallized pure LiNi0.5Mn0.5O2 can be obtained through a co-precipitation method combined with the solid state process.
and there is no impurity phase. As mentioned, it is hard to synthesize the pure phase of LiNi0.5Mn0.5O2 by solid state method [24]. However, the XRD pattern indicates that well crystallized pure LiNi0.5Mn0.5O2 can be obtained through a co-precipitation method combined with the solid state process.
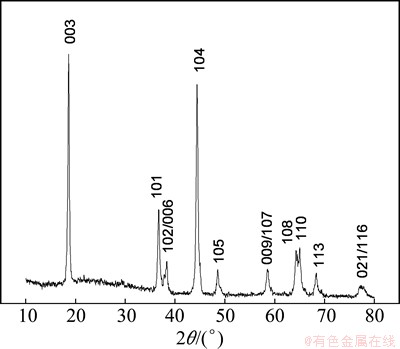
Fig. 1 X-ray diffraction pattern of as-prepared material by co-precipitation method
The morphology of LiNi0.5Mn0.5O2 particles was observed by SEM (Fig. 2). It can be seen clearly that the LiNi0.5Mn0.5O2 powder is composed of particles. The particle size covers a narrow distribution range from 100 nm to 200 nm.

Fig. 2 SEM image of LiNi0.5Mn0.5O2 powder
Figure 3 displays the CV curve of LiNi0.5Mn0.5O2 electrode in neutral 5 mol/L LiNO3 solution at -0.1 to 1.4 V. The scannig rate is 0.6 mV/s. As can be seen, a pair of redox peaks, with the anodic peak at about 0.71 V and cathodic peak at about 0.49 V, are observed obviously. Based on previous reports [9,10], appearance of redox peak indicates a reversible intercalation and de-intercalation of Li ions in solid phase with charge transfer at the electrode/electrolyte interface. The corresponding equations for the redox reaction can be written as follows:
In oxidation process,
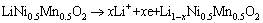 (1)
(1)
In reduction process,
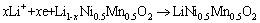 (2)
(2)
It is interesting to note that the potentials of Li+ ion intercalation and de-intercalation of LiNi0.5Mn0.5O2 electrode in aqueous LiNO3 solutions are within the safety range of water decomposition. Accordingly, LiNi0.5Mn0.5O2 could be proposed as one kind of promising cathode materials for aqueous lithium ion battery. When the scanning voltage is more than 1.2 V, a large irreversible peak appears, which is probably due to the oxygen evolution [7,9,20]. As seen by results above, LiNi0.5Mn0.5O2 electrode shows good electrochemical reversible behaviors in LiNO3 aqueous electrolyte.

Fig. 3 CV curve of LiNi0.5Mn0.5O2 electrode in neutral 5 mol/L LiNO3 solution at -0.1 to 1.4 V with scanning rate of 0.6 mV/s
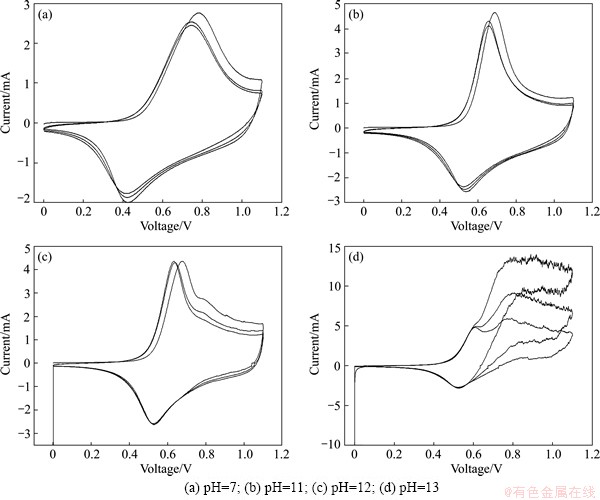
Fig. 4 CV comparison of LiNi0.5Mn0.5O2 electrodes in 5 mol/L LiNO3 solution with various pH values between 0 and 1.1 V (vs SCE) at 0.6 mV/s
The pH value of the solution electrolyte has been proved as a very important factor to the electrochemical performance [25]. Therefore, effect of 5 mol/L LiNO3 electrolytes with different pH values (7, 11, 12, 13) to electrochemical behavior is further studied in details. Related results are shown in Fig. 4. The scanning voltage range is from 0 to 1.1 V and the scanning rate is 0.6 mV/s. As observed, the potential of oxidation peak in LiNO3 electrolytes (pH=11, 12, 13) shifts backward and that of reduction peak moves forward in comparison with that in neutral electrolyte, suggesting that the reversibility of redox reaction could be improved while increasing the pH of solution electrolyte. In this work, there is no oxygen evolution at oxidation curves in electrolyte with pH 7 and 11. Surprisingly, when the pH is increased to 13, large second irreversible reaction happens with the oxygen release. As shown in Fig. 4, it is concluded that it will lead to oxygen release in a lower decomposition potential when excessively increasing the pH of LiNO3 electrolyte. That is to say, adding too much amount of OH- into the electrolyte would result in inferior cyclic stability of electrode. According to the difference of CV curves in LiNO3 with various pH values, it is clearly discovered that reduction process becomes more and more stable with increasing pH value. At the same time, a little oxygen evolution happens in oxidation curve of that with pH 12. After three cycles, the oxygen evolution process in Fig. 4(c) seems so small that it is hard to see. From the results above, it can not be distinguished easily that either electrolyte with pH value of 11 or 12 is the best choice to improve the electrochemical behavior of LiNi0.5Mn0.5O2. For LiNi0.5Mn0.5O2 in electrolyte with pH 12, obviously, the pH of electrolyte decreases a little with the oxygen release and then seems stably after several CV curves. Herein, long cyclic test will be carried out to compare the electrochemical stability of electrode in electrolyte with different pH values (11 and 12). Considering the complicate effect if the aqueous lithium ion battery with anode material is built, the three-electrode cell is still used to evaluate the electrochemical stability of LiNi0.5Mn0.5O2 electrode.
From the above information, the electrodes which have been cycled three CV curves in Fig. 4 are carried out another 17 CV curves in the same condition, respectively. The following 17 CV curves are compared in Fig. 5. From CV curves with pH 7, the couple of redox peak corresponding to the intercalation and de-intercalation of lithium ion disappears nearly after cycles, revealing the poor cyclic stability. As we know, bulk cathode materials for aqueous lithium ion battery such as LiMn2O4 [2,9] and LiNi1/3Co1/3Mn1/3O2 [7,11] without any modification in neutral aqueous electrolyte show poor cyclic performance. When the pH is increased to 11, the cyclic stability is somewhat enhanced. Not like that in neutral solution, there is still an obvious pair of redox peak located at 0.87 V and 0.36 V, respectively, after 20 CV curves. To our surprise, CV curves with pH 12 indicate a couple of better defined and reversible redox peak and much less fading with increasing cycle, compared with those in electrolyte with pH 7 and 11. The potentials of such redox peaks after 20 CV curves are about 0.7 and 0.46 V, respectively. In addition, no oxygen evolution exists in oxidation curves in electrolyte with pH 12. It is further confirmed the pH of that electrolyte lowers a little with the oxygen release and then becomes stable after some CV curves. Hereby, it is concluded that 5 mol/L LiNO3 with pH 12 is the most suitable for LiNi0.5Mn0.5O2 cycling in LiNO3 aqueous solution.
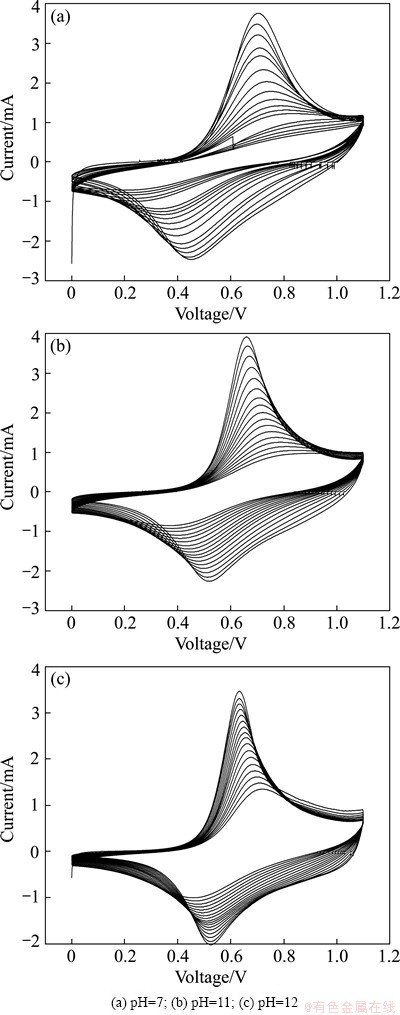
Fig. 5 Next 17 CV curves of LiNi0.5Mn0.5O2 electrodes in 5 mol/L LiNO3 solution between 0 and 1.1 V (vs SCE) at 0.6 mV/s with various pH values
To understand the possible reason of improved cyclic stability of LiNi0.5Mn0.5O2 in electrolyte with pH 12, EIS results for electrodes after 20 CV curves in 5 mol/L LiNO3 electrolytes with pH 7 and 12 are measured at the charged state of 0.5 V ( vs SCE), as shown in Fig. 6. Both AC impedance spectra of electrodes in LiNO3 electrolytes with different pH values consist of a high-frequency arc and a low-frequency line. It has been confirmed that the high frequency contribution is due to the charge-transfer process and the low-frequency line corresponds to diffusion process of lithium ion in electrode solid [26,27]. Apparently, after 20 CV curves, high-frequency arc of electrode in aqueous electrolyte with pH 7 is much larger than that electrode in with pH 12. In another word, charge-transfer resistance of LiNi0.5Mn0.5O2 in 5 mol/L LiNO3 with pH 12 after 20 CV curves is suppressed effectively. Using the equivalent circuit (inset of Fig. 6), the charge-transfer resistances of LiNi0.5Mn0.5O2 in 5 mol/L LiNO3 with pH 7 and 12 are calculated to be 79.5 and 11.7 W, respectively. In some previous reports, change of charge-transfer resistance was considered the important factor to capacity fading for cathode materials [27-30]. SUN et al [27] considered that the improved electrochemical performance of AlF3-coated LiCoO2 could be explained by the lower charge transfer resistance (Rct) and reduced Co dissolution of the coated electrode. LI et al [29] deduced that LiNi0.5Mn0.4Co0.1O2 with upgraded rate capabilities, compared with non-doped LiNi0.5Mn0.5O2, was attributed to the decrease in charge-transfer resistance. In brief, suppressed charge-transfer resistance is the major cause why electrochemical stability of LiNi0.5Mn0.5O2 electrode in electrolyte with pH 12 improves remarkably. It is well known that charge transfer resistance involves many factors such as electronic conductivity, crystal structure, and the inter-particle contacts (active particle-active particle, carbon-active particle, and carbon-carbon grain contracts) [31]. For that reason, the electrodes after cycling are further characterized by XRD and SEM toexplain the origin of change in charge transfer resistance.
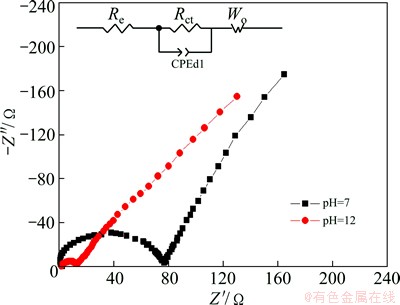
Fig. 6 AC impedance spectra of LiNi0.5Mn0.5O2 electrodes at 0.5 V (vs SCE) after 20 CV curves in 5 mol/L LiNO3 solution with pH 7 and 12, respectively, associated with equivalent circuit inset
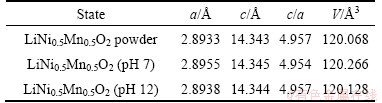
With regard to the poor cyclic stability of cathode material in neutral aqueous solution, probable capacity fade mechanisms of aqueous lithium ion battery have been proposed by some research groups and are mainly focused on the transition metal ion dissolution in aqueous solution, structural changes of electrode materials, as well as the decomposition of water [2,13,30]. In this work, structural change of LiNi0.5Mn0.5O2 electrodes in electrolyte with different pH values should be considered. Figure 7 shows the XRD patterns of LiNi0.5Mn0.5O2 electrodes after 20 CV curves in 5 mol/L LiNO3 solution with various pH values (7, 12) between 0 and 1.1 V (vs SCE). With the exception of those diffraction peaks marked with *, which are due to the stainless steel mesh, the other lines in Fig. 7 are attributed to LiNi0.5Mn0.5O2. Evidently, both samples keep good α-NaFeO2-type structure and no impurity phases are detected. The lattice constants of LiNi0.5Mn0.5O2 electrodes are recorded in Table 1. It needs to note that the lattice constants of the electrode after cycling in the aqueous electrolyte with pH 12 agree well with those of pristine LiNi0.5Mn0.5O2 powder. When cycling in neutral electrolyte, the electrode shows the large change with the crystallite volume from 120.068 to 120.266  3. It is well known that the intensity ratio of I003/I104 in XRD line depends on the degree of displacement between the cations located at 3a and 3b sites [32]. The higher the ratio is, the less the cationic disorder in the α-NaFeO2-type structure is. As shown in Fig. 7, the degree of cationic disorder is greatly reduced in LiNi0.5Mn0.5O2 that cycling in electrolyte with pH 12, compared with that in neutral electrolyte. The structure change of LiNi0.5Mn0.5O2 cycling in neutral aqueous electrolyte could probably be resulted from the H+ intercalation and exchange [7].
3. It is well known that the intensity ratio of I003/I104 in XRD line depends on the degree of displacement between the cations located at 3a and 3b sites [32]. The higher the ratio is, the less the cationic disorder in the α-NaFeO2-type structure is. As shown in Fig. 7, the degree of cationic disorder is greatly reduced in LiNi0.5Mn0.5O2 that cycling in electrolyte with pH 12, compared with that in neutral electrolyte. The structure change of LiNi0.5Mn0.5O2 cycling in neutral aqueous electrolyte could probably be resulted from the H+ intercalation and exchange [7].
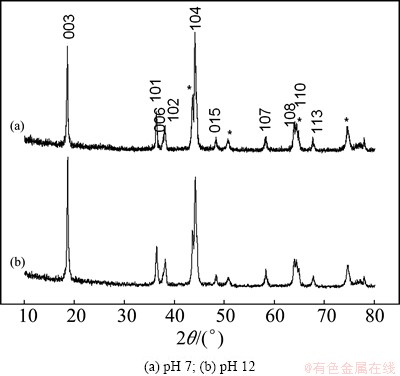
Fig. 7 XRD patterns of LiNi0.5Mn0.5O2 electrodes after 20 CV curves in 5 mol/L LiNO3 solution between 0 and 1.1 V (vs SCE) with different pH values
Table 1 Crystallographic data of LiNi0.5Mn0.5O2 after CV curves in electrolyte with different pH values
The morphology comparison (Fig. 8) of LiNi0.5Mn0.5O2 electrodes after 20 CV curves in 5 mol/L LiNO3 electrolyte with different pH values highlights the difference between the two materials. In the case of LiNi0.5Mn0.5O2 after cycling in neutral electrolyte, the particles seem to be self-aggregated, and their contact with binder (PTFE) and acetylene black becomes poor because of the presence of many large voids, which may be attributed to the attack of H2O [13,29]. As demonstrated in Fig. 8, PTFE binder is apparently observed. On the other hand, LiNi0.5Mn0.5O2 electrode in electrolyte with pH 12 shows excellent particle distribution and better surface smoothness. Besides, no obvious crack is observed on the surface of LiNi0.5Mn0.5O2 electrode. Results above suggest that electrode surface after cycling in electrolyte with pH 12 is more stable than that in neutral electrolyte, which agrees well with the suppressed charge transfer resistance, thus resulting in improved cyclic stability. In other words, the attack of H2O in electrolyte with pH 12 is probably greatly suppressed. According to results above, it is believed that the mechanism of suppressed charge transfer resistance should be originated from the less change in the electrode surface and structure. It should be pointed out that WANG et al [7] once found that capacity fading of LiNi1/3Co1/3Mn1/3O2 was attributed to H+ intercalation when cycling in neutral Li2SO4 electrolyte; however, no study mentioned the change of electrode surface.
4 Conclusions
1) LiNi0.5Mn0.5O2 was prepared by surfactant assisted co-precipitation method.
2) XRD and SEM results indicate that LiNi0.5Mn0.5O2 is pure phase and covers a narrow distribution range from 100 to 200 nm.
3) The as-prepared LiNi0.5Mn0.5O2 exhibits good electrochemical reversible behavior in 5 mol/L LiNO3 solution electrolyte. Meantime, the electrode in LiNO3 electrolyte with pH value of 12 has the best electrochemical cyclic stability.
4) EIS result indicates that the improved cyclic stability of LiNi0.5Mn0.5O2 in aqueous electrolyte with pH 12 could be explained by the suppressed charge-transfer resistance, which is probably ascribed to the more stable electrode surface morphology and less structure change according to SEM and XRD results.

Fig. 8 SEM images of LiNi0.5Mn0.5O2 electrodes after 20 CV curves in 5 mol/L LiNO3 solution between 0 and 1.1 V (vs SCE) with various pH values
References
[1] LI W, DAHN J R, Wainwright D S. Rechargeable lithium batteries with aqueous electrolytes [J]. Science, 1994, 264: 1115-1118.
[2] LI W, Dahn J R. Lithium-ion cells with aqueous electrolytes [J]. J Electrochem Soc, 1995, 142: 1742-1746.
[3] LI W, Mckinnon W R, Dahn J R. Lithium intercalation from aqueous solution [J]. J Electrochem Soc, 1994, 141: 2310-2316.
[4] Schlijrb H, Bungs M, Plieth W. Synthesis and electrochemical studies of manganese oxides with spinel structure in aqueous electrolyte (9 M KOH) [J]. Electrochimica Acta, 1997, 42: 2619-2625.
[5] Wang G X, Zhong S, Bradhurst D H, Dou S X, Liu H K. Synthesis and characterization of LiNiO2 compounds as cathodes for rechargeable lithium batteries [J]. Journal of Power Sources, 1998, 74: 198-201.
[6] Nakayam N, Nozawa T, Iriyama Y, Abe T, Ogumi Z, Kikuchi K. Interfacial lithium-ion transfer at the LiMn2O4 thin film electrode/aqueous solution interface [J]. Journal of Power Sources, 2007, 174: 695-700.
[7] Wang Y G, Luo J Y, Wu W, Wang C X, Xia Y Y. Hybrid aqueous energe storage cellsusing activated carbon and lithium-ion interealated compound III. Capacity fading mechanism of LiCo1/3Nil/3Mnl/3O2 at different pH electrolyte solutions [J]. J Electrochem Soc, 2007, 154: 228-234.
[8] Wang G J, Zhao N H, Yang L C, Wu Y P, Wu H Q, HOLZE R. Characteristic of an aqueous rechargeable lithium battery (ARLB) [J]. Electrochimica Acta, 2007, 52: 4911-4915.
[9] Wang H B, Zeng Y Q, Huang K L, Liu S Q, Chen L Q. Improvement of cycle performance of lithium ion cell LiMn2O4/LixV2O5 with aqueous solution electrolyte by polypyrrole coating on anode [J]. Electrochimica Acta, 2007, 52: 5103-5107.
[10] Wang G J, Fu L J, Wang B, Zhao N H, Wu Y P, Holze R. An aqueous rechargeable lithiumbattery based on Li[Ni1/3Co1/3Mn1/3]O2 positive electrode [J]. J Appl Electrochem, 2008, 38: 579-581.
[11] Wang H B, Huang K L, Zeng Y Q, Chen L Q. Stabilizing cycle life of aqueous lithium ion cell LiNi1/3Co1/3Mn1/3O2/LixV2O5 by polyaniline coating on anode [J]. Electrochemical and Solid-State Letters, 2007, 10: 199-203.
[12] Wang G J, Fu L J, Zhao N H, Yang L C, Wu Y P, Wu H Q. An aqueous rechargeable lithium battery with good cycling performance [J]. Angew Chem Int Ed, 2007, 46: 295-297.
[13] Luo J Y, Xia Y Y. Aqueous lithium-ion battery LiTi2(PO4)3/LiMn2O4 with high power and energy densities as well as superior cycling stability [J]. Adv Funct Mater, 2007, 17: 3877-3884.
[14] Kohler J, Makihara H, Uegatio H, Inoue H, Toki M. LiV3O8: Characterization as anode material for an aqueous rechargeable Li-ion battery system [J]. Electrochimica Acta, 2000, 46: 59-65.
[15] Mi C H, Zhang X G, Li H L. Electrochemical behaviors of solid LiFePO4 and Li0.99Nb0.01FePO4 in Li2SO4 aqueous electrolyte [J]. Journal of Electroanalytical Chemistry, 2007, 602: 245-254.
[16] Manickam M, Singh P, Thurgate S, Prince K. Redox behavior and surface characterization of LiFePO4 in lithium hydroxide electrolyte [J]. Journal of Power Sources, 2006, 158: 646-649.
[17] Liu X H, Saito T, Doi T, Okada S, Yamaki. Electrochemical properties of rechargeable aqueous lithium ion batteries with an olivine-type cathode and a Nasicon-type anode [J]. Journal of Power Sources, 2009, 189: 706-710.
[18] MANJUNATHA H, VENKATESHA T V, SURESH G S. Electrochemical studies of LiMnPO4 as aqueous rechargeable lithium-ion battery electrode [J]. J Solid State Electrochem, 2012, 16: 1941-1952.
[19] TANG W, LIU L L, ZHU Y S, SUN H, WU Y P, ZHU K. An aqueous rechargeable lithium battery of excellent rate capability based on a nanocomposite of MoO3 coated with PPy and LiMn2O4 [J]. Energy Environ Sci, 2012, 5: 6909-6913.
[20] Wang H B, Huang K L, Zeng Y Q, Yang S, Chen L Q. Electrochemical properties of TiP2O7 and LiTi2(PO4)3 as anodes for lithium ion battery with aqueous solution electrolyte [J]. Electrochimica Acta, 2007, 52: 3280-3286.
[21] ZHOU D H, LIU S Q, WANG H Y, YAN G Q. Na2V6O16·0.14H2O nanowires as a novel anode material for aqueous rechargeable lithium battery with superior cycling performance [J]. J Power Sources, 2013, 227: 111-117.
[22] LI H Q, ZHAI T Y, HE P, WANG Y G, HOSONOA E, ZHOU H S. Single-crystal H2V3O8 nanowires: a competitive anode with large capacity for aqueous lithium-ion batteries [J]. J Mater Chem, 2011, 21: 1780-1787.
[23] Spahr M E, Novak P, Schnyder B, Hass O, Nesper R. Cycling performance of novel lithium insertion electrode materials based on the Li-Ni-Mn-O system [J]. Journal of Power Sources, 1997, 68: 629-633.
[24] Makimura Y, Ohzuku T. Lithium insertion material of LiNi1/2Mn1/2O2 for advanced lithium-ion batteries [J]. Journal of Power Sources, 1997, 119-121: 156-160.
[25] WANG YG, YI J, XIA Y Y. Recent progress in aqueous lithium-ion batteries [J]. Adv Energy Mater, 2012, 2: 830-840.
[26] Kang Y J, Kim J H, Lee S W, Sun Y K. The effect of Al(OH)3 coating on the Li[Li0.2Ni0.2 Mn0.6]O2 cathode material for lithium secondary battery [J]. Electrochim Acta, 2005, 50: 4784-4791.
[27] Sun Y K, Han J M, Myung S T, Lee S W, Amine K. Significant improvement of high voltage cycling behavior AlF3-coated LiCoO2 cathode [J]. Electrochem Commun, 2006, 8: 821-826.
[28] Shaju K M, Subba R G V, Chowdari B V R. InfluenceofLi-ionkineticsinthecathodicperformanceoflayeredLi(Ni1/3Co1/3Mn1/3)O2 [J]. J Electrochem Soc, 2004, 151: 1324-1332.
[29] Li D C, Sasaki Y, Kobayakawa K, Sato Y. Morphological, structural, and electro chemical characteristics of LiNi0.5Mn0.4M0.1O2 (M=Li, Mg, Co, Al) [J]. Journal of Power Sources, 2006, 157: 488-493.
[30] Javalakshmi M, Rao M M, Scholz F. Electrochemical behavior of solidlithium manganate (LiMn2O4) in aqueous neutral electrolyte solutions [J]. Langmuir, 2003, 19: 8403-8408.
[31] Fan J, Fedkiw P S. Electrochemical impedance spectra of full cells: relation to capacity and capacity-rate of rechargeable Li cells using LiCoO2, LiMn2O4, and LiNiO2 cathodes [J]. Journal of Power Sources, 1998, 72: 165-173.
[32] Julien C, Ziolkiewicz S, Lemal M, Massot M. Synthesis, structure and electrochemistry of LiMn2-yAlyO4 prepared by a wet-chemistry [J]. J Mater Chem, 2001, 11: 1837-1842.
王海燕,何菡娜,周 南,金冠华,唐有根
中南大学 化学化工学院,有色金属资源化学教育部重点实验室,长沙 410083
摘 要:研究LiNi0.5Mn0.5O2电极在LiNO3水溶液中的电化学行为,同时分析该电极在不同pH值电解液中的循环衰减原因。循环伏安测试显示LiNi0.5Mn0.5O2在浓度为5 mol/L的LiNO3水溶液中具有较好的锂离子脱嵌能力。对比发现,LiNi0.5Mn0.5O2电极在浓度为5 mol/L,pH值为12的LiNO3水溶液中具有最好的循环稳定性能。通过交流阻抗法、X射线衍射分析及电极形貌的对比分析发现:电极在浓度为5 mol/L,pH值为12的LiNO3水溶液中循环时,电极的表面形貌和电极结构都能得到较好的保持,电极的电荷传递阻抗得到明显抑制,因此在该pH值电解液中的循环稳定性最好。
关键词:锂离子电池;LiNi0.5Mn0.5O2;循环衰减机制;循环伏安法;LiNO3溶液
(Edited by Chao WANG)
Foundation item: Project (21301193) supported by the National Nature Science Foundation of China; Project (2013M530356) supported by the China Postdoctoral Science Foundation Funded; Project (CUSZC201303) supported by the Scientific Research Foundation of Central South University, and the Open-End Found for Valuable and Precision Instruments of Central South University
Corresponding author: Hai-yan WANG; Tel: +86-731-88830886; Fax: +86-731-88879616; E-mail: wanghy419@126.com
DOI: 10.1016/S1003-6326(14)63077-6

