
27Al MAS-NMR study of inorganic polymer formation at ambient temperature
Sang-Wook AHN1, Hee-Soo LEE2, Wan-Hee YANG3, Jung-Woo LEE3, Young-Keun JEONG1
1. National Core Research Center for Hybrid Materials Solution, Pusan National University, Busan 609-735, Korea;
2. School of Material Science and Engineering, Pusan National University, Busan 609-735, Korea;
3. INTChem Co., Ltd., Suwon 443-373, Korea
Received 21 April 2010; accepted 10 September 2010
Abstract: Inorganic polymers are a novel class of materials formed by the polymerization of silicon, aluminium and oxygen species to form an amorphous three-dimensional framework structure. The basis of this process is the alkaline solutions to induce a certain amount of Si and Al atoms to dissolve from a feedstock such as aluminosilicate. A study of 27Al MAS-NMR was carried out in an attempt to understand the reaction mechanism of the inorganic polymerization at ambient temperature. Scanning electron microscopy (SEM) and X-ray diffractometry (XRD) were also employed to establish the composition and microstructure of the inorganic polymerization. Specimens were prepared with different Al/Si mole ratios from the starting materials. The higher the Al content, the more sufficient the Al atoms that can combine with SiO4, and the longer the reaction time, the more the bonded Si—O—Al—O polymer structure, and then the higher the Al content, the fewer the octahedral Al with a uniform Si—O—Al—O structure in four directions, because four Al atoms are combined with SiO4, resulting in a uniform Si—O—Al—O structure in four directions. The results show that they have an amorphous microstructure.
Key words: inorganic polymer; amorphous; tetrahedral
1 Introduction
Aluminosilicate inorganic polymer is a new class of inorganic material that is widely used in a variety of applications due to its durability, high compressive strength, high heat resistance, high fire resistance and high chemical resistance. Commercial uses include building products, waste stabilization and high temperature lightweight applications[1]. What is important and interesting about this material is its infinite potential as a replacement for cement because its raw materials are industrial by-products.
The term ‘inorganic polymer’ describes a family of mineral binders that are reported to have a polymeric Si—O—Al framework structure similar to that found in zeolites[2]. This inorganic polymer has three- dimensional amorphous inorganic structure by the component mole ratio of Al to Si under highly alkaline conditions[3]. This theory was suggested by Davidovits in 1983 who presented a reaction mechanism with the copolymer structure. These materials may be synthesized at ambient temperature or higher by alkaline activation of aluminosilicates obtained from industrial by-products such as coal ash and blast furnace slag[2, 4]. In general, ordinary Portland cement depends on the presence of Ca+ in chemical reaction, but inorganic polymer does not affect CSH creation in reaction products. However, inorganic polymer obtains its unique, excellent structural characteristics due to the binding of Al and Si from polymerization reaction under a strong alkali condition.
Studies on the accurate combination structure of inorganic polymers are still going on. The basic structure of inorganic polymer is amorphous, and it is depicted as a fully amorphous three-dimensional polymeric chain and ring structure consisting of Si—O—Al—O bonds [1, 3, 5-6]. Chemical reaction between various aluminosilicate oxides with silicate under highly alkaline conditions yields a sialate network consisting of SiO4 or AlO4 tetrahedral linked alternately by sharing all the oxygen[1, 3, 5]. For balanced electric charge of Al3+ through combination with the negative charge generated here, positive ions Na+ or K+ must be present, resulting in Si—O—Al—O polymeric bonds[1, 3, 5].
This study was carried out to investigate the inorganic polymer combination structure. For this purpose, XRD, SEM, and 27Al MAS-NMR were used to analyze at various mole ratios of Al and Si which are the main components of inorganic polymer. Through the analysis of XRD and SEM we can observe the formation of semi-crystalline or polycrystalline phases of the inorganic polymer reaction products, and through the analysis of 27Al MAS-NMR we can understand the combination structure of the valence electron of Al atoms of inorganic polymer formation at ambient temperature.
2 Experimental
2.1 Materials
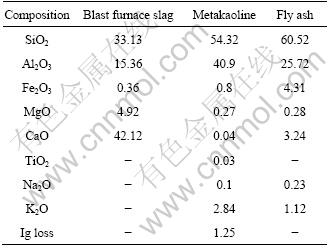
Table 1 lists the properties of the raw materials used in this study. Table 2 lists the chemical composition of each raw material used. The raw materials used in the experiments were commercial products. For the activator needed for reaction with the raw materials and formation of inorganic polymer structure, alkali silicate solutions were produced and used. We mixed sodium silicate solution with sodium hydroxide (NaOH) solution to produce alkali silicate solution[7-8]. The Na2O/SiO2 mole ratio of this alkali silicate solution was 1.7:3.0, which was calculated from the total content of Na2O and SiO2 in the sodium hydroxide solution and the sodium silicate solution. This is the typical composition that was determined to induce the optimum reaction of the inorganic polymer through a preliminary study. The solid content of the final alkali silicate solution was 25% (mass fraction). This is a condition for easy production derived to achieve a flow similar to that of the paste used commercial portland cement.
Table 1 Properties of raw materials

Table 2 Chemical compositions of raw materials (mass fraction, %)
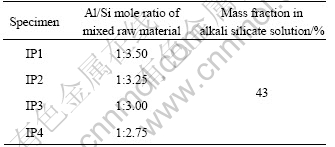
The Al/Si mole ratios for each specimen are listed in Table 3. Each specimen has a different Al/Si mole ratio, which is the mole ratio of Al to Si components in the mixture of blast furnace slag, metakaoline, and fly ash[9-12]. The mole ratio of Al to Si of 1:2.75-1:3.50 was designed to achieve a higher physical performance as the commercial portland cement in consideration of the relationship between chemical resistance and physical performance of the specimens[13]. Furthermore, the mixing quantity of alkali silicate solution was determined so as to achieve a flow similar to the paste that used commercial portland cement.
Table 3 Al/Si mole ratio for each specimen
2.2 Procedure
The raw materials, blast furnace slag, metakaoline, and fly ash were mixed, and the mixture was input to the alkali silicate solution. They were mixed into a paste. The paste was then filled into a cubic mold with the sizes of length, width and height of 50 mm. The specimens were then cured at (20±2) °C and relative humulidity of 70%. Each specimen was analyzed for XRD, SEM, and 27Al MAS-NMR according to age in day and their compressive strengths were measured.
2.3 Measurements
The X-ray powder diffraction pattern was recorded on a Rigaku Corp D-MAX2500-PC spectrometer using Cu Kα radiation(40 kV, 30 mA) with a scanning rate of 2 (°)/min from 5° to 70°. A JEOL JSM-5600 microscope was used for SEM analysis at the accelerating voltage of 20 kV. It was used for image observation of the samples at the accelerating voltage of 5 kV. The resolution was 3.5 nm and the size of measurement space was 150 mm or less in diameter. Samples for JSM-5600 analysis were placed on the sample holders supported by carbon tape followed by 1 min sputtering coating of gold. The 27Al MAS-NMR spectra were obtained at 59.61 and 78.18 MHz on a Varian 300/solid-state spectrometer employing magic angle spinning at 6.9 kHz. The measuring range was (50-150)×10-6. The NMR peaks were fitted by Gaussian lines. Through this 27Al MAS-NMR we can determine whether the Al atom has the tetrahedral or octahedral coordinate covalent combination structure. The following conditions were applied to the solid-state spectrometer: 27Al MAS-NMR spectra at spinning speed in access of 8 kHz, radiation pulse of 3.2 μs (90? pulse), high power proton decoupling during the signal accumulation, spectral width of 100 kHz, and around 16 scans collected with the repetition time of 1 s. The compressive strength was measured at various ages of the specimens using an Universal Testing Machine that can measure up to 10 kN of compressive strength as the measuring instruments.
3 Results and discussion
3.1 Structural analysis
The XRD patterns of specimen with different Si/Al mole ratios are shown in Fig.1. We can see that specimen IP1 has a peak at 7 d with overall crystalline trend, and the longer the age is, the weaker the trend of crystalline peak. The reason for this is that in the early reaction, the inorganic polymer has the SiO4 structure with four oxygen atoms with highly crystalline characteristic, and as the age gets older, it changes to the amorphous structure of Si—O—Al—O by the combination of Al atoms with the oxygen atoms in SiO4. Specimens IP2 and IP3 also show some crystalline peaks during the young ages, but the older the age is, the more amorphous structure they exhibit. Furthermore, the specimen IP4 that has the high Al content forms SiO4 in the inorganic polymer reaction. However, because it has sufficient Al atoms for inorganic polymer combination, the inorganic polymer structure of Si—O—Al—O is mostly formed from the early stage of reaction. Because the increased Al content facilitates the formation of inorganic polymer, the specimen can easily exhibit a physical performance in the early stage. This can be used as the basis for identification of tendency according to Al content during the chemical property evaluation of specimens.
According the XRD patterns of specimens IP1, IP2 and IP3 aged for 7, 14 and 28 d, the crystalline peaks appear at the 2θ of 26° and 32°, and these peaks nearly fall in with the mullite peaks. This seems to be due to the non-reacted raw materials during the formation of inorganic polymer structure of the raw materials used in the reaction. The reason for this is that the lower the Al content is, the smaller the quantity required for bonding with Si—O and the more insufficient the formation of the perfect Si—O—Al—O inorganic polymer structure, which results in the peak measurement of the components of the remnant powder raw materials. We could see that the higher the Al content and the older the age after reaction is, the fewer the mullite peaks. In the final analysis, the higher the Al content is, the more sufficient the Al atoms that can combine with SiO4, and the longer the reaction time, the more the bonded Si— O—Al—O polymers, forming a broad and amorphous structure. The specimen IP4 with high Al content has amorphous structure at young ages after reaction. The older the age is, the more SiO4 in the inorganic polymers shows the Si—O—Al—O combination structure, inorganic polymers of framework structure. Moreover, there are no crystalline peaks such as C2S(2CaO·SiO2), C3S(3CaO·SiO2) and mono sulfates which typically appear in the hydration reaction of commercial portland cement.

Fig.1 XRD patterns of inorganic polymer with age in days: (a) Specimen IP1; (b) Specimen IP2; (c) Specimen IP3; (d) Specimen IP4
3.2 Microstructure
The SEM images of inorganic polymer binder specimen with different Al/Si mole ratios are compared in Fig.2. The inorganic polymer has amorphous structure with different cement hydrate of crystalline. And then we can confirm to amorphous structure similar to glass structure. It is found that the inorganic polymer binder specimens are not crystalline in general; they have homogeneous amorphous structure.
Inorganic polymer particles were checked at varying Al/Si mole ratios, and they all showed amorphous structure regardless of different Al/Si mole ratios. Furthermore, there are no peaks of calcium hydroxide, ettringite, mono sulfate, C2S(2CaO·SiO2), and C3S- (3CaO·SiO2) which generally appear in commercial portland cement hydroxides. This shows that inorganic polymer binders have a polymer combination structure under the highly alkali condition due to the sodium silicate solution, and do not form particles from hydration reaction among the components. Furthermore, the older the age was, the less clear changes were observed in the amorphous particles. Besides, micro cracks were seen in a part of the particles which were formed from the reaction, which seems to be caused by the evaporation of water contained in the alkali silicate solution. More studies are needed to reduce the micro cracks for improvement of physical performance of inorganic polymer binder.
3.3 27Al MAS-NMR analysis
27Al MAS-NMR can be used to examine the difference in the number of coordinate covalent bonds of Al atoms which can provide important information in the polymer formation process. Chemical reaction of aluminosilicate oxides results in the tetrahedron structure of SiO4 or the network structure of AlO4 which copolymerize with oxygen. The 27Al MAS-NMR analysis was carried out for each specimens aged 28 d. The results are compared in Fig.3. At the tetrahedral Al peak, Al atoms are combined with four Si—O, which is a gel shape of a three-dimensional structure with the basic structure of Si—O—Al—O. The analysis of Al NMR found Al(3Si) tetrahedrons peaks at 65×10-6. As a result, the higher the Al content, the fewer the octahedral Al because four Al atoms are combined with SiO4, resulting in uniform Si—O—Al—O structure in four directions which is the optimum amorphous combination structure.
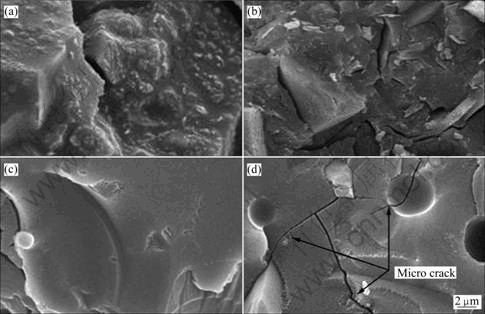
Fig.2 SEM images of inorganic polymer binder with different Al/Si mole ratios aged for 7 d: (a) Specimen IP1; (b) Specimen IP2; (c) Specimen IP3; (d) Specimen IP4

Fig.3 27Al MAS-NMR spectra of inorganic polymer binder with different Al/Si mol ratios: (a) Specimen P1; (b) Specimen IP2; (c) Specimen IP3; (d) Specimen IP4
Furthermore, the 27Al MAS-NMR analysis found that Al atoms have the tetrahedral structure that has four valence electrons.
3.4 Compressive strength
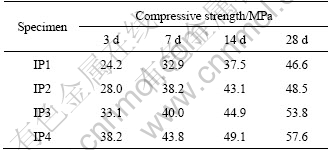
The compressive strengths of the specimens with different Al/Si mole ratios were measured (Table 4). It is found that the higher the Al content is, the higher the compressive strength of specimen aged 3 d which is the early strength. The compressive strength was also high at 28 d according to the same trend. Furthermore, the compressive strength at 3 d is 51.9% that of at 28 d for IP1 and 57.7% for IP2, 61.5% for IP3, and 66.3% for IP4. This shows that the higher the Al content, the better the physical properties appeared.
Table 4 Compressive strength of specimen with different Al/Si mole ratios
4 Conclusions
1) The structural analysis found that the higher the Al content, the more amorphous structure the inorganic polymers had and the clearer the amorphous shape of inorganic polymers formed in the early stage of reaction. The Al content did not change much and the amorphous shape was maintained. Furthermore, micro cracks were found in the inorganic polymers due to the evaporation of water during the drying process.
2) When the Al content increased, the conditions for polymer combination of the Si—O—Al—O frame network structure met for polymers of the SiO4 combination structure, forming a networked amorphous structure based on this combination structure. Furthermore, it was found that the octahedral structures decreased over time with the bonding of Al and SiO4 as the octahedral polymer structure changed to tetrahedral through the bonding of O and Al. Thus, the Si atoms of 4 valence form stable polymers, maintaining a stable amorphous inorganic polymer structure for the long term.
3) The higher the Al content, the better compressive strengths were observed. The higher the Al content, the more amorphous inorganic polymers formed in the early stage, which allows excellent mechanical properties. From these results, we confirmed that inorganic polymers have excellent physical properties with amorphous structure within Al/Si mole ratio of 1:2.75-1:3.50. Also, it seems to have excellent properties in chemical resistance and fire resistance.
Acknowledgements
This work was supported by Energy Resource Technology Development Project [The Development and commercialization of Inorganic Polymer Ceramic Panel] of Korea Energy Management Corporation, and supported by NCRC(National Core Research Center) Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology.
References
[1] DAY K W. Concrete mix design quality control and specification[M]. 3rd ed. London: E&FN Spon, 2006: 311.
[2] SINGH P S, TRIGG M, BURGAR I, BASTOW T. Geopolymer formation processes at room temperature studied by 29Si and 27Al MAS-NMR [J]. Materials Science and Engineering, 2005, 396(1/2): 392-402.
[3] SCHNEIDER J, CINCOTTO M A, PANEPUCCI H. 29Si and 27Al high-resolution NMR characterization of calcium silicate hydrate phases in activated blast-furnace slag pastes [J]. Cement and Concrete Research, 2001, 31(7): 993-1001.
[4] CHANG J J. A study on the setting characteristics of sodium silicate-activated slag pastes [J]. Cement and Concrete Research, 2003, 33(7): 1005-1011.
[5] XU Hua, van DEVENTER J S J. Microstructural characterization of geopolymer synthesized from kaolinite/stilbite mixtures using XRD, MAS-NMR, SEM/EDX, TEM/EDX, and HERM [J]. Cement and Concrete Research, 2002, 32(11): 1705-1716.
[6] SCHMUCKER M, MACKENZIE K J D. Microstructure of sodium polysialate siloxo geopolymer[J]. Ceramics International, 2005, 31(3): 433-437.
[7] PHAIR J W, van DEVENTER J S J. Effect of the silicate activator pH on the microstructural characteristics of waste-based geopolymers [J]. International Journal of Mineral Processing, 2002, 66(1/4): 121-143.
[8] LEE W K W, van DEVENTER J S J. Structural reorganisation of class F fly ash in alkaline silicate solutions [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2002, 211(1): 49-66.
[9] BANKOWSKI P, ZOU L, HODGES R, SINGH P S, TRIGG M. Brown coal fly ash stabilisation by inorganic polymers [C]// Proceedings of the International Conference on Geopolymer. Melbourne, 2002.
[10] GORETTA K C, CHEN N, GUTIERREZ-MORA F, ROUTBORT J L, LUKEY G C, van DEVENTER J S J. Solid-particle erosion of a geopolymer containing fly ash and blast-furnace slag [J]. Wear, 2004, 256(7/8): 714-719.
[11] van JAARSVELD J G S, van DEVENTER J S J, LUKEY G C. The characterisation of source materials in fly ash-based geopolymers [J]. Materials Letters, 2003, 57(7): 1272-1280.
[12] LEE W K W, van DEVENTER J S J. The effects of inorganic salt contamination on the strength and durability of geopolymers [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2002, 211(2/3): 115-126.
[13] van JAARSVELD J G S, van DEVENTER J S J, LUKEY G C. The effect of composition and temperature on the properties of fly ash- and kaolinite-based geopolymers [J]. Chemical Engineering Journal, 2002, 89(1/3): 63-73.
(Edited by LONG Huai-zhong)
Corresponding author: Young-Keun JEONG; Tel: +82-51-5102483; E-mail: nano@pusan.ac.kr