J. Cent. South Univ. Technol. (2011) 18: 386-391
DOI: 10.1007/s11771-011-0708-4
Phenotypic and genetic characterization of a novel strain of Acidithiobacillus ferrooxidans (AF2)
LIU Qian(刘倩)1,2, ZHOU Hong-bo(周洪波)1, YANG Bo(杨波)2, AO Jing-qun(敖敬群)2, CHEN Xin-hua(陈新华)2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Marine Biogenetic Resources, Third Institute of Oceanography,
State Oceanic Administration, Xiamen 361005, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract: A comparative study on the phenotypic and genetic characteristics among Acidithiobacillus ferrooxidans (AF2), a typic strain ATCC23270 and a previously isolated strain AF3 was performed. AF2 can use ferrous ion (Fe2+) or elemental sulfur (S0) as sole energy source, but oxidizes S0 more effectively than Fe2+, which is different from ATCC23270 and AF3. The G+C content of AF2 is 51.8% (molar fraction), however, ATCC23270 and AF3 strains have G+C content of 63.7% and 64.8% (molar fraction), respectively. The DNA-DNA hybridization results show that AF2 has 41.53% and 52.38% genome similarity to ATCC 23270 and AF3, respectively, but AF3 has a high genome similarity of 89.86% to ATCC 23270 strain. Rusticyanin (rus) and subunit III of aa3-type cytochrome oxidase (coxC) genes are not detected in AF2, but Fe2+ oxidase (iro) gene can be detected. To understand the genomic organization of iro gene, a cosmid library of AF2 genome was constructed and iro gene-containing clone was screened. The sequencing result shows that although the nucleotide sequence of iro gene in AF2 is completely identical to that of ATCC 23270 strain, its genomic organization is different from that of ATCC 23270. In AF2, iro is located at downstream of purA gene, while it is located at downstream of petC-2 gene in ATCC 23270 strain. These results indicate that AF2 is a novel strain of A. ferrooxidans, and that phenotypic differences among the strains of A. ferrooxidans are closely correlated with their genetic polymorphisms.
Key words: Acidithiobacillus ferrooxidans; phenotypic and genetic characterization; Iro gene; cosmid library; iron respiratory chain
1 Introduction
Acidithiobacillus ferrooxidans (A. ferrooxidans), a chemolithoautotrophic, mesophilic, acidophilic Gram- negative bacterium, is important in bioleaching industry, since it can derive energy from the oxidation of ferrous iron, elemental sulfur and reduced sulfur compounds. Attempts to understand the energetic metabolism of A. ferrooxidans, especially the mechanism of Fe2+ oxidization, have been initiated and led to the proposition of several respiratory chains composed of several characterized redox proteins in A. ferrooxidans [1]. The distinguishing feature of the species is its ability to derive energy from the oxidation of ferrous ions. Various strains of this species have been isolated from natural (rocks, ores and mine waters) and technological (ore concentrates and pulps of the gold and non-ferrous industries) sources. Four strains of A. ferrooxidans, AF1, AF2, AF3 and AFC, were isolated from different sites in China using the 9K medium. All of these four isolates are Gram-negative, motile, acidophilic and chemolithoauto- trophic bacteria rods, and each of them can obtain energy by the oxidation of Fe2+, S0 and pyrite. Sequencing of the 16S rDNA also demonstrates that these four strains belong to A. ferrooxidans [2]. Twenty-three strains of A. ferrooxidans were examined with regards to the growth temperature, ability to utilize Fe2+ and S0, G+C content and interstrain hybridization of their genomic DNA, showing the presence of the genomic and physiological diversity amongst these A. ferrooxidans strains [3]. The DNA-DNA hybridization results showed that 17 of 23 strains are divided into four genomovars, while six independent, considerably divergent strains cannot be assigned to any of the genomovars. However, these isolates are divided into five groups based on their sequences of 16S rDNA, and three of them are more special than the others [4]. These studies suggest that the microevolution of a strain in each ecological niche is accompanied by changes in the nucleotide sequence of its chromosomal DNA, and the difference in nucleotide sequence causes the phenotypic polymorphism of the strains [5]. However, the exact biological mechanism underlying the diversity of these strains is still not well understood.
Various molecular methods, such as rRNA analysis, G+C content analysis and southern hybridization, have been used to achieve a better understanding of the phylogenetic relationships among A. ferrooxidans [6]. In this study, the characterization of three strains of A. ferrooxidans was reported, and the comparative analysis of their physiological and genetic differences was performed. To investigate the possible role of iro and analyze its electron transfer chain, a genomic cosmid library of the AF2 strain was constructed and the iro gene-containing clone was sequenced and analyzed.
2 Experimental
2.1 Strains and their culture condition
A. ferrooxidans strains, AF2 and AF3, were previously isolated from the Daye Mine of Hubei Province and the Yufu Mine of Hunan Province in China, respectively [2]. The typic strain, ATCC23270, was purchased from American Type Culture Collection. A. ferrooxidans was grown aerobically on 9K medium supplemented with 3 g/L (NH4)2SO4, 0.5 g/L K2HPO4, 0.5 g/L MgSO4, 0.1 g/L KCl, 0.01 g/L Ca(NO3)2 and 44.7 g/L FeSO4·7H2O. The initial pH of the medium was adjusted to 2.5. Growth on elemental sulfur was carried out by adding 1% (m/V) sulfur prills in 9K medium to replace the FeSO4·7H2O. Strains were cultivated in 250 mL flasks containing 100 mL 9K medium on a shaker at 160 r/min and 30 °C.
2.2 Oxidizing activity analysis
When ferrous ion was used as the sole energy source, the content of Fe3+ in the medium was determined as an indicator for growth of A. ferrooxidans strains. The total Fe (Fe2++ Fe3+) content was determined by using atomic absorption spectrometry according to the protocol recommended by the manufacturer (The Second Optic Apparatus, Beijing, China). The ρ(Fe2+)/ρ(Fe3+) ratio (the content of Fe3+) was determined by complexometric titration using potassium dichromate. When S0 was used as the sole energy source, the pH value of the medium was measured as an indicator for the growth of A. ferrooxidans strains. The pH value was determined with a pH/ISE testing instrument (828 model, Orion Research).
2.3 Transmission electron microscopy (TEM) analysis
Cells of A. ferrooxidans were cultured in 9K medium with ferrous ions as the sole energy source. Afterwards, they were fixed in glutaraldehyde (2.5% in 0.1 mol/L phosphate buffer, pH 7.4) for 1 h, rinsed three times for 10 min with 0.1 mol/L phosphate buffer (pH 7.4) and post-fixed with 1% osmium tetraoxide for 2 h at 4 °C. After the fixation, the cells were rinsed three times with 0.1 mol/L phosphate buffer (pH 7.4) and then dehydrated in graded acetone. After the dehydration, the cells were treated with propylene oxide twice for 10 min at 4 °C. The cells were sequentially infiltrated with a mixture of propylene oxide: Durcupan’s ACM epoxy resin (3:1, 1:1 and 1:3) for 45 min. Polymerization of the resin to form specimen blocks was performed in an oven at 60 °C for 72 h. The specimen blocks were hand trimmed with a razor blade and sectioned with a diamond knife. Thin sections (70-80 nm) were placed on copper grids with pore size of 50 μm. The sections were stained for 15-20 min in uranyl:ethyl alcohol (1:1), washed three times and then incubated in a drop of Reynold’s lead citrate and examined using a JEM-1230 transmission electron microscope (Japan).
2.4 Genomic DNA extraction and determination of G+C content
Genomic DNA was extracted from three A. ferrooxidans strains above as described by Harrison (1982). By using high performance liquid chromatography (HPLC), the G+C content of DNA was tested. DNA sample extracted was dissolved in distilled water to a final concentration of 1 mg/mL, and then the solution was heated at 100 °C for 5 min. After rapid cooling on ice, 10 mL of denatured DNA solution was reacted with 10 mL of nuclease P1 as recommended by the manufacturer. Nucleosides were separated by reversed-phase HPLC according to the previously described method [7]. Wild type lambda phage DNA was used as the standard, and the G+C content (molar fraction) was determined using the method described previously [8].
2.5 Genomic DNA-DNA hybridization
Nitrocellulose filters were removed from the filter holders and sequentially placed on Whatman 3MM paper saturated with 0.2 mol/L NaOH-0.6 mol/L NaCl and 1 mol/L Tris-0.6 mol/L NaCl to denature genomic DNA from each strain above. These filters were then baked for 2 h at 80 °C in a vacuum oven [9]. Filters were prehybridized and hybridized. The solution for prehybridization consists of 6x SSC (1x SSC is 0.15 mol/L NaCl plus 0.015 mol/L sodium citrate), 1x Denhardt solution, 1 mmol/L EDTA (pH 8.0), 0.5% sodium dodecyl sulfate. For hybridization, the nicktranslated probe was added directly to the plastic bag containing the filters and prehybridization solution. Prehybridization was usually carried out for 1-2 h at 65 °C; hybridization, at 65 °C overnight; and washing, at 65 °C in 0.2x SSC for 30 min. Melanie 4.0 software was used to read and analyze the hybridization signal [10-11].
2.6 Construction of cosmid library of AF2 strain genome
The cosmid library of AF2 genome was constructed by a pWEB Cosmid Cloning Kit. Briefly, the genomic DNA from AF2 was partially digested with Sau3AI and size-fractionated by PFGE. The 30-45 kb DNA fragments were collected and were ligated with pWEB∷TNC. Ligated DNA was packaged in vitro with a packaging kit (Epicentre), and transduced into E.coli EPI100-T1. The library was plated at concentrations to yield 300 colonies per plate. The bacteria from each plate were combined, and the complete plasmid DNA isolated from 12 plate pools was screened by diagnostic PCR using the specific primers. Positive pools were plated with numbers of 50 per plate and rescreened. This procedure was repeated once until single positive colony could be identified. Positive cosmids were sonicated, end-repaired by BAL-31 and Klenow fragment, and size- fractionated by gel electrophoresis to yield fragments of 1-2 kb in length. These fragments were ligated into the EcoRV site of pBluescript II SK (Stratagene) and end-sequenced. Remaining gaps were filled by using specifically designed primers and by targeted subcloning [12].
2.7 Sequence analysis of iro gene-containing clone
The iro gene probes were prepared with a Biotin labeling Kit, according to the manual instructions. The resulting probes were used for screening the cosmid library above. According to the order of the cell membrane in the nylon, in-situ method of cracking released cosmid plasmid DNA, for hybridization. After further verifying the fragment insertion by colony PCR, positive clones containing inserts of about 40 kb were sequenced in Beijing Genomics Institute (Beijing, China). Sequence analysis was performed by using BLASTX, PROSITE, FRAMEPLOT, and the LaserGene DNAStar software package.
3 Results and discussion
3.1 Morphological observation by transmission electron microscopy (TEM)
Micrographs of A. ferrooxidans cells by transmission electron microscopy (TEM) show that all the three strains are rod-shaped, like other A. ferrooxidans strains identified before (data not shown). There is no apparent difference to be observed among them (Fig.1).
3.2 Fe2+ and S0 oxidation activity analysis
As shown in Fig.2(a), when using Fe2+ as the energy source in 9K medium, the tested strains can use Fe2+ as the sole energy source. To compare their Fe2+ oxidation activities, the content of Fe3+ in the medium is determined as an indicator for the growth of A. ferrooxidans strains. It is found that AF2 is the least active one (Fig.2(a)).
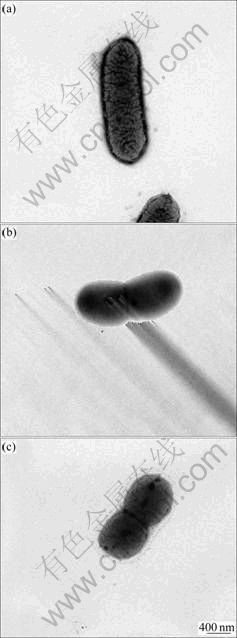
Fig.1 Morphologies of A. ferrooxidans strains by transmission electron microscopy: (a) A. ferrooxidans ATCC23270; (b) AF2; (c) AF3
When using S0 as the energy source instead of Fe2+ in the 9K medium, all three strains can use S0 as the sole energy source. To compare their oxidation activities, the oxidation efficiency of S0 (expressed as the pH change of the medium due to sulphuric acid formation) is determined. AF2 is also the least active one (Fig.2(b)). But according to the description of CHEN [2], AF2 is shown to have a higher activity when it uses S0 as the energy source instead of Fe2 +.
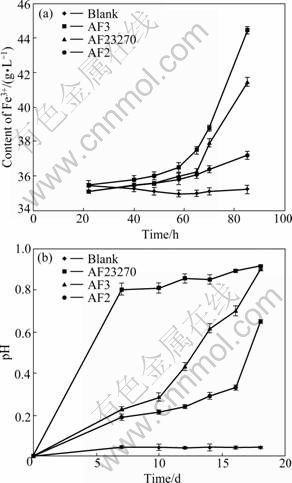
Fig.2 Fe2+ and S0 oxidation activities of three strains of A. ferrooxidans: (a) Fe2+ oxidation activity; (b) S0 oxidation activity
3.3 Determination of G+C content
The G+C contents of the genomic DNA of three strains were examined by HPLC analysis. Its greatest values are probably its usefulness as a taxonomic marker and its usefulness in distinguishing phenotypically similar microorganisms. The G+C contents of AF3 and ATCC23270 are 64.8% and 63.7% (molar fraction), respectively, which correspond well to the G+C content range of 57%-65% (molar fraction) in A. ferrooxidans [13-14]. However, the G+C content of AF2 is 51.8% (molar fraction), which is about 5.2% (molar fraction) lower than this range.
3.4 Genomic DNA-DNA hybridization
The DNA-DNA hybridization of genomic DNA shows that AF2 has only 41.53% and 52.38% similarities with ATCC23270 and AF3, respectively. But AF3 shows 89.86% similarity with ATCC23270 (Table 1). AF2, ATCC23270 are formerly considered to be the same species as A. ferrooxidans, while the results reveal that they show poor DNA sequence similarity. In contrast, ATCC23270 and AF3 are more phylogenetically close.
Table 1 DNA-DNA hybridization results (%) among ATCC23270, AF32 and AF3 strains

3.5 Sequence analysis of iro gene-containing clone
The cosmid clone containing iro gene was then sequenced. Sequence analysis shows that the cosmid clone is 40 kb in length and contains hflK protein (hflK), hflC protein (hflC), ATP phosphoribosyltransferase regulatory subunit (hisZ), adenylosuccinate synthetase (purA), iro, tRNA-Leu, ribonuclease R (vacB), pyridine nucleotide-disulfide oxidoreductase as well as functional genes (Fig.3(a)). But ATCC23270 has a different gene distribution of iro gene (Fig.3(b)). In strain AF2, iro is located at the downstream of purA, and the upstream from a putative leucyl-tRNA gene, encoding adenylosuccinate synthase. The genetic organization of the iro locus differs depending on the strain. In ATCC23270, iro gene is located at downstream of petC-2 (Fig.3(b)), encoding the cytochrome c1 of the second cytochrome bc1 complex of A. ferrooxidans. At the downstream of the iro, no tRNA gene is detected, but a putative gene encoding a theoretical protein is found. These findings underline the genotypic heterogeneity within the A. ferrooxidans species.

Fig.3 Gene distribution of A. ferrooxidans AF2 (a) and ATCC23270 (b): 1—hflK protein (hflK); 2—hflC protein (hflC); 3—ATP phosphoribosyltransferase regulatory subunit (hisZ); 4—Adenylosuccinate synthetase (purA); 5—Iron oxidase (iro); 6—tRNA-Leu; 7—Ribonuclease R (vacB); 8—Conserved hypothetical protein; 9—Pyridine nucleotide- disulfide oxidoreductase; 10—Conserved hypothetical protein; 1′—Conserved hypothetical protein; 2′—Ribosomal RNA large subunit methyltransferase J (rrmJ); 3′—Hypothetical protein; 4′—Ubiquinol-cytochrome c reductase, cytochrome c1 subunit (petC-2); 5′—Iron oxidase (iro); 6′—Hypothetical protein; 7′— Ubiquinol-cytochrome c reductase, cytochrome b subunit (petB-2); 8′—Ubiquinol-cytochrome c reductase, iron-sulfur subunit (petA-2); 9′—Oxidoreductase, short-chain dehydro- genase-reductase family; 10′—Cytochrome c4 (cycA-2)
3.6 Discussion
In the present study, three strains of A. ferrooxidans, ATCC23270, AF2, and AF3, are physiologically and genetically analyzed and compared. All of them are Gram-negative, motile, acidophilic and chemolitho- autotrophic bacteria rods (data not shown), and can obtain energy by the oxidation of Fe2+ and S0. When using Fe2+ as the sole energy source, AF3 is the most active one, and AF2 is the least one. When using S0 as the energy source instead of Fe2+, the highest utilization rate of S0 is obtained by strain ATCC23270, followed by AF3 and AF2. AF2 appears to be different from the other strains in substrate utilization, as it oxidizes S0 more effectively than Fe2+. This is consistent with the previous reports that strains isolated from various ecological niches have differences in rates of growth, oxidation of Fe2+ and S0 [3-4, 15-16]. G+C content is also a fundamental property of cellular deoxyribonucleic acid (DNA) and is correlated with the amino acid composition of proteins, codon usage in messenger ribonucleic acid, auxotrophy for specific bases, and other properties of general biological interest [17]. These results are also in agreement with the results obtained from the G+C content and southern hybridization analysis of their genomic DNA, which show that AF2 has a lower G+C content of 51.8% (molar fraction) (for AF3 and ATCC23270, the values are 64.8% and 63.7% in molar fraction, respectively), and shows lower DNA similarities with ATCC23270 and AF3 (only 41.53% and 52.38%, respectively). Our results strongly suggest that strain AF2 is, both phenotypically and genetically, significantly different from the other strains.
To gain further insight into the molecular biology of the energy production process of these strains, the iro genes involved in electron transfer are cloned and sequenced. The iro gene can be cloned from all of the three strains and their nucleotide sequences are completely identical to each other. The iro genes in all of the three strains consist of a 273 bp open reading frame and share 100% sequence identity (Genbank accession numbers of iro genes of AT23270, AF2 and AF3 strains are Yp_002427108.1, DQ909081, DQ909082, respectively).
There are two arrangement ways for iro gene in A. ferrooxidans genome. It is located at the downstream of purA (Fig.3(a) 4) in strain AF2, however, located at the downstream of petC-2 (Fig.3(b) 4′) in A. ferrooxidans ATCC 23270 (the typic strain). These findings underline the genotypic heterogeneity within the A. ferrooxidans strains. Iro is located at the downstream of purA in AF2, which is synthesized under the iron conditions. It is suggested as an intermediate electron carrier in ferrous oxidation [18]. In ATCC23270, iro is not located at the downstream from purA but is instead at the downstream from petC-2, which is synthesized under iron as well as sulfur, or thiosulfate growth conditions. However, it is synthesized more in sulfur oxidation than in iron oxidation [18]. The Iro protein is involved in the electron transfer chain by transferring electrons from a cytochrome bc1 complex to a terminal oxidase [19]. From this, iro gene is a significant gene in the iron respiratory electron transport chain in A. ferrooxidans.
The rus gene and the coxC gene cannot be amplified from AF2. It has been found that both coxC and rus belong to the same operon (rus operon), which encodes two C-type cytochromes, Cyc1 and Cyc2 and the other three subunits of aa3-type cytochrome oxidase, coxB, A and D [20]. Rus operon expression has been identified to be significantly induced by ferrous iron, suggesting that the rus-operon-encoded products have been involved in the oxidation of ferrous iron [20-21]. And rusticyanin is synthesized in iron oxidation rather than sulfur oxidation, which constitutes up to 5% of all soluble proteins in iron-grown cells [22-23]. However, they are not presented in AF2. Indeed, AF2 is able to grow on ferrous iron, although slower than the other three strains. It is even possible that these discrepancies may represent the actual differences in the electron transfer pathways among A. ferrooxidans strains. Therefore, it is speculated that a new iron respiratory chain, different from that encoded by the rus operon, may be presented in AF2. Either from the phenotype or genetic terms, AF2 is significantly different from ATCC23270 and AF3. AF2 is a novel strain of A. ferrooxidans.
4 Conclusions
1) Three strains of A. ferrooxidans, ATCC23270, AF2 and AF3, are Gram-negative, motile, acidophilic and chemolithoautotrophic bacteria rods (data not shown), and all of them can obtain energy from the oxidation of Fe2+ and S0. But, AF2 appears to be different from the other strains in substrate utilization, as it oxidizes S0 more effectively than Fe2+.
2) AF2 has a lower G+C content of 51.8% (molar fraction) (for AF3 and ATCC23270, the values are 64.8% and 63.7% in molar fraction, respectively), and shows lower DNA similarities with ATCC23270 and AF3 (only 41.53% and 52.38%, respectively).
3) The iro genes in all of the three strains consist of a 273 bp open reading frame and share 100% sequence identity. But, the distribution of iro gene is different between AF2 and ATCC23270.
4) AF2 is able to grow on ferrous iron. But the rus gene and the coxC gene cannot be amplified from this strain. Thus, a new iron respiratory chain, different from that encoded by the rus operon may be presented in AF2.
5) A. ferrooxidans (AF2) is identified to be physiologically and genetically significantly different from the other strains such as ATCC 23270 and AF3. AF2 strain is a novel strain of A. ferrooxidans.
References
[1] YAMANAKA T, FUKUMORI Y. Molecular aspects of the electron transfer system which participates in the oxidation of ferrous ion by Thiobacillus ferrooxidans [J]. FEMS Microbiol Rev, 1995, 17(4): 401-413.
[2] CHEN Hong, YANG Bo, CHEN Xin-hua. Identification and characterization of four strains of Acidithiobacillus ferrooxidans isolated from different sites in China [J]. Microbiol Res, 2009, 164(6): 613-629.
[3] THOMPSON F L, HOSTE B, VANDEMEULEBROECKE K, SWINGS J. Genomic diversity amongst Vibrio isolates from different sources determined by fluorescent amplified fragment length polymorphism [J]. Syst Appl Microbiol, 2001, 24(4): 520-538.
[4] KARAVAIKO G I, TUROVA T P, KONDRAT’EVA T F, LYSENKO A M, KOLGANOVA T V, AGEEVA S N, MUNTYAN L N, TAT’YANA A P. Phylogenetic heterogeneity of the species Acidithiobacillus ferrooxidans [J]. Int J Syst Evol Micr, 2003, 53(10): 113-119.
[5] RAWLINGS D E, KUSANO T. Molecular genetics of Thiobacillus ferrooxidans [J]. Microbiol Rev, 1994, 58(1): 39-55.
[6] LANE D J, STAHL D A, OLSEN G J, HELLER D J, PACE N R. Phylogenetic analysis of the genera Thiobacillus and Thiomicrospira by 5S rRNA sequences [J]. J Bacteriol, 1985, 163(1): 75-81.
[7] HUANG P C, ROSENBERG E. Determination of DNA base composition via depurination [J]. Anal Biochem, 1966, 16(1): 107-113
[8] MESBAH M, PREMACHANDRAN U, WILLIAM B W. Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography [J]. Int J Syst Bacteriol, 1989, 39(10): 159-167.
[9] ANDO T, MONROE S S, GENTSCH J R, JIN Q, LEWIS D C, GLASS R I. Detection and differentiation of antigenically distinct small round-structured viruses (Norwalk-like viruses) by reverse transcription-PCR and southern hybridization [J]. Clin Microbiol, 1995, 33(1): 64-71.
[10] GELLEN L S, WALL-MANNING G M, SISSONS C H. Checkerboard DNA-DNA hybridization technology using digoxigenin detection [J]. Methods Mol Biol, 2007, 353(5): 39-67.
[11] WILLEMS A, DOIGNON-BOURCIER F, GORIS J, COOPMAN R, DE LAJUDIE P, DEVOS P, GILLIS M. DNA-DNA hybridization study of Bradyrhizobium strains [J]. Int J Syst Evol Micr, 2001, 51(4): 1315-1322.
[12] JORN P. A polyketide synthase-peptide synthetase gene cluster from an uncultured bacterial symbiont of Paederus beetles [J]. Proc Natl Acad Sci, 2002, 99: 14002-14007.
[13] NORRIS P R, CLARK D A, OWEN J P, WATERHOUSE S. Characteristics of sulfobacillus acidophilus sp. nov. and other moderately thermophilic mineral-sulphide-oxidizing bacteria [J]. Microbiol, 1996, 142: 775-783.
[14] RAWLINGS D E. Characteristics and adaptability of iron- and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates [J]. Microb Cell Fact, 2005, 4(13): 1-15.
[15] AGEEVA S N, KONDRAT'EVA T F, KARAVAIKO G I. Phenotypic characteristics of Thiobacillus ferrooxidans strains [J]. Microbiol, 2001, 70(2): 186-194
[16] HARRISON A P. Genomic and physiological diversity amongst strains of Thiobacillus ferrooxidans and genomic comparison with Thiobacillus thiooxidans [J]. Arch Microbiol, 1982, 131(10): 68-76.
[17] MOSTAFA M, USHA P, WILLIAM B. Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography [J]. Int J Syst Bacteriol, 1989, 39(2): 159-167.
[18] BRUSCELLA P, APPIA-AYME C, LEVICAN G, RATOUCHNIAK J, JEDLICKI E, HOLMES D S, BONNEFOY V. Differential expression of two bc1 complexes in the strict acidophilic chemolithoautotrophic bacterium Acidithiobacillus ferrooxidans suggests a model for their respective roles in iron or sulfur oxidation [J]. Microbiol, 2007, 153(10): 102-110.
[19] BRUSCELLA P, CASSAGNAUD L, RATOUCHNIAK J, BRASSEUR G, LOJOU E, AMILS R, BONNEFOY V. The HiPIP from the acidophilic Acidithiobacillus ferrooxidans is correctly processed and translocated in Escherichia coli, in spite of the periplasm pH difference between these two micro-organisms [J]. Microbiol, 2005, 151(10): 1421-1431.
[20] APPIA-AYME C, GUILIANI N, RATOUCHNIAK J, BONNEFOY V. Characterization of an operon encoding two c-type cytochromes, an aa(3)-type cytochrome oxidase, and rusticyanin in Thiobacillus ferrooxidans ATCC 33020 [J]. Appl Environ Microb, 1999, 65(11): 4781-4787.
[21] YARZABAL A, APPIA-AYME C, RATOUCHNIAK J, BONNEFOY V. Regulation of the expression of the Acidithiobacillus ferrooxidans rus operon encoding two cytochromes c, a cytochrome oxidase and rusticyanin [J]. Microbiol, 2004, 150(7): 2113-2123.
[22] COX J C, BOXER D H. The purification and some properties of rusticyanin, a blue copper protein involved in iron(II) oxidation from Thiobacillus ferrooxidans [J]. Biochem J, 1978, 174(12): 497-502.
[23] WANG J, WANG Y J, GUO H. Ballistic-electron transport through a coupled-quantum-wire system [J]. Phys Rev B: Condens Matter, 1992, 46(4): 2420-2427.
(Edited by YANG Bing)
Foundation item: Project(200805032) supported by the Scientific Research Program of Marine Public Welfare Industry of China
Received date: 2010-03-18; Accepted date: 2010-09-17
Corresponding author: CHEN Xin-hua, PhD; Tel: +86-592-2195297; E-mail: chenxinh@tom.com