硝苯柳胺的合成
来源期刊:中南大学学报(自然科学版)1999年第5期
论文作者:易健民 施天益 张一甫 成本诚
文章页码:519 - 521
关键词:硝苯柳胺;次氯酸叔丁酯;水杨酸;合成
Key words:niphensamide; t-butyl-hypochlorite; salicylic acid; synthesis
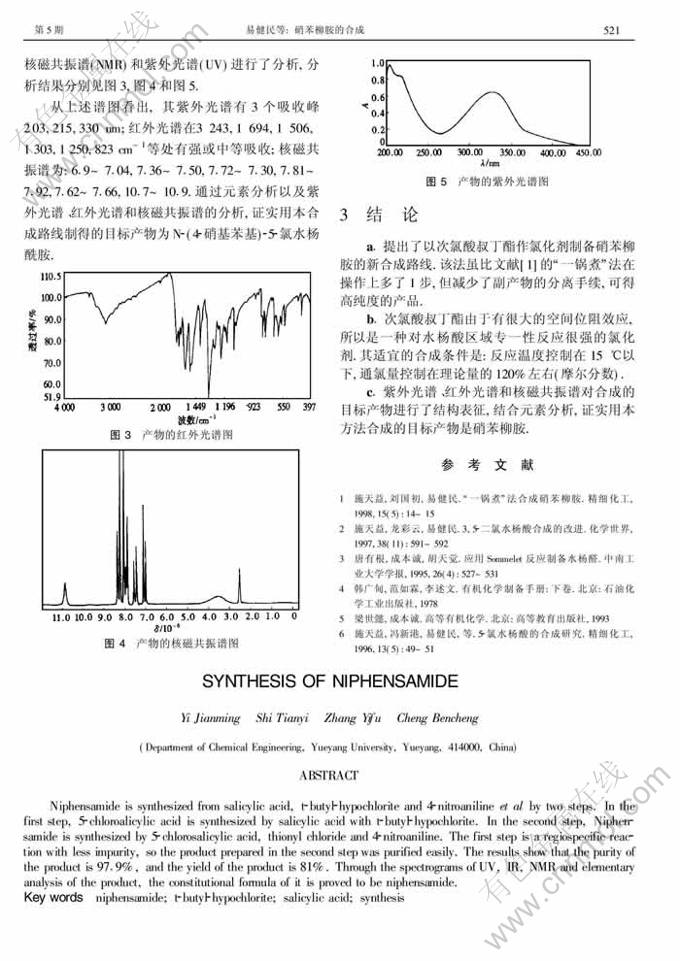
摘 要:以水杨酸、次氯酸叔丁酯和对硝基苯胺 为原料分2步合成了硝苯柳胺.第1步用次氯酸叔丁酯和水杨酸反应合成5-氯水杨酸;第2步用5-氯水杨酸、二氯亚砜和对硝基苯胺反应合成硝苯柳胺.第1步是一种区域专一性反应,杂质极少,从而使第2步反应产物易于提纯.此外,用紫外光谱、红外光谱和核磁共振谱对硝苯柳胺的结构进行了表征.结果表明,产品纯度高达97.9% ,且其收率也可达81%.
Abstract: Niphensamide is synthesized from salicylic acid, t butyl hypochlorite and 4-nitroaniline et al by two steps. In the first step, 5-chloroalicylic acid is synthesized by salicylic acid with t butyl hypochlorite. In the second step, Niphens-amide is synthesized by 5-chlorosalicylic acid, thionyl chloride and 4-nitroaniline. The first step is a regiospecific reac-tion with less impurity, so the product prepared in the second step was purified easily. The results show that the purity of the product is 97.9%, and the yield of the product is 81%. Through the spectrograms of UV, IR, NMR and elementary analysis of the product, the constitutional formula of it is proved to be niphensamide.