Degradation of organic wastewater containing Cu-EDTA by Fe-C micro-electrolysis
来源期刊:中国有色金属学报(英文版)2012年第4期
论文作者:陈润华 柴立元 王云燕 刘恢 舒余德 赵静
文章页码:983 - 990
关键词:微电解;羟基自由基;EDTA;废水
Key words:micro-electrolysis; ·OH radical; EDTA; wastewater
摘 要:为了破坏冶炼废水中重金属有机螯合物,例如Cu-EDTA配离子废水,研究一种破络并预处理的新方法。该方法基于铁碳微电解反应原理,·OH在酸性有氧气存在的条件下产生,并在铁碳表面攻击吸附的有机基团导致螯合物的破坏,从而使铜离子将从有机物中剥离下来,然而EDTA将被·OH降解。研究pH值、温度、微电解反应时间、Fe/C质量比对铜离子脱除率及总有机碳(TOC)残余含量的影响,通过扫描电子显微镜分析(SEM)、能谱分析(EDS)、红外光谱分析(FTIR)研究处理前、后样品的表面官能团变化及形貌推断铁碳微电解反应的机理。并进行工业条件优化,得到最佳工艺条件:pH值为2,温度为常温,Fe/C质量比≥0.02,时间为60 min,有氧气存在。在该条件下TOC浓度为200 mg/L、铜离子浓度为60 mg/L的废水反应完成后TOC和Cu残余浓度分别减低到40.66和1.718 mg/L;羟基自由基降解反应机理合理解释了该实验现象。
Abstract: In order to break the complex bonds and treat the organic wastewater containing heavy metal, such as Cu-EDTA solution, a novel process of Fe-C micro-electrolysis was proposed. Based on the principle of iron-carbon micro-electrolysis reaction, ·OH radicals which were generated under the acidic aerobic condition during the micro-electrolysis process attacked to the organic groups of coordination compounds, which resulted in complex bonds breaking. Therefore copper (II) ions were removed via nascent gelatinous ferric hydroxide and ferrous hydroxide, and EDTA was degraded by ·OH radicals. Effects of pH value, temperature, electrolysis time and mass ratio of Fe to C on residual concentrations of total organic carbon (TOC) and Cu(II) were studied. The mechanism of Fe-C micro-electrolysis was investigated and verified by analyzing micrographs of scanning electron microscopy (SEM), energy dispersive analysis (EDS) and Fourier transform infrared spectrometry (FTIR).The removal efficiency is optimal at pH value of 2.0, temperature of 25℃, the mass ratio Fe to C of 0.02, and reaction time of 60 min. Under above conditions, the concentration of TOC decreases from 200 mg/L to 40.66 mg/L and the residual concentration of Cu(II) decreases from initial 60 mg/L to 1.718 mg/L.

![]()

Trans. Nonferrous Met. Soc. China 22(2012) 983-990
CHEN Run-hua, CHAI Li-yuan, WANG Yun-yan, LIU Hui, SHU Yu-de, ZHAO Jing
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 18 April 2011; accepted 21 June 2011
Abstract: In order to break the complex bonds and treat the organic wastewater containing heavy metal, such as Cu-EDTA solution, a novel process of Fe-C micro-electrolysis was proposed. Based on the principle of iron-carbon micro-electrolysis reaction, ?OH radicals which were generated under the acidic aerobic condition during the micro-electrolysis process attacked to the organic groups of coordination compounds, which resulted in complex bonds breaking. Therefore copper (II) ions were removed via nascent gelatinous ferric hydroxide and ferrous hydroxide, and EDTA was degraded by ?OH radicals. Effects of pH value, temperature, electrolysis time and mass ratio of Fe to C on residual concentrations of total organic carbon (TOC) and Cu(II) were studied. The mechanism of Fe-C micro-electrolysis was investigated and verified by analyzing micrographs of scanning electron microscopy (SEM), energy dispersive analysis (EDS) and Fourier transform infrared spectrometry (FTIR).The removal efficiency is optimal at pH value of 2.0, temperature of 25 ℃, the mass ratio Fe to C of 0.02, and reaction time of 60 min. Under above conditions, the concentration of TOC decreases from 200 mg/L to 40.66 mg/L and the residual concentration of Cu(II) decreases from initial 60 mg/L to 1.718 mg/L.
Key words: micro-electrolysis; ?OH radical; EDTA; wastewater
1 Introduction
Heavy metals belong to persistent pollutants in environment because of their high toxicity. The discharge of heavy metals containing wastewater causes waste of resources and influences the safety of drinking water. Copper, one of heavy metals, is considered the substance which has a deleterious effect on the aquatic environment and should be subject to authorization with specification of discharge standards [1]. According to Chinese health standard, the limit of Cu in drinking water is 1.0 mg/L [2-5]. However, concentrations of Cu(II) effluents discharged from painting, printed circuit board manufacturing, electroplating, dyeing, photography, metal finishing, or surface treatment industry usually contain different complex agents [6,7], an amine carboxylic derivative commonly known as EDTA [8-10]. This kind of wastewater contains not only lots of organic matters but also a high concentration of heavy metals.
It is well known that, in the presence of chelating agents like EDTA, the performance of metal removal can be adversely affected due to the formation of soluble complexes like Cu-EDTA (CH2N)2[(CH2COO)2Cu]2, which could not be efficiently removed by the traditional chemical precipitation or physical chemistry technologies [11]. It is also hard to remove organic matter by biological method, because the value of BOD/COD is below 0.3 and the concentration of heavy metal is too high for the organism to be alive [12,13]. When EDTA becomes an important interference in the metal removal process, the breaking of the complex bonds is needed. Due to the strong stability of this wastewater, the traditional methods were not valid [14].
Based on the electrochemical redox reaction principle, Fe-C micro-electrolysis had developed gradually since early 1970s with the application of iron to wastewater treatment. Except for the electrochemical redox reaction, during the process many reactions also contribute to the degradation of pollutants, such as electrical enrichment, coagulation, adsorption and filtration reaction [15-18]. There are lots of other advantages. For example, the reactor has a simple structure and is easily manufactured and operated [19]. The process also keeps low processing costs and can be combined with other ways easily. So much effort has been done to study the treatment of heavy metal chelating organic wastewater using Fe-C micro- electrolysis method [20-23].
The aim of this work is to study the degradation of copper irons and total organic carbon (TOC) in the simulated (CH2N)2[(CH2COO)2Cu]2 wastewater in the Fe-C micro-electrolysis process in order to get the optimal conditions. The mechanism of ?OH generation in the process was proposed. Scanning electron microscopy (SEM), energy-dispersive analysis (EDS) and Fourier transform infrared spectrometry (FTIR) analyses were also used to further illustrate Fe-C micro-electrolysis mechanism.
2 Experimental
2.1 Materials and analytical methods
All reagents used in this work were of analytic grade. The stock of Cu-EDTA wastewater containing 60 mg/L Cu(Ⅱ) and 200 mg/L TOC was prepared by dissolving solid anhydrous CuSO4 and EDTA-2Na in ultrapure water. 1 mol/L NaOH and 1 mol/L HCl solutions were used to adjust pH value of the solution. The granule activated carbon (GAC) was supplied by Yushan Activated Carbon Company (Jiangxi, China). The average particle size of GAC is 2.360-1.000 μm. Components of iron powder used in the series of experiments are as follows: iron >98%, insoluble sulfate 0.1%, sulfide 0.06% and copper 0.005%.
The pH of the solution during the experiment was determined using a PHS-3C meter (Shanghai Rex Instrument Factory). The concentration of copper (Ⅱ) ions (Cu2+) in solution was measured by a WFX-120 atomic absorption spectrophotometer. The TOC of wastewater and effluent was measured by a TOC-VCPH (Shimadzu Instrument Factory) meter. The surface structures and components of samples were analyzed by JSM-6360LV scanning electronic microscope (SEM) coupled with energy dispersive spectrometer (EDS). Infrared spectra of GAC after adsorbing EDTA and Cu-EDTA in solid phase were obtained on a Fourier transform infrared spectrometer by using the KBr method.
2.2 Experimental set-up
As shown in Fig. 1, the experimental apparatus was a novel Fe-C micro-electrolysis reactor. Both the cell support and microporous diaphragm were made of PVC plastic. The upper structure was a batch cylindrical cell with the inner diameter of 100 mm and the height of 300 mm. The bottom part was a conical structure and it was separated with the upper by a microporous diaphragm, which can diffuse the compressed oxygen/nitrogen into upper department. The iron/carbon packing layer was placed on the diaphragm. There were three sampling outlets at different heights of the upper. A constant flow pump was used for recycling the solution. The oxygen or nitrogen tank was connected to inlet of the reactor.
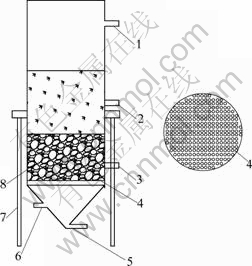
Fig. 1 Schematic diagram of Fe-C micro-electrolysis reactor: 1—Inlet; 2, 3—Sampling port; 4—Diaphragm frame; 5—Aeration port; 6—Outlet; 7—Support; 8—Fe-C packing layer
At the beginning, mixture with different mass ratios of iron to carbon was placed on the diaphragm as a packing layer. A total of 300 mL of wastewater was placed in the cell being in a temperature controlled water bath. Then the experiment was run when the constant flow pump and gas tank were switched on. The resulted solution was filtered to remove any traces of activated carbon or Fe powder, and then was analyzed for the concentration of TOC and Cu(II). The experimental results were assessed mainly based on the residual concentrations of TOC and Cu(II).
3 Results
3.1 Effect of pH
The residual concentrations of TOC and Cu(II) at different pH values were analyzed and the results are presented in Fig. 2 and Fig. 3. As shown in Fig. 2, in all cases the amount of TOC increased with increasing the pH value of the solution. In particular, there was an abrupt decrease of TOC removal rate from 85.04% to 40.61% when the pH value varied from 2 to 6. The maximum value for TOC removal was achieved at pH 2. Till the solution was alkaline, there was almost no TOC degradation at all while desorption and dissolution of EDTA absorbed on the activated carbon would make TOC up.
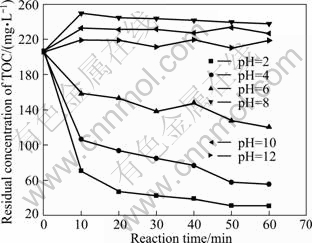
Fig. 2 Effect of pH value on residual concentration of TOC (Experimental conditions: m(Fe)/m(C)=3:40, reaction time =60 min, temperature=25 ℃, oxygen existing)
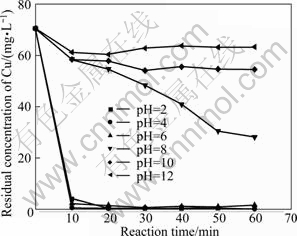
Fig. 3 Effect of pH value on residual concentration of Cu(II) (Experimental conditions: m(Fe)/m(C)=3:40, reaction time =60 min, temperature=25 ℃, oxygen existing)
As can be seen in Fig. 3, when pH was up to 6 from 2, the residual concentration of copper ions after treatment for 60 min increased from 0.52 mg/L to 1.72 mg/L. And then with pH increasing to 10, the residual concentration of copper achieved 63.54 mg/L rapidly, while even prolonging the reaction time, it was useless for the improvement of removal efficiency. pH of 2 is the best but pH of 4 is selected for rest experiments, taking into account the cost of treatment.
3.2 Effect of temperature
Figures 4 and 5 show the residual concentrations of TOC and Cu(II) under various temperatures, respectively. As shown in Fig. 4, the residual mass concentrations of TOC increased obviously with an increase in temperature. After 60 min treatment, at the temperature of 0 ℃, the concentration of TOC is 40.66 mg/L, while it is 122.10 mg/L at 60 ℃. As shown in Fig. 5, the effect of temperature on the residual concentrations of Cu(II) is not remarkable. Reaction is very rapid during the first 30 min resulting in Cu(II) concentration of 1.21 mg/L, and then slows down abruptly. The reaction equilibrium is obtained at 30 min. To sum up, prolonging the time after 60 min would not make any sense on Fe-C micro-electrolysis. Though the theoretical optimum temperature is 0 ℃, considering the actual situation, 15 ℃ is appropriate for rest experiments.
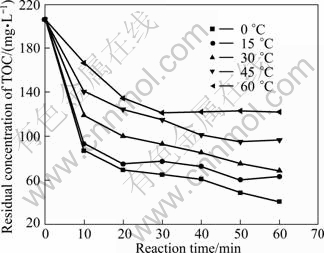
Fig. 4 Effect of temperature on residual concentration of TOC (Experimental conditions: m(Fe)/m(C)=3:40, pH =4.0, reaction time =60 min, oxygen existing)
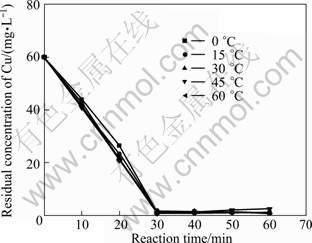
Fig. 5 Effect of temperature on residual concentration of Cu (Experimental conditions: m(Fe)/m(C)=3:40, pH =4.0, reaction time=60 min, oxygen existing)
3.3 Effect of dissolved oxygen
Aerobic and anaerobic experiments were carried out, respectively, and the results are shown in Figs. 6 and 7. As can be seen in Fig. 6, after 30 min treatment, the residual TOC is 152.60 mg/L for the anaerobic experiment with nitrogen pumping in, while it is 44.98 mg/L for the aerobic experiment with oxygen pumping in. It is shown clearly in Fig.7 that the dissolved oxygen also has a great impact on the Cu(II) removal efficiency. After 30 min treatment, the concentrations of Cu(II) were 20.68 mg/L and 0.42 mg/L for oxygen and nitrogen conditions, respectively. So it can be concluded that the dissolved oxygen has a beneficial effect on the Fe-C micro-electrolysis.
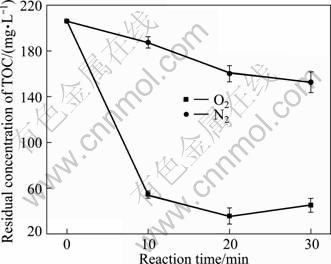
Fig. 6 Effect of ventilation condition on residual concentration of TOC (Experimental conditions: m(Fe)/m(C)=3:40, pH =4.0, reaction time=30 min, temperature=25 ℃)
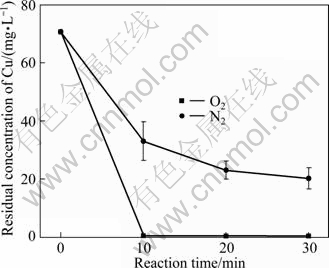
Fig. 7 Effect of ventilation condition on residual concentration of Cu (Experimental conditions: m(Fe)/m(C)=3:40, pH=4.0, reaction time=30 min, reaction temperature=25 ℃)
3.4 Effect of mass ratio of iron to carbon
Mass ratio of Fe to C is an important operational parameter for an economical wastewater treatment process. Figures 8 and 9 show the effect of mass ratio of Fe to C on the residual concentrations of TOC and Cu(II). It is indicated that the amount of adding iron had little effect on both the degradation of Cu(II) and EDTA. When the mass ratio m(Fe)/m(C)=0, there is certain degradation efficiency due to the adsorption of activated carbon. But along with the mass ratio varying from 0.01 to 0.04, the curves of residual Cu(II) are almost the same under different ratios and so do the curves of residual TOC. After 60 min, prolonging the reaction time makes no sense to the degradation of Cu-EDTA. In order to obtain the complete reaction, 0.01 is selected as the tested mass ratio of Fe to C ratio for rest experiments.
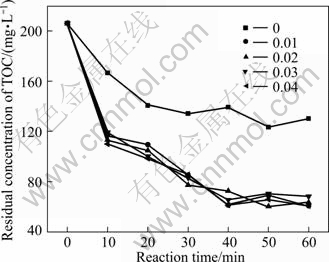
Fig. 8 Effect of mass ratio of Fe to C on residual concentration of TOC (Experimental conditions: pH=4.0, reaction time =60 min, temperature=25 ℃, activated carbon=40 g, oxygen existing)
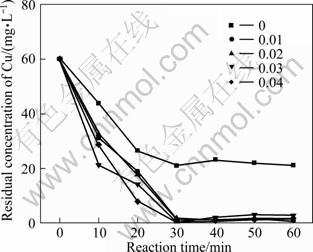
Fig. 9 Effect of mass ratio of Fe to C on residual concentration of Cu (Experimental conditions: pH=4.0, reaction time=60 min, temperature=25 ℃, oxygen existing)
3.5 Duplicate experiments under optimal conditions
After series of experiments, the optimal conditions were got as follows: pH 2.0, room temperature, the mass ratio of Fe to C being 0.02, oxygen existing and 60 min of reaction time. The residual concentrations of Cu(II) and TOC were 1.718 mg/L and 40.66 mg/L, respectively. Under the above conditions, experiments were also carried out in duplicate and analysis of each parameter was done in triplicate for each run. The coefficient of variation obtained was no more than 5% for the three determinations and for the experiment runs carried out in duplicate.
4 Fe-C micro-electrolysis mechanism of Cu-EDTA
4.1 SEM and EDAX analyses
In this study, the surface morphologies of activated carbon before and after Fe-C micro-electrolysis were compared by SEM analysis. Figures. 10(a) and (b) indicate the presence of many amorphous particles and agglomerates. From Fig. 10(a), it can be seen that small particles of amorphous precipitate adhere to the surface of agglomerates. This may be due to the presence of iron powder on the activated carbon surface, which can be further confirmed from the EDS analysis. This can be thought to be successful adsorption of iron powder onto the activated carbon surface before Fe-C micro- electrolysis.
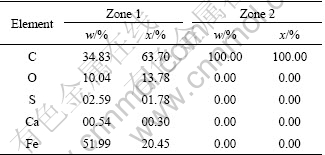
The EDS patterns for activated carbon before and after Fe-C micro-electrolysis are illustrated in Figs. 10(c) and (d), and the detailed elemental constitution is shown in Table1. In addition to C signal, Fe, S, O and Ca signals can be observed in Fig. 10(c), which are known as the principal elements of iron powder. As illustrated in Fig. 10(d), only C signal can be observed. The evanescence of Fe and other signals provides direct evidence that Fe powders have been consumed completely during Fe-C micro-electrolysis.
Table 1 Analytic results of activated carbon in Fig. 10
4.2 FTIR analysis
The FTIR spectra obtained for activated carbon after adsorbing EDTA and Cu-EDTA are shown in Fig. 11. As shown in Fig. 11, the assignment of FTIR bands and detailed wave number shifted for the two samples almost have no significant difference. So the FTIR studies reveal that during the process of activated carbon adsorbing Cu-EDTA, Cu(II) has been liberated to the solution from the complex of Cu-EDTA.
4.3 Degradation mechanism of Cu-EDTA
4.3.1 Generation of ?OH free radicals
The micro-cell reaction that occurred in the Fe-C-O2 water system fits the following reaction [21]:
![]() (1)
(1)
Then, the produced Fe2+ and H2O2 can react to generate ?OH in absorbed form by Fenton reaction according to Eq. (2):
![]() (2)
(2)
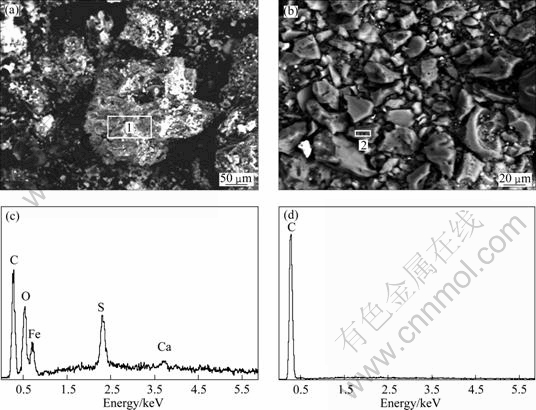
Fig. 10 SEM images (a, b) and EDS patterns (c, d) of activated carbon before (a and c) and after (b and d) micro-electrolysis
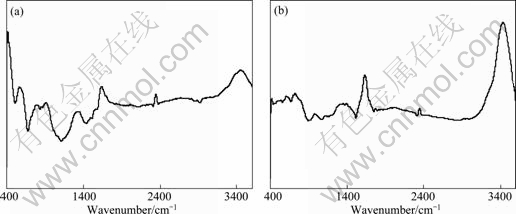
Fig. 11 FTIR spectra of activated carbon after adsorbing EDTA(a) and Cu-EDTA(b)
When the equilibrium is reached between reactions (1) and (2), the concentration of ?OH radicals can be calculated by the following Eq. (3):
![]() (3)
(3)
where ![]() is Gibbs free energy;
is Gibbs free energy; ![]() is the amount of DO adsorbed at equilibrium; R is the proportionality constant; and T is the temperature.
is the amount of DO adsorbed at equilibrium; R is the proportionality constant; and T is the temperature.
4.3.2 Degradation process of EDTA
Since the standard electrode potential of ?OH is 2.80 V and thus ?OH radicals have a strong electron affinity, the ?OH can attack on the site with high electron density on EDTA. Ultimately, EDTA is degraded and the degradation process can be described as follows:
![]()
![]() —COOH
—COOH
![]() —
—![]() (4)
(4)
4.3.3 Dissociation of EDTA-chelated copper ions
pH value of the solution can affect the extent of the adsorption and micro-electrolysis because it strongly influences the metal ions (Cu2+) precipitation, speciation and the protonation degree of the ligand (EDTA). At high pH values, coordination reaction takes place between Cu2 + and EDTA:
![]() (5)
(5)
where Y represents organic groups. At pH of 2.0, Cu liberated from CuY2- results in the predominant species of Cu(II) in the wastewater containing Cu-EDTA. The reaction pathway may follow Eq. (6).
![]() (6)
(6)
This indicates that when pH is 2.0, the species of EDTA in Cu-EDTA solution is H4Y and EDTA is adsorbed on activated carbon also in form of H4Y, which is no relevant to Cu(II). It can be further confirmed from the EDS analysis because the EDS analytic results indicated that there is no Cu signal on the surface of activated carbon shown in Fig. 10 and Table 1. In addition, Fig. 11 shows that there is no obvious difference between the FTIR diagrams of activated carbon after adsorbing H4Y (EDTA) and Cu2Y (Cu-EDTA), which can also be used to further confirm the deduction that Cu is freed from CuY2- to be Cu(II) during the adsorption process when pH is 2.0.
5 Discussion
5.1 Effect of pH
As Eq. (3) shown, the concentration of free ?OH radicals increased with pH decreasing. So the removal rate of EDTA enhanced and the residual concentration reduced with pH falling. As the ligand (EDTA) was removed, Cu(II) lost its ligand and hydrolytic removal of free Cu(II) increased with decreasing pH.
5.2 Effect of temperature
It can be deduced from Eq. (3) that the amount of ?OH radicals decreased with the advance of temperature, resulting in the fact that the removal rate of EDTA went down. But the effect of temperature on the Cu(II) removal was negligible, as shown in Fig. 6. The reason is that though ?OH radicals under lower temperatures were not enough for the complete mineralization of EDTA to be CO2 and water, there were sufficient EDTA to decompose into intermediate products and then the complexation was also destructed entirely. Ultimately, in spite of the change of temperature, the complexation is always destructed entirely and the removal of Cu(II) has nothing to do with the temperature.
5.3 Effect of dissolved oxygen
During the Fe-C micro-electrolysis, the existence of dissolved oxygen enhanced the removal efficiency significantly. Equation (3) indicated that the increase of oxygen pressure led to more ?OH radicals, which resulted in the increased removal rate of EDTA and Cu(II).
6 Conclusions
1) Based on the mechanism of the electrochemical redox reaction, a novel cylindrical Fe-C micro- electrolysis reactor for the treatment of organic wastewater containing Cu-EDTA was designed and developed. It also can be applied to treating other kinds of organic complex wastewater.
2) The removal efficiency for organic wastewater containing Cu-EDTA using Fe-C micro-electrolysis reactor is optimal under the following conditions: pH of 2.0, temperature of 25 ℃, the mass ratio of Fe to C being 0.02, and 60 min of reaction time. Under the above conditions, the concentration of TOC decreases from 200 mg/L to 40.66 mg/L and the residual concentration of Cu(II) maintains 1.718 mg/L in contrast to the initial concentration of 60 mg/L.
3) The degradation mechanism of Cu-EDTA was speculated. During the Fe-C micro-electrolysis process, due to pH 2.0, Cu liberated from Cu-EDTA results in the predominant species of Cu(II) in the solution. So, the Cu-EDTA is decomposed into Cu(II) and EDTA, and then Cu(II) and EDTA are oxidized respectively mainly by ?OH. The degradation process of EDTA is described in equation: EDTA→R3N→R2NH→ H2NR→![]() +HOOC—
+HOOC—![]() + HOOC—COOH→CO2+ H2O+NH3. The concentration of ?OH radicals can be calculated by equation:
+ HOOC—COOH→CO2+ H2O+NH3. The concentration of ?OH radicals can be calculated by equation:
![]()
![]() ,
,
according to which the experimental data can be explained reasonably.
References
[1] Wuhan University. Analytical chemistry [M]. Beijing: People’s Press, 1978: 22-26. (in Chinese)
[2] ZHANG Ya-ping, WEI Zhao-hai. Oxidation degradation of cationic red GTL by photo-Fenton [J]. Journal of Central South University: Science and Technology, 2008, 39(4): 688-693. (in Chinese)
[3] BRILLAS E, CALPE J C. Mineralization of 2,4-D by advanced electrochemical oxidation processes [J]. Water Research, 2000, 34(8): 2253-2262.
[4] YANG Shu-ming, HUANG Chang-dun. Textile printing and dyeing industry wastewater treatment technology [M]. Beijing: Chemical Industry Press, 2002: 29-32. (in Chinese)
[5] ZHOU Pei-guo, FU Da-fang. Application and development for microelectrolysis technology [J]. Technigues and Equipment for Environmental Pollution Control, 2001, 2(4): 18-24. (in Chinese)
[6] XU Fu-yuan, SONG Tian-shun. Oxidation degradation of cationic red GTL by photo-Fenton [J]. Journal of Central South University: Science and Technology, 2008, 38(6): 480-485. (in Chinese)
[7] ZHANG Bo, HE Yi-liang. The pretreatment effect of ferric-carbon micro electrolysis and coagulation sedimentation for chemical industrial organic wastewater [J]. Journal of Lanzhou Railway University, 2001, 20(3): 95-98. (in Chinese)
[8] PITTER P, SYKORA V. Biodegradability of ethylene diamine-based complexing agents and related compounds [J]. Chemosphere, 2001, 44(4): 823-826.
[9] WALSH F, READE G. Design and performance of electrochemical reactors for efficient synthesis and environmental treatment, Part 2: Typical reactors and their performance [J]. Analyst, 1994, 119(5): 797-803.
[10] WANG You-le, ZHANG Qing-fang. Development of industrial wastewater treatment with micro-electrolysis [J]. Journal of Gansu University of Technology, 2003, 29(1): 67-69. (in Chinese)
[11] ZHANG Ya-nan, DUAN Shun-shan, LIU Guo-guang. Study on pretreatment for pharmacadical wastewater by iron scrap process [J]. Ecologic Science, 2002, 21(1): 62-64. (in Chinese)
[12] MA Qian, YE Shao-dan, LI Yi-jiu. Study on the wastewater treated by the iron chipping micro-electrolysis [J]. Industrial Water Treatment, 2003, 23(5): 38-40. (in Chinese)
[13] DING Zhi-hui, LIU Ji-kai. Progress on the research of pyrethroids [J]. Yunnan Chemical Technology, 2001, 28(2): 22-24. (in Chinese)
[14] CHANG Wen-xing, QU Chang-hong. Introduction on treatment methods of high concentrated pesticide wastewater [J]. Environmental Protection Science, 2001, 27(5): 25-26. (in Chinese)
[15] CHENG Ming, HE Wen-ying, PENG Guang-ming. A test of treatment of wastewater from pesticide glyphosate production [J]. Industrial Water & Wastewater, 2003, 34(1): 30-32. (in Chinese)
[16] LIU Hong, ZHANG Lin-xia, WU Ke-ming. Study on the treatment of coke plant wastewater by adsorption catalytic oxidation process [J]. Industrial Water Treatment, 2003, 5(5): 35-37. (in Chinese)
[17] ZOU Ding-ping. Structure analysis of organic compounds [M]. Beijing: Science Press, 2005: 53. (in Chinese)
[18] JONSON T L. Knitics of halogenated organic compound degradation by iron metal [J]. Environmental Science Technology, 1996, 30: 2634-2640.
[19] LI Di. Electrochemical principle [M]. Beijing: Beijing University of Aeronautics and Astronautics Press, 2008: 441-450. (in Chinese)
[20] ZHA Quan-xing. Introduction to electrode kinetics [M]. Beijing: Science Press, 2002: 192-197. (in Chinese)
[21] QU Jiu-hui. LIU Hui-juan. Principle and Technology of electrochemical water treatment [M]. Beijing: Science Press, 2007: 216-438. (in Chinese)
[22] CHEN Run-hua, CHAI Li-yuan, WANG Yun-yan. Degradation of organic wastewater containing EDTA by Fe-C micro-electrolysis [J]. Journal of Central South University: Science and Technology, 2011: 42(6): 1516-1521. (in Chinese)
[23] DENG Wan-hao. Degradation of organic wastewater containing EDTA by fenton & ferrite process [D]. Guangzhou: Sun Yat-sen University, 2004 (in Chinese)
陈润华,柴立元,王云燕,刘 恢,舒余德,赵 静
中南大学 冶金科学与工程学院,长沙 410083
摘 要:为了破坏冶炼废水中重金属有机螯合物,例如Cu-EDTA配离子废水,研究一种破络并预处理的新方法。该方法基于铁碳微电解反应原理,·OH在酸性有氧气存在的条件下产生,并在铁碳表面攻击吸附的有机基团导致螯合物的破坏,从而使铜离子将从有机物中剥离下来,然而EDTA将被·OH降解。研究pH值、温度、微电解反应时间、Fe/C质量比对铜离子脱除率及总有机碳(TOC)残余含量的影响,通过扫描电子显微镜分析(SEM)、能谱分析(EDS)、红外光谱分析(FTIR)研究处理前、后样品的表面官能团变化及形貌推断铁碳微电解反应的机理。并进行工业条件优化,得到最佳工艺条件:pH值为2,温度为常温,Fe/C质量比≥0.02,时间为60 min,有氧气存在。在该条件下TOC浓度为200 mg/L、铜离子浓度为60 mg/L的废水反应完成后TOC和Cu残余浓度分别减低到40.66和1.718 mg/L;羟基自由基降解反应机理合理解释了该实验现象。
关键词:微电解;羟基自由基;EDTA;废水
(Edited by LI Xiang-qun)
Foundation item: Project (50925417) supported by China National Funds for Distinguished Young Scientists; Project (50830301) supported by the National Natural Science Foundation of China; Project (2007BAC25B01) supported by the National Key Project of Science and Technical Supporting Programs Funded by Ministry of Science and Technology of China during the 11th Five-year Plan; Project (2009ZX07212-001-01) supported by the Major Science and Technology Program for Water Pollution Control and Treatment, China
Corresponding author: WANG Yun-yan; Tel: +86-731-88830875; E-mail: wyy@csu.edu.cn
DOI: 10.1016/S1003-6326(11)61274-0

