
Leaching process of rare earths from weathered crust elution-deposited rare earth ore
TIAN Jun(田 君)1, 2, CHI Ru-an(池汝安)3, YIN Jing-qun(尹敬群)2
1. School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Institute of Applied Chemistry, Jiangxi Academy of Sciences, Nanchang 330029, China;
3. Key Laboratory for Green Chemical Process of Ministry of Education,
Wuhan Institute of Technology, Wuhan 430073, China
Received 6 July 2009; accepted 16 January 2010
Abstract: In order to strengthen the leaching procedure, the chemical processes of leaching rare earths (RE) from the weathered crust elution-deposited rare earth ore were investigated frow the viewpoints of kinetics, hydrodynamic and mass transfer. The results show that the leaching hydrodynamics follows the Darcy law. The leaching kinetics can be described by the shrinking core model; the leaching process is controlled by diffusion of porous solid layer; and the mass transfer can be described with Van Deemter equation. This provides a theoretic basis and a scientific approach with high efficiency and optimized extraction conditions in industrial practice.
Key words: weathered crust elution-deposited rare earth ore; leaching; hydrodynamics; kinetics; mass transfer
1 Introduction
The weathered crust elution-deposited rare earth ores only exist in China[1], mainly located in Jiangxi, Fujian, Hunan, Guangdong, Yunnan and Guangxi province[2], and are the main resources of mid-heavy rare earth (RE) in the world[3].
For a long time, many efforts have been engaged in the research and development of a series of hydrometallurgical processes for the specially weathered crust elution-deposited rare earth resources[4]. The research has shown that the rare earth in the weathered crust elution-deposited rare earth ore mainly exists with the ion phase adsorbed on clay minerals[5], which can be leached by ion-exchange method based on the adsorbed characteristics of rare earth ions[6].
The leaching effect is not only controlled by the properties of the rare earth ore, but also influenced by the kinetics, hydrodynamics and mass transfer of the leaching operation[2, 7-8]. In order to know the rare earth leaching procedure, choose the more suitable technology and strengthen the leaching procedure, the kinetics, hydrodynamics and mass transfer of leaching rare earths (RE) from the weathered crust elution-deposited rare earth ore were investigated in this work. It would be useful to providing a scientific approach to and a theoretic basis for leaching rare earth from this ore with high performance and low consumption, and can be applied to optimize the rare earth extraction conditions and to improve the rare earth recovery in the extraction process[9-10].
2 Leaching chemistry in weathered crust elution-deposited rare earth ore
RE in the weathered crust elution-deposited rare earth ore mainly exists as the ion-exchangeable phase adsorbed on clay minerals. Because of this property, ion-exchange leaching is the only method to extract RE from this type of ore[11]. The weathered crust elution-deposited rare earth ore mainly contains quartz, potash feldspar, plagioclase, kaolin, white mica, whose chemical composition is SiO2 70%, A12O3 15%, K2O 3%-5%, Fe2O3 2%-3%, CaO 0.2%-0.5%, MgO 0.1%-0.3% and other impurities. The RE grade is very low being only 0.05%-0.3%. The clay minerals can be regarded as nature inorganic ion-exchanger. RE was adsorbed by aluminosilicate mineral which can be described as [Al2Si2O5(OH)4]m?nRE3+ for kaolinite, [Al(OH)6Si2O5(OH)3]m?nRE3+ for halloysite, and [KAl2(AlSi3O10)(OH)2]m?nRE3+ for muscovite.
RE ions would be exchanged when this ore is leached with electrolyte solution, similar to the ion-exchange procedure. When meeting cation, ion-exchange reaction will occur. Therefore, the RE adsorbed can be released from the clays into the slurry in the presence of electrolyte solution. The leaching chemical reaction can be described as[12]:
[Al2Si2O5(OH)4]m?nRE3+(s)+3nNH4+(aq)=
[Al2Si2O5(OH)4]m?(NH4+)3n(s)+nRE3+(aq) (1)
[Al(OH)6Si2O5(OH)3]m?nRE3+(s)+3nNH4+(aq)=
[Al(OH)6Si2O5(OH)3]m?(NH4+)3n(s)+nRE3+(aq) (2)
[KAl2(AlSi3O10)(OH)2]m?nRE3+(s)+3nNH4+(aq)=
[KAl2(AlSiO5O10)(OH)2]m?(NH4+)3n(s)+nRE3+(aq) (3)
3 Leaching hydrodynamics of weathered crust elution-deposited rare earth ore
Weathered crust elution-deposited rare earth ore is a kind of unconsolidated granular-bed. The RE leaching process is affected not only by ore properties and concentration of leaching agent, but also by hydrodynamics, kinetics and mass transfer of the leaching operation. The leaching hydrodynamics of a certain heavy weathered crust ore and a kind of middle heavy weathered crust ore were studied systematically[8]. According to the Darcy law, the permeability σ is
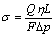 (4)
(4)
where Q, η, L, F, Δp are the velocity of leachate liquid flowing, the kinematic viscosity of leaching agent solution, the packing ore height, the cross-sectional area, and the pressure difference, respectively.
The permeability with the packed porosity (φ) for various particle size of the weathered crust elution-deposited rare earth ore is shown in Fig.1.
As shown in Fig.1, the permeability is not only related to ore granule size, but also to the packed porosity. The smaller the ore granule size is, the less the permeability will be because the fluid channels become narrower and more flexuous with the smaller particle size of the ore.
The permeability of the weathered crust elution-deposited rare earth ore is affected by the concentration of leaching reagent due to the solution viscosity. The velocities of leachate liquid flowing (Q) changed with the concentration of leaching reagent under various pressure differences (Δp) are calculated in Fig.2.
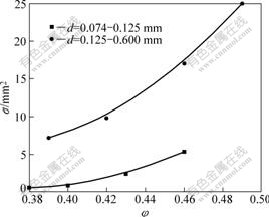
Fig.1 Relationship between k and φ
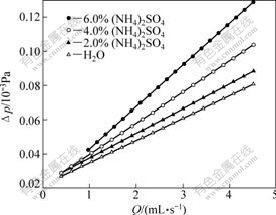
Fig.2 Relationship between Q and Δp under different concentrations of reagent
As shown in Fig.2, the relationship between Q and Δp follows the Darcy law. The higher the concentration of leaching reagent, the less the leachate liquid flowing velocity at a certain pressure difference, because the higher the concentration of the leaching reagent, the higher the fluid viscosity (η(6%(NH4)2SO4)=0.939 5 Pa?s, η(4%(NH4)2SO4)=0.917 7 Pa?s, η(2%(NH4)2SO4)=0.874 9 Pa?s, η(H2O)=0.851 3 Pa?s).
4 Kinetics of leaching RE from weathered crust elution-deposited rare earth ore
The study on leaching kinetics of this ore aids to strengthen the leaching procedure and improve the leaching effect. The RE leaching from the weathered crust elution-deposited rare earth ore is a typical liquid-solid leaching process, which can be described by the shrinking-core model, as shown in Fig.3, when the ore particle is regard as spherical type[13].
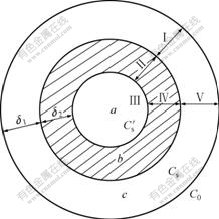
Fig.3 Illustrative diagram of leaching process (a Non-leached particle of rare earth ore; b Remainder of solid; c Diffusion region of solvent; C0 Concentration of leaching reagent in fluid phase; Cs Concentration of leaching reagent on surface of particle; 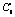 Concentration of leaching reagent in region of reaction; δ1 Effective thickness of diffusion region; and δ2 Thickness of solid remainder)
Concentration of leaching reagent in region of reaction; δ1 Effective thickness of diffusion region; and δ2 Thickness of solid remainder)
The leaching kinetics can be subdivided into outer/inner diffusion and chemical control model. When more than one step limits the leaching kinetics, this process is considered to be mixed controlled[14].
Attempts were made to fit all experimental data with different kinetic models and various rate-controlling mechanism. The kinetics equation is obtained by try and error method[15]. A series of straight line can be obtained with plotting 1-(2/3)α-(1-α)2/3 vs t shown in Fig.4 at different leaching temperatures for the weathered crust elution-deposited rare earth ore. It follows the inner diffusion control model[16].
Therefore, the kinetics equation can be expressed as
1-(2/3)α-(1-α)2/3=kt (5)
where k is the linear rate constant; and α is the rare earth leached fraction.
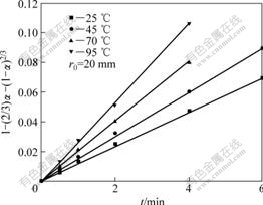
Fig.4 Plots of 1-(2/3)α-(1-α)2/3 vs time for weathered crust elation-deposited rare earth ore at different temperatures
A series of straight line can also be obtained with plotting 1-(2/3)α-(1-α)2/3 vs t shown in Fig.5 under different particle sizes of the ore. This further proves that the leaching process is controlled by inner diffusion.
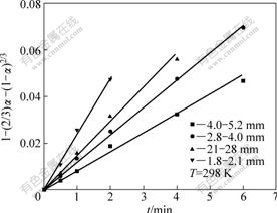
Fig.5 Plots of 1-(2/3)α-(1-α)2/3 vs time for weathered crust elution-deposited rare earth ore with different particle sizes
According to Arrhenius equation, it is represented as
 (6)
(6)
where k is the rate constant; A′ is the pre-exponential factor; n is the leaching reaction grade number; r0 is the initial radius of the ore sample particle; n is the order of the ore particle size; R is the universal gas constant; T is leaching reaction temperature; and E is apparent activation energy.
The k values of leaching process at different temperatures were calculated from these slopes of the straight lines given in Fig.4. The E value of leaching process can be obtained from the slope of straight line in the Arrhenius diagram (plots of lnk vs 1/T), which is 9.24 kJ/mol. This also proves that leaching process is controlled by inner diffusion for its activation energy is between 4 kJ/mol and 12 kJ/mol[17]. n and A′ can be calculated from the straight line slope for relationship between lnk and lnr0 with different particle sizes, which are -1.29 and 1.50, respectively. Then, the kinetics equilibrium equation can be obtained as
 (7)
(7)
5 Mass transfer in leaching of weathered crust elution-deposited rare earth ore
The mass transfer in leaching of the weathered crust elution-deposited rare earth ore had been studied with chromatographic plate theory[2]. The effects of the flowrate (U) on the height equivalent to a theoretical plate (HETP) were investigated for various ores packed column height (L). The results are shown in Table 1.
As shown in Table 1, the higher the HETP, the stronger the mass transfer diffusion and the worse the leaching efficiency.
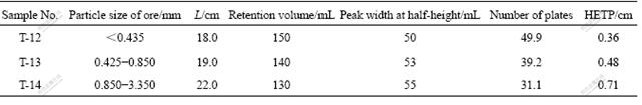
The effects of the particle size of ore on the height equivalent to a theoretical plate (HETP) were calculated with experiment data under different particle sizes of ore. The results are listed in Table 2.
According to Van Deemter equation[18], there is
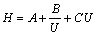 (8)
(8)
where H is the height equivalent to theoretic plate; and A, B, C are the parameters related to chromatographic efficiency. A is the radial diffusion and vortex diffusion coefficient, which represents the peak width changing with the uniformity of ore packing and reflects the intensity of channel flowing phenomenon in leaching process. B is the lengthways coefficient. The smaller the leaching flowrate, the higher the B/U value or the stronger the lengthways diffusion. C is the mass transfer impedance coefficient, which is caused by flowing impedance for the leaching reagent and product distributing between liquid and solid phases and is affected by the leaching chromatographic efficiency. Therefore, the quicker the leaching flowrate, the larger the mass transfer impulse.
As can be noted from Table 2, the HETP increases with increasing the particle size of ore, because in Van Deemter equation the larger the dp or the A (=2λdp), the higher the HETP.
The mass transfer in leaching of the weathered crust elution-deposited rare earth ores is described with Van Deemter equation. The results are shown in Fig.6.
As seen from Fig.6, the HETP decreases with increasing the flowrate at initial step, but when the flow-
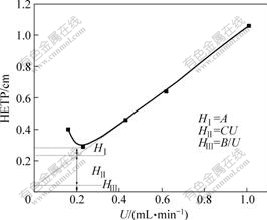
Fig.6 Plot of HETP vs various U for weathered crust elution-deposited rare earth ores
rate surpasses a certain value, it begins to increase in latter step.
The results show that for the same ore and the same leaching reagent, there is an optimum flowrate in the leaching process. In industrial practice, when the flowrate is too rapid and the ore particle size is too large, the “channel flowing” phenomenon will happen. When there are fine particles of ore and huge content of clay in ore, the ore becomes pasty in pool. The flowrate becomes too slow, and then the leaching reagent and leaching product diffuse difficultly because a layer of fine slurry formed on surface of the ore[9]. The leaching chromatographic efficiency and the RE recovery are worse relatively.
According to properties of the ore, the leaching efficiency can be improved with the optimum flowrate and ore particle size. Besides, not only is RE concentration of the leachate increased with less leaching reagent and leachate, but also the impurities would not be leached during the RE leaching[19].
Table 1 HETP under various flowrates for weathered crust elution-deposited rare earth ore
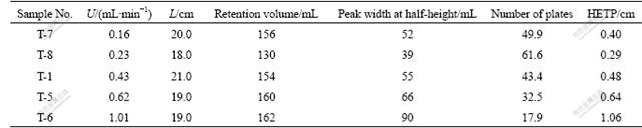
Table 2 HETP under different particle sizes for weathered crust elution-deposited rare earth ore
6 Conclusions
1) The leaching hydrodynamics on the weathered crust elution-deposited rare earth ore shows that the relationship between leachate liquid flowing velocity and pressure difference follows the Darcy law. The higher the concentration of leaching reagent is, the less the permeability is.
2) The leaching kinetics on the weathered crust elution-deposited rare earth ore can be described by the shrinking core model. The leaching kinetics is controlled by diffusion of porous solid layer. The apparent activation energy is 9.24 kJ/mol, and an empirical equation of the leaching kinetics is established as
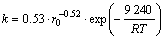 .
.
3) The mass transfer in leaching of the weathered crust elution-deposited rare earth ore could be described with chromatographic plate theory. The effects of the flowrate on the height equivalent to a theoretical plate have been analysed with Van Deemter equation.
4) The kinetics, hydrodynamic and mass transfer in leaching RE from the weathered crust elution-deposited rare earth ore provide a theoretic basis and a scientific approach with high efficiency and low consumption for this ore, which can be applied to optimize the RE extraction conditions, improve the RE recovery and inhibit impurities leaching in extraction process.
References
[1] CHI Ru-an, TIAN Jun. Weathered crust elution-deposited rare earth ores [M]. New York: Nova Science Publishers, 2008: 1-29.
[2] TIAN Jun, YIN Jing-qun. Study on the mass transfer in leaching of the south china rare earth ore [J]. Engineering Chemistry & Metallurgy, 1996 17(3): 264-268. (in Chinese)
[3] CHI Ru-an, TIAN Jun. Review of weathered crust rare earth ore [J]. Journal of the Chinese Rare Earth Society, 2007, 25(6): 641-650. (in Chinese)
[4] TIAN Jun, YIN Jing-qun, OUYANG Ke-xian, CHI Ru-an. Development progress and research connotation of green chemistry of extraction process of rare earth from weathering crust elution-deposited rare earth ores in China [J]. Chinese Rare Earths, 2006, 27(1): 70-2, 102. (in Chinese)
[5] CHI Ru-an, TIAN Jun, LI Zhong-jun, PENG Cui, WU Yuan-xin, LI Shi-rong, WANG Cun-wen, ZHOU Zhi-ang. Existing state and partitioning of rare earth on weathered ores [J]. Journal of Rare Earths, 2005, 23(6): 756-759.
[6] HE Lun-yan, FENG Tian-ze, FU Shi-yi. Study on process of extraction of rare earths from the ion-adsorption type rare earth ore ore by (NH4)2SO4 leaching [J]. Chinese Rare Earths, 1983, 4(3): 1-5. (in Chinese)
[7] TIAN Jun, LU Sheng-liang, YIN Jing-qun. Kinetic study on leaching a south china rare earth ore [J]. Engineering chemistry & Metallurgy, 1995, 16(3): 354-358. (in Chinese)
[8] TIAN Jun, CHI Ru-an, ZHU Guo-cai, XU Sheng-ming, QIU Xin, ZHANG Zhi-geng. Leaching hydrodynamics of weathered elution-deposited rare earth ore [J]. Transactions of Nonferrous Metals Society of China, 2001, 11(3): 434-437.
[9] TIAN Jun, YIN Jing-qun. Study on kinetic and the masstransfer in leaching of the south China rare earth ore [J]. Chinese Journal of Rare Metals, 1996, 20(5): 330-342. (in Chinese)
[10] HUANG Xiao-wei, LONG Zhi-qi, LI Hong-wei, YING Wei-juan, ZHANG Guo-cheng, XUE Xiang-xin. Development of rare earth hydrometallurgy technology in China [J]. Journal of Rare Earths, 2005, 23(1): 1-5.
[11] CHI Ru-an, TIAN Jun, GAO Hong, ZHOU Fang, LIU Min, WANG Cun-wen, WU Yuan-xin. Kinetics of leaching flavonoids from pueraria lobata with ethanol [J]. Chinese J Chem Eng, 2006, 14(3): 402-406.
[12] CHI Ru-an, TIAN Jun. Hydrometallurgy of the weathered crust elution-deposited rare earth ores in China [M]. Beijing: Science Press, 2006: 115-122. (in Chinese)
[13] SOHN H Y, WADSWORTH M E. Rate process of extraction metallurgy [M]. New York: Plenum press, 1979.
[14] NUNEZ C, CRUELLS M, GARCIA-SOTO L. A general shrinking-particle model for the chemical dissolution of all types of cylinders and discs [J]. Hydrometallurgy, 1994, 36(3): 285-294.
[15] CHI Ru-an, ZHU Guo-cai, XU Sheng-ming, TIAN Jun, LIU Jun, XU Zheng-he. Kinetics of manganese reduction leaching from weathered rare-earth mud with sodium sulfite [J]. Metall Mater Trans B, 2002, 33(1): 41-46.
[16] CHI Ru-an, ZHU Guo-cai, TIAN Jun. Leaching kinetics of rare earth from black weathering mud with hydrochloric acid [J]. Transactions of Nonferrous Metals Society of China, 2000, 10(4): 531-533.
[17] CHI Ru-an, TIAN Jun, ZHU Guo-cai, WU Yuan-xin, LI Shi-rong, WANG Cun-wen, ZHOU Zhi-ang. Kinetics of rare earth leaching from a mangnese-removed weathered rare-earth mud in hydrochloric acid solutions [J]. Separation Science and Technology, 2006, 41(6): 1099-1113.
[18] DEVAULT D. Theory of chematography [J]. J Am Chem Soc, 1943, 65: 532-540
[19] MOHAMMAD A, ZAFARAND I Z, A.TARIQ M. Selective leaching kinetics and upgrading of low-grade calcareous phosphate rock in succinic acid [J]. Hydrometallurgy, 2005, 80(4): 286-292.
(Edited by YANG Bing)
Foundation item: Projects(50664004, 50474022, 50574069) supported by the National Natural Science Foundation of China; Projects(Q959612, Q972026) supported by the Natural Science Foundation of Jiangxi Province, China
Corresponding author: TIAN Jun; Tel: +86-791-8177783; E-mail: tianjun63@126.com
DOI: 10.1016/S1003-6326(09)60232-6