应用基于人工神经网络建立的新型物理图形预测Al-Zn-Mg-Cu合金固溶过程的组织演变
来源期刊:中国有色金属学报(英文版)2015年第3期
论文作者:刘蛟蛟 李红英 李德望 武 岳
文章页码:944 - 953
关键词:铝合金;固溶处理;电阻率;人工神经网络;显微组织演变
Key words:aluminum alloy; solution treatment; electrical resistivity; artificial neural network; microstructure evolution
摘 要:采用原位电阻测试法、金相显微镜观察、扫描电镜观察、透射电镜观察和拉伸测试技术研究固溶条件对Al-Zn-Mg-Cu合金显微组织和拉伸性能的影响。基于实验数据建立人工神经网络模型,将该模型用于预测实验合金在固溶过程中的电阻率变化。结果表明,所建立的模型能很好地预测合金在固溶过程中的电阻率变化。预测结果与实验值的相关系数为0.9958,相对误差为0.33%。采用预测数据可以建立一种新型的“固溶-电阻率”物理图形。该图形显示,实验合金的最佳固溶温度区间为465~475 °C,保温时间为50~60 min;在该区间内第二相的溶解与再结晶对合金性能的影响将达到平衡。
Abstract: The effects of the solid solution conditions on the microstructure and tensile properties of Al-Zn-Mg-Cu aluminum alloy were investigated by in-situ resistivity measurement, optical microscopy (OM), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and tensile test. A radial basis function artificial neural network (RBF-ANN) model was developed for the analysis and prediction of the electrical resistivity of the tested alloy during the solid solution process. The results show that the model is capable of predicting the electrical resistivity with remarkable success. The correlation coefficient between the predicted results and experimental data is 0.9958 and the relative error is 0.33%. The predicted data were adopted to construct a novel physical picture which was defined as “solution resistivity map”. As revealed by the map, the optimum domain for the solid solution of the tested alloy is in the temperature range of 465-475 °C and solution time range of 50-60 min. In this domain, the solution of second particles and the recrystallization phenomenon will reach equilibrium.
Trans. Nonferrous Met. Soc. China 25(2015) 944-953
Al-Zn-Mg-Cu alloy during solid solution process
Jiao-jiao LIU1,2, Hong-ying LI1,2, De-wang LI1,2, Yue WU3
1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Nonferrous Metal Materials Science and Engineering, Ministry of Education, Central South University, Changsha 410083, China;
3. Beijing Institute of Aeronautical Materials, Aviation Industry Corporation of China, Beijing 100095, China
Received 10 April 2014; accepted 28 December 2014
Abstract: The effects of the solid solution conditions on the microstructure and tensile properties of Al-Zn-Mg-Cu aluminum alloy were investigated by in-situ resistivity measurement, optical microscopy (OM), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and tensile test. A radial basis function artificial neural network (RBF-ANN) model was developed for the analysis and prediction of the electrical resistivity of the tested alloy during the solid solution process. The results show that the model is capable of predicting the electrical resistivity with remarkable success. The correlation coefficient between the predicted results and experimental data is 0.9958 and the relative error is 0.33%. The predicted data were adopted to construct a novel physical picture which was defined as “solution resistivity map”. As revealed by the map, the optimum domain for the solid solution of the tested alloy is in the temperature range of 465-475 °C and solution time range of 50-60 min. In this domain, the solution of second particles and the recrystallization phenomenon will reach equilibrium.
Key words: aluminum alloy; solution treatment; electrical resistivity; artificial neural network; microstructure evolution
1 Introduction
The AA7050 aluminum alloy belongs to 7xxx series aluminum alloys, which has high specific strength, high fracture toughness, good resistance to exfoliation corrosion and stress corrosion cracking. In order to obtain improved mechanical properties, aluminum alloys are often subjected to different heat treatments. Generally, the solution treatment is a primary and key step [1,2]. Coarse intermetallic particles (larger than 1 μm) are generally detrimental to the properties, especially to the toughness, and in 7xxx alloys these particles are especially the Fe-rich and S phases [3,4]. During the solution treatment, the soluble phase formed during solidification can be redissolved into the matrix. At a higher solution temperature, the soluble phase can redissolve more sufficiently. However, the higher temperature of the solution treatment inevitably increases the percentage of the recrystallized grains. Available literatures indicate that recrystallization reduces toughness and increases quench sensitivity of the alloys [5,6]. Consequently, detailed investigation on the microstructure of 7xxx alloys with an aim to optimize the properties is of key interest to materials researchers.
The resistivity evolution of aluminum alloys has proved to be a useful tool to characterize the precipitation processes during the heat treatment [7,8]. Numerous works have been reported to reveal the correlation between electrical resistivity and microstructure features of aluminum alloys.  and ROMHANJI [9] characterized the microstructural changes in Al-6.8%Mg alloy (mass fraction) by using electrical resistivity measurements. STARINK and LI [10] developed an electrical resistivity model for peak-aged and overaged Al-Zn-Mg-Cu alloys. RAEISINIA et al [11] used electrical resistivity measurements to examine the precipitation reactions in the AA6111 aluminum alloy. Furthermore, EIVANI et al [12] studied the correlation among electrical resistivity, particle dissolution, precipitation of dispersoids, and recrystallization behavior of AA7020 aluminum alloy. They reported that the electrical resistivity measurement was a reliable technique to examine the microstructure evolution during solid solution. Generally, the electrical resistivity for a 7xxx aluminum alloy during the solid solution procedure can encompass a range of complex non-linear and interactive effects. The artificial neural networks technology can provide a novel approach to materials modeling, especially for complex and non-linear relationships [13]. The model has proved to be capable of learning from a data set to describe the non-linear and interactive effects with remarkable success, even if the form of non-linear relationship is unknown and some of the experimental data points are faulty [14]. These advantages make the artificial neural network technique a robust technique for obtaining the functional relationship in many engineering problems [15].
and ROMHANJI [9] characterized the microstructural changes in Al-6.8%Mg alloy (mass fraction) by using electrical resistivity measurements. STARINK and LI [10] developed an electrical resistivity model for peak-aged and overaged Al-Zn-Mg-Cu alloys. RAEISINIA et al [11] used electrical resistivity measurements to examine the precipitation reactions in the AA6111 aluminum alloy. Furthermore, EIVANI et al [12] studied the correlation among electrical resistivity, particle dissolution, precipitation of dispersoids, and recrystallization behavior of AA7020 aluminum alloy. They reported that the electrical resistivity measurement was a reliable technique to examine the microstructure evolution during solid solution. Generally, the electrical resistivity for a 7xxx aluminum alloy during the solid solution procedure can encompass a range of complex non-linear and interactive effects. The artificial neural networks technology can provide a novel approach to materials modeling, especially for complex and non-linear relationships [13]. The model has proved to be capable of learning from a data set to describe the non-linear and interactive effects with remarkable success, even if the form of non-linear relationship is unknown and some of the experimental data points are faulty [14]. These advantages make the artificial neural network technique a robust technique for obtaining the functional relationship in many engineering problems [15].
The objective of the present work is to characterize microstructural evolution of a commercial AA7050 aluminum alloy during solid solution treatment and to establish a convenient model which can be devoted to optimizing the solution parameters. By adopting the artificial neural networks, a new physical picture is plotted which can be used to optimize the solution parameters.
2 Experimental
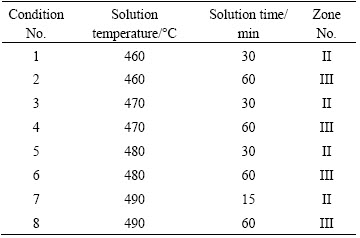
The investigations were carried out on an AA7050 aluminum alloy with composition of 6.1% Zn, 2.15% Mg, 2.37% Cu, 0.04% Zr, 0.06% Ti, 0.09% Mn, 0.12% Si, and 0.15% Fe (mass fraction). Electric testing samples were taken from quarter-plane along the rolling direction and machined as strips. In-situ electrical resistivity measurements were acquired by a custom four-point probe technique. The electrical measurements were performed in a real-time controlled thermal set-up developed in the laboratory and the electrical circuit was powered by the DC power. The data acquisition was performed by a personal computer. The solution treatment procedures are listed in Table 1.
The grain structure was observed with a Leica DMI 3000 optical microscope (OM) under polarized light. The morphology of the residual phase was examined on Sirion 200 scanning electron microscope (SEM). The second phase particles were identified by energy dispersive X-ray spectrometry (EDX). Thin foils for TEM were prepared from 3 mm discs by using twin-jet electropolishing in a 25% HNO3+75% CH3OH solution at -30 °C. TEM observations were performed with an FEI Tecnai G220 microscope, operating at 200 kV. The tensile samples were machined along the transverse (T) orientation and solution-treated at temperatures of 460, 470, 480 and 490 °C for 60 min. Subsequently, they were quenched to room temperature. After the quenching, all samples were kept at room temperature for 24 h and then aged at 120 °C for 24 h. Tensile tests were carried out on an MTS810 material testing system to evaluate the mechanical properties of the samples.
Table 1 Solution treatment schedules of AA7050 aluminum alloy rolled plate
The radial basis function artificial neural network (RBF-ANN) model was established with two inputs (solution temperature and solution time) and one output (electrical resistivity) for the prediction of resistivity for the alloy. Before the training of the neural network, both input and output variables were normalized within the range from 0.1 to 0.9 in order to obtain a usable form for the network to read. The following equation was used widely for unification:
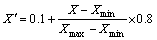 (1)
(1)
where X′ is the normalized result; Xmax is the maximum value of the variable; Xmin is the minimum value of the variable.
To verify the derived constitutive model, comparisons between the experimental and predicted results were carried out. The accuracy of the RBF-ANN model was evaluated by using correlation coefficient (R) and average absolute relative error (AARE). They were expressed as follows:
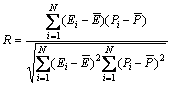 (2)
(2)
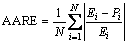 (3)
(3)
where Ei is the experimental data and Pi is the predicted value calculated from constitutive equations; 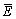 and
and 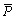 are the mean values of E and P, respectively; N is the number of data employed in the investigation. The correlation coefficient is a commonly used statistical parameter and provides information about the performance of linear relationship between the observed and calculated values. However, a higher value of R may not always indicate a better performance [13]. This is because the tendency of the calculated results can be biased toward higher or lower values. The AARE is computed through a one-by-one comparison of the relative error and therefore is an unbiased statistical parameter for measuring the predictability of a model [14].
are the mean values of E and P, respectively; N is the number of data employed in the investigation. The correlation coefficient is a commonly used statistical parameter and provides information about the performance of linear relationship between the observed and calculated values. However, a higher value of R may not always indicate a better performance [13]. This is because the tendency of the calculated results can be biased toward higher or lower values. The AARE is computed through a one-by-one comparison of the relative error and therefore is an unbiased statistical parameter for measuring the predictability of a model [14].
3 Results and discussion
3.1 Resistivity-time curves
Figure 1 shows the typical electrical resistivity-time curves of the AA7050 aluminum alloy during different solution treatments. It can be seen that the resistivity increases rapidly with the extension of the solution time at the beginning of the solution treatment, and then it increases gently, and finally keeps unchanged or decreases to some extent. During the solution treatment, the microstructure evolution of the alloy may include recrystallization, dissolution of second phases and over-burning. Different evolutions have different effects on the electrical resistivity [12]. At the beginning, fine secondary phase particles are dissolved into the matrix dramatically, which results in the rapid increase of resistivity. With further treatment, the recrystallization occurs, which leads to a gentle increase of the resistivity. Furthermore, as exhibited in the curve of 480 °C, the recrystallization plays a main role at solution time of 45 min which leads to a slight decrease in the resistivity of the alloy. However, the resistivity of samples approaches a constant at 490 °C, which reveals that the alloy has over burnt at this temperature.
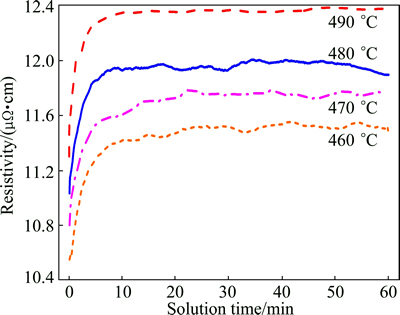
Fig. 1 Electrical resistivity-time curves for different solution treatments of AA7050 aluminum alloy
3.2 Neural networks modeling and solution resistivity map
Figure 2(a) shows the predicted and experimental electrical resistivity curves of the alloy at solution temperatures of 460, 470, 480 and 490 °C, respectively. As shown in Fig. 2(a), there is a satisfactory agreement between the experimental and the predicted values. Figure 2(b) shows the linear relationship between the experimental value and the predicted value, and the R value is also given in Fig. 2(b). A good correlation, with the R value of 0.9958, between experimental and predicted values was obtained. And the average absolute relative error (AARE) was 0.33%, which was calculated by Eq. (3). The results indicate the excellent predictability of the developed RBF-ANN model.
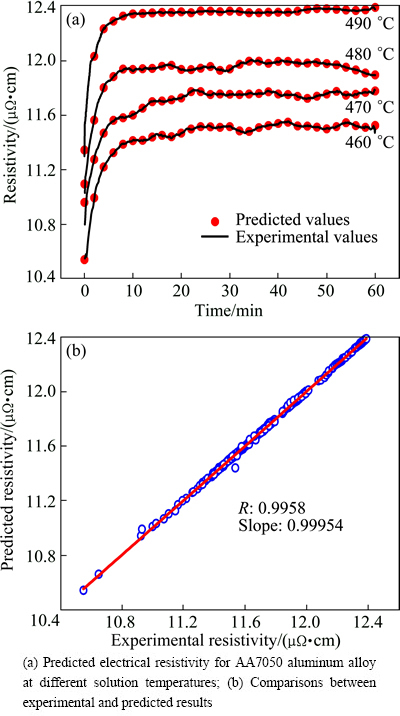
Fig. 2 Verification for predicted results of RBF-ANN model
Based on the excellent predictability of the developed RBF-ANN model, the electrical resistivity of AA7050 aluminum alloy at finer intervals of temperature and time was predicted in the tested temperature range and time range. In order to compare the electrical resistivity caused by the microstructure evolution at different temperatures, the electrical resistivity, ρi(T), which was contributed by the change of temperature should be removed. The ρi(T) was measured at a rapid heating rate, which would inhibit second phases dissolved into the Al matrix and the time for recovery/ recrastallization phenomenon was limited. As can be seen from Fig. 3, the result of ρi(T) has shown a satisfactory agreement with the matthiesen’s rule.
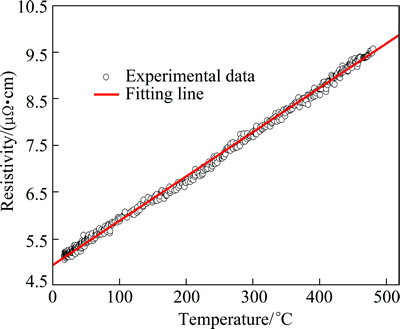
Fig. 3 Electrical resistivity of AA7050 alloy during rapid heating process
The predicted values would subtract the values of ρi(T) at the corresponding temperatures. And then, the result can be plotted as a contour map, which is shown in Fig. 4. Figure 4 is named as “solution resistivity map” and it can be used to discuss the variation of the resistivity versus solution temperature and time. For the analysis of the electrical resistivity variation, the entire map could be broadly divided into three zones.
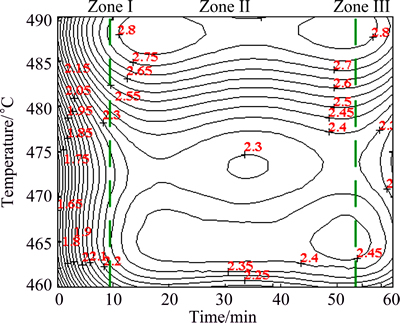
Fig. 4 Solution electrical resistivity map for AA7050 aluminum alloy
Zone I. This zone occurs in first few minutes of the solution treatment. In this domain, the resistivity increases dramatically. The dissolution of the fine AlZnMgCu phase, which would increase the scattering probability of the conduction electron, is the major source of the increase of the solution resistivity in this regime [16].
Zone II. This domain occurs in the solution time range of 15-50 min, where the solution time has slight effect on the solution resistivity. This is because the influences of the recrystallization and the dissolution of second phases on the resistivity tend to balance. However, in Zone II, significant differences of the solution resistivity features have been observed in different temperature ranges. At 460-465 °C, the resistivity increases rapidly with the increase of temperature because the increasing temperature can accelerate the fine secondary phase particles dissolved into the matrix. And then, the resistivity changes gently in the temperature range of 465-475 °C as a result of the recrystallization of the alloy. At higher temperatures (475-485 °C), the residual particles which prefer dissolving at relatively high temperatures (such as S phase (Al2CuMg)) will lead to the increase of resistivity. When the temperature is higher than 485 °C, the domain exhibits a solution electrical resistivity peak with maximum value of 2.8 μΩ·cm, which may be corresponding to the over burning of the alloy.
Zone III. This zone occurs at the last few minutes of the solution treatment. In this region, as the solution time is extended, due to the recrystallization of the alloy, the solution resistivity of the alloy decreases more or less at different temperatures. However, the decrease of electrical resistivity becomes more intense in the temperature range of 475-485 °C and this range can be defined as the drastic recrystallization domain. Thus, the solution parameters should be avoided to choose from these temperatures.
By synthesizing the analysis of the electrical resistivity evolution in these three zones, the optimum domain for the solid solution treatment of AA7050 aluminum alloy is found to be in the temperature range of 465-475 °C and the solution time range of 50-60 min. The over-burnt phenomenon occurs at the temperature higher than 485 °C, and at these temperatures the alloy could be over-burnt in a few minutes.
3.3 Microstructure validation
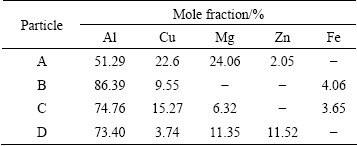
Figure 5 shows the original microstructures of the AA7050 aluminum alloy rolled plate. It can be observed that a number of coarse second phase particles distribute along the rolling direction. Except for the spherical particles, other particles appear as irregularly shaped blocks. The EDX analysis reveals that these second particles are AlZnMgCu, AlCuMg, and Fe-rich phases (Table 2). Large amounts of fine white phases are distributed in the matrix (Fig. 5(b)). According to the EDX analysis, they are also AlZnMgCu phase. Most second phase particles are expected to be dissolved into the matrix by the solution treatment [17].
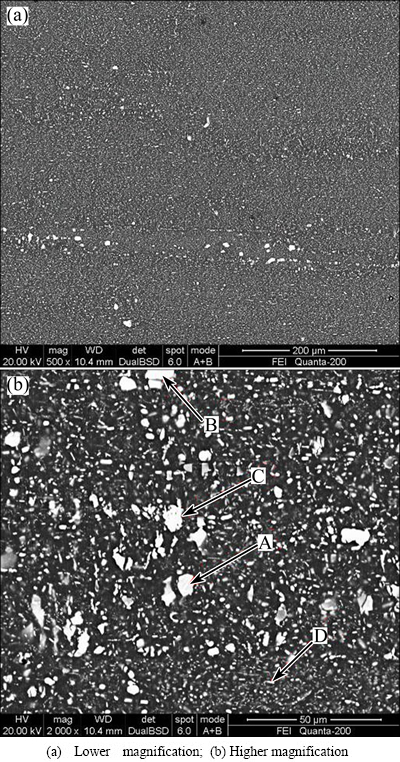
Fig. 5 Morphologies of second phase particles of AA7050 aluminum alloy rolled plate
Table 2 EDX results of second phase particles of AA7050 aluminum alloy rolled plate in Fig. 5(b)
In order to verify the accuracy of the map, some typical solution conditions (listed in Table 1) were chosen to conduct the microstructure observations by OM, SEM and TEM. The evolution of the resistivity is relatively simple in Zone I and the solution treatment is not possible to be finished in such a few minutes. Therefore, the microstructure of the specimens in this zone would not be discussed in the present work and microstructural analysis was carried out for the specimens solution-treated in Zones II and III.
Figure 6 shows the typical microstructures in Zone II including OM and SEM images. Figures 6(a), (c), (e) and (g) show optical micrographs of the specimens (Conditions 1, 3, 5 and 7), respectively. Fibrous structures are observed in Fig. 6(a), and no clear recrystallization has been observed in Condition 1. However, as shown in Figs. 6(c), (e) and (g), fine equiaxed grains are observed, which reveals that recrystallization of the alloy has occurred under these conditions. The typical SEM images in Zone II of the AA7050 aluminum alloy are shown in Figs. 6(b), (d), (f) and (h). Compared with the AA7050 aluminum alloy rolled plate, the fine secondary phase particles are dissolved into the matrix of the solution-treated samples. And the volume fraction of the coarse particles decreases with the increase of the solution temperature. However, in Condition 7 (490 °C for 15 min), some voids are observed, which is due to the melting of S phase at around 490 °C for the alloy. This implies that the alloy has been over-burnt in a few minutes at the solution temperature of 490 °C.
The representative microstructures in Zone III (Conditions 2, 4, 6 and 8) of the solution-treated specimens are shown in Fig. 7. Partial recrystallized microstructures are observed in Conditions 2 and 4. Some fine equiaxed grains can be observed between the elongated grains, as shown in Fig. 7(a). As exhibited in Fig. 7(c), the volume fraction of equiaxed grains significantly increases compared with those in Condition 2 and small sub-grains with a size of 5-10 μm are also observed in fibrous structures. As can be seen in Figs. 7(e) and (g), the recrystalization is almost finished in Conditions 6 and 8 and the grain size of the alloy increases with the increase of solution temperature. Backscattered images under these conditions are shown in Figs. 7(b), (d), (f) and (h), respectively. After solution treated at 460 °C for 60 min, the detected particles are mostly Cu-rich phase (diameter ~3 μm) and Fe-rich particles (typically ~15 μm). The EDX results are listed in Table 3. It can be seen that the chemical components of these particles are similar to particles A and B observed in the cold-rolled samples. However, the mole fractions of Mg and Zn elements in Cu-rich phase decrease and the mole fraction of Fe element in Fe-rich phase increases. In the Cu-rich phase, the mole ratio of Cu to Mg is close to 1:1. Moreover, except for Al matrix, only a small amount of Zn element exists in the phase. Therefore, this intermetallic phase is considered to be S (Al2CuMg) phase [18-20]. In the temperature range of 460-480 °C, S phase is still observed and the complete dissolution of this phase is not possible at these solution temperatures [3,21]. During solution treatment, S (Al2CuMg) phase gradually disappears and finally dissolves into matrix completely at 490 °C (Fig. 6(h)). Only an irregular phase exists in Conditions 8. From the EDX analysis of this irregular phase, the mole ratio of Cu to Fe is 2:1. Moreover, there is no other element in this phase except for Al matrix. Therefore, this intermetallic phase is considered to be Al7Cu2Fe phase [18-20]. Al7Cu2Fe phase exhibits no change with the increase of solution temperature and time. Because Al7Cu2Fe phase is an indissolvable impurity intermetallic phase, it can hardly be eliminated by solution treatment.

Fig. 6 Typical microstructures of Zone II in Fig. 4
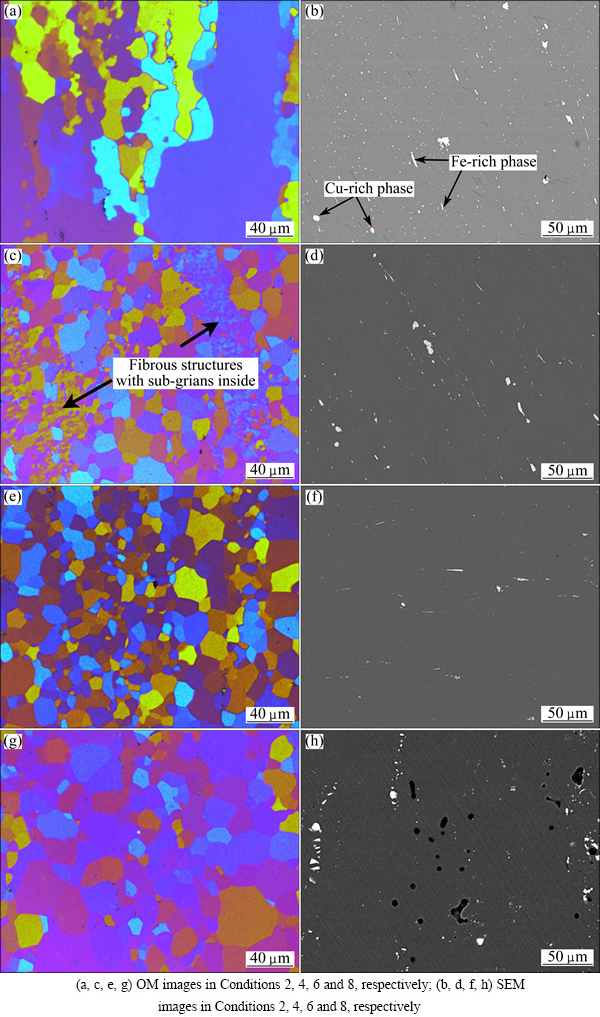
Fig. 7 Typical microstructures of zone III in Fig. 4
The sub-grain micrographs of the AA7050 aluminum alloy solution-treated under different conditions are shown in Fig. 8. For the sample treated at 460 °C, the sub-grains can be distinguished even though a large number of dislocations surround them. And the size of the sub-grains is 5-10 μm for the sample solution-treated at 470 °C. These sub-grains are located in large elongated grains, which are similar to the results observed in OM images. Al3Zr dispersoids are observed at the sub-grain boundaries, which could fix the grain boudnaries and prevent the growth of grains effectively (marked in Fig. 8(b)). The sizes of the subgrains increase remarkably with the solution temperature increasing to 480 °C (Fig. 8(c)). For the solution treatment at 490 °C, remarkable recrystallization occurs and the triple grain boundary is easily observable under the TEM (Fig. 8(d)).
Table 3 EDX results of residual particles in AA7050 alloy

3.4 Tensile properties
Figure 9 shows the tensile properties and elogation to failure of the aged AA7050 aluminum alloy solution- treated under different conditions. For the solution- treated samples, the strength firstly increases and then decreases with increasing solution temperature. The ultimate tensile strength (Rm) and yield strength (Rp0.2) reach their peaks at the solution temperature of 470 °C, which are 580 MPa and 510 MPa, respectively. While the maximum elogation to failure of 17.7% is obtained at the solution temperature of 460 °C.
It is obvious that the strengthening mechanisms for the AA7050 aluminum alloy are mainly precipitation strengthening and fine grain strengthening when the alloy is solution treated under different conditions and then aging-treated under the same condition. The precipitation strengthening is ascribed to the dissolving of the second phase particles during the solution treatment, which offer great amounts of solution atoms and are beneficial for the precipitation in the subsequent aging treatment [22,23]. With increasing the solution temperature, more and more residual phases are dissolved into the matrix, providing more and more solution atoms, which results in a higher strength of the AA7050 aluminum alloy. But the volume fraction of the recrystallized grains and the size of the sub-grains increase significantly with increasing the solution temperature, leading to a larger average grain size and a lower fine grain strengthening. As a result, a proper solution treatment can achieve improved tensile strength by coordinating the relationship between precipitation strengthening and fine grain strengthening. And the optimized solution parameters of AA7050 alluminum alloy can be predicted by the “solution resistivity” map as shown in Fig. 4.
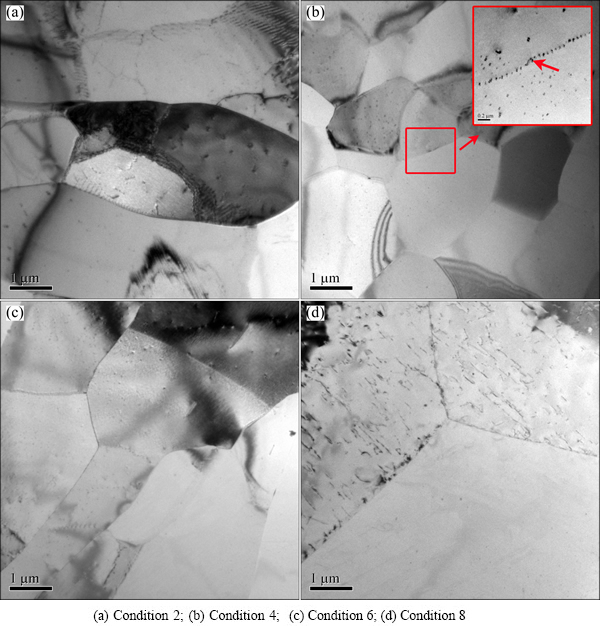
Fig. 8 TEM micrographs of AA7050 aluminum alloy solution-treated under different conditions
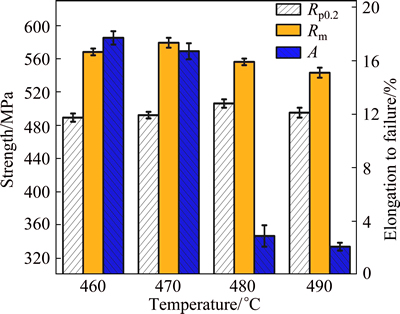
Fig. 9 Tensile properties and elongation to failure of aged AA7050 aluminum alloy solution-treated under different conditions
4 Conclusions
1) A RBF-ANN model was constructed to predict the electrical resistivity of the AA7050 aluminum alloy in the temperature range of 460-490 °C and the solution time range of 0-60 min. The predicted result shows a good agreement with the experimental result, where the correlation coefficient is found to be 0.9958 and the relative error is 0.33%.
2) The predicted data in the experimental range were used to plot a “solution resistivity map” for the AA7050 aluminum alloy, which has a potential to predict the microstructure evolution of the alloy during different solid solution processes. The map exhibits that the suitable solution temperature range is 465-475 °C and the solution time should be controlled in the range of 50-60 min. The over-burnt phenomenon occurs at temperatures higher than 485 °C and at these temperatures the alloy can be over-burnt in a few minutes.
3) The residual phase can be redissolved into the matrix with increasing the solution temperature, meanwhile, the volume fraction of the recrystallized grains and the size of the sub-grains increase dramatically. The specimens solution-treated at 470 °C for 60 min have gained better mechanical properies after the ageing treatment. And the predicted results of “solution resistivity map” has verified the experimental results.
References
[1] HAN N M, ZHANG X M, LIU S D, HE D G, ZHANG R. Effect of solution treatment on the strength and fracture toughness of aluminum alloy 7050 [J]. J Alloys Compd, 2011, 509: 4138-4145.
[2] LI Pei-yue, XIONG Bai-qiang, ZHANG Yong-an, LI Zhi-hui. Temperature variation and solution treatment of high strength AA7050 [J]. Transactions Nonferrous Meals Society of China, 2012, 22: 546-554.
[3] ROMETSCH P A, ZHANG Y, KNIGHT S. Heat treatment of 7xxx series aluminium alloys—Some recent developments [J]. Transactions Nonferrous Metals Society of China, 2014, 24: 2003-2017.
[4] SHA G, WANG Y B, LIAO X Z, DUAN Z C, RINGER S P, LANGDON T G. Microstructural evolution of Fe-rich particles in an Al-Zn-Mg-Cu alloy during equal-channel angular pressing [J]. Mater Sci Eng A, 2010, 527: 4742-4749.
[5] ZHENG Yu-lin, LI Cheng-bo, LIU Sheng-dan, DENG Yun-lai, ZHANG Xin-ming. Effect of homogenization time on quench sensitivity of 7085 aluminum alloy [J]. Transactions Nonferrous Metals Society of China, 2014, 24: 2275-2281.
[6] FLOWER H M. High performance materials in aerospace [M]. London: Chapman & Hall, 1995.
[7] RAEISINIA B, POOLE W J. Electrical resistivity measurements: A sensitive tool for studying aluminium alloys [J]. Mater Sci Forum, 2006, 519-521: 1391-1396.
[8] OLAFSSON P, SANDSTROM R, KARLSSON A. Comparison of experimental, calculated and observed values for electrical and thermal conductivity of aluminium alloys [J]. J Mater Sci, 1997, 32: 4383-4390.
[9]  M, ROMHANJI E. Characterization of microstructural changes in an Al-6.8wt.% Mg alloy by electrical resistivity measurements [J]. Mater Sci Eng A, 2008, 492: 460-467.
M, ROMHANJI E. Characterization of microstructural changes in an Al-6.8wt.% Mg alloy by electrical resistivity measurements [J]. Mater Sci Eng A, 2008, 492: 460-467.
[10] STARINK M J, LI X M. A model for the electrical conductivity of peak-aged and overaged Al-Zn-Mg-Cu alloys [J]. Metall Mater Trans A, 2003, 34: 899-911.
[11] RAEISINIA B, POOLE W J, LOYD D J. Examination of precipitation in the aluminum alloy AA6111 using electrical resistivity measurements [J]. Mater Sci Eng A, 2006, 420: 245-249.
[12] EIVANI A R, AHMED H, ZHOU J, DUSZCZYK J. Correlation between electrical resistivity, particle dissolution, precipitation of dispersoids, and recrystallization behavior of AA7020 aluminum alloy [J]. Metall Mater Trans A, 2009, 40: 2435-2446.
[13] LIN Y C, CHEN X M. A critical review of experimental results and constitutive descriptions for metals and alloys in hot working [J]. Mater Des, 2011, 32: 1733-1759.
[14] SUN Y, ZENG W D, ZHAO Y Q, QI Y L, MA X, HAN Y F. Development of constitutive relationship model of Ti600 alloy using artificial neural network [J]. Comput Mater Sci, 2010, 48: 686-691.
[15] LIN Y C, FANG X, WANG Y P. Prediction of metadynamic softening in a multi-pass hot deformed low alloy steel using artificial neural network [J]. J Mater Sci, 2008, 43: 5508-5515.
[16] ROSSITER P L. The electrical resistivity of metals and alloys [M]. Cambridge: Cambridge University Press, 1987.
[17] ZHANG D L, ZHENG L H, STJOHN D H. Effect of a short solution treatment time on microstructure and mechanical properties of modified Al-7wt.%Si-0.3wt.%Mg alloy [J]. J Light Met, 2002, 2: 27-36.
[18] DENG Y, YIN Z M, CONG F G. Intermetallic phase evolution of 7050 aluminum alloy during homogenization [J]. Intermetallics, 2012, 26: 114-121.
[19] XU D K, ROMETSCH P A, BIRBILIS N. Improved solution treatment for an as-rolled Al-Zn-Mg-Cu alloy. Part I. Characterisation of constituent particles and overheating [J]. Mater Sci and Eng A, 2012, 534: 234-243.
[20] ZHANG Y, MILKEREIT B, KESSLER O, SCHICK C, ROMETSCH P A. Development of continuous cooling precipitation diagrams for aluminium alloys AA7150 and AA7020 [J]. J Alloys Compd, 2014, 584: 581-589.
[21] LI X M, STARINK M J. Identification and analysis of intermetallic phases in overaged Zr-containing and Cr-containing Al-Zn-Mg-Cu alloys [J]. J Alloys Compd, 2011, 509: 471-476.
[22] ZHU Z, STARINK M J. Solution strengthening and age hardening capability of Al-Mg-Mn alloys with small additions of Cu [J]. Mater Sci Eng A, 2008, 488: 125-133.
[23] DIXIT M, MISHRA R, SANKARAN K K. Structure-property correlations in Al 7050 and Al 7055 high-strength aluminum alloys [J]. Mater Sci Eng A, 2008, 478: 163-172.
刘蛟蛟1,2,李红英1,2,李德望1,2,武 岳3
1. 中南大学 材料科学与工程学院,长沙 410083;
2. 中南大学 有色金属材料科学与工程教育部重点实验室,长沙 410083;
3. 中国航空工业集团公司 北京航空材料研究院,北京 100095
摘 要:采用原位电阻测试法、金相显微镜观察、扫描电镜观察、透射电镜观察和拉伸测试技术研究固溶条件对Al-Zn-Mg-Cu合金显微组织和拉伸性能的影响。基于实验数据建立人工神经网络模型,将该模型用于预测实验合金在固溶过程中的电阻率变化。结果表明,所建立的模型能很好地预测合金在固溶过程中的电阻率变化。预测结果与实验值的相关系数为0.9958,相对误差为0.33%。采用预测数据可以建立一种新型的“固溶-电阻率”物理图形。该图形显示,实验合金的最佳固溶温度区间为465~475 °C,保温时间为50~60 min;在该区间内第二相的溶解与再结晶对合金性能的影响将达到平衡。
关键词:铝合金;固溶处理;电阻率;人工神经网络;显微组织演变
(Edited by Wei-ping CHEN)
Foundation item: Project (51344004) supported by the National Natural Science Foundation of China
Corresponding author: Hong-ying LI; Tel: +86-731-88836328; E-mail: lhying@csu.edu.cn
DOI: 10.1016/S1003-6326(15)63683-4

