Preparation and application of perovskite-type oxides for electrocatalysis in oxygen/air electrodes
来源期刊:中南大学学报(英文版)2019年第6期
论文作者:庄树新 何佳怡 张伟鹏 周南 路密 廉冀琼 孙婧婧
文章页码:1387 - 1401
Key words:perovskite-type oxides; electrocatalysts; preparation; oxygen/air electrodes
Abstract: Recent advances in the preparation and application of perovskite-type oxides as bifunctional electrocatalysts for oxygen reaction and oxygen evolution reaction in rechargeable metal-air batteries are presented in this review. Various fabrication methods of these oxides are introduced in detail, and their advantages and disadvantages are analyzed. Different preparation methods adopted have great influence on the morphologies and physicochemical properties of perovskite-type oxides. As a bifunctional electrocatalyst, perovskite-type oxides are widely used in rechargeable metal-air batteries. The relationship between the preparation methods and the performances of oxygen/air electrodes are summarized. This work is concentrated on the structural stability, the phase compositions, and catalytic performance of perovskite-type oxides in oxygen/air electrodes. The main problems existing in the practical application of perovskite-type oxides as bifunctional electrocatalysts are pointed out and possible research directions in the future are recommended.
Cite this article as: ZHUANG Shu-xin, HE Jia-yi, ZHANG Wei-peng, ZHOU Nan, LU Mi, LIAN Ji-qiong, SUN Jing-jing. Preparation and application of perovskite-type oxides for electrocatalysis in oxygen/air electrodes [J]. Journal of Central South University, 2019, 26(6): 1387-1401. DOI: https://doi.org/10.1007/s11771-019-4095-6.

ARTICLE
J. Cent. South Univ. (2019) 26: 1387-1401
DOI: https://doi.org/10.1007/s11771-019-4095-6
ZHUANG Shu-xin(庄树新)1, HE Jia-yi(何佳怡)1, ZHANG Wei-peng(张伟鹏)1,
ZHOU Nan(周南)2, LU Mi(路密)1, LIAN Ji-qiong(廉冀琼)1, SUN Jing-jing(孙婧婧)1
1. Key Laboratory of Functional Materials and Applications of Fujian Province,
School of Materials Science and Engineering, Xiamen University of Technology, Xiamen 361024, China;
2. College of Science, Hunan Agricultural University, Changsha 410128, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: Recent advances in the preparation and application of perovskite-type oxides as bifunctional electrocatalysts for oxygen reaction and oxygen evolution reaction in rechargeable metal-air batteries are presented in this review. Various fabrication methods of these oxides are introduced in detail, and their advantages and disadvantages are analyzed. Different preparation methods adopted have great influence on the morphologies and physicochemical properties of perovskite-type oxides. As a bifunctional electrocatalyst, perovskite-type oxides are widely used in rechargeable metal-air batteries. The relationship between the preparation methods and the performances of oxygen/air electrodes are summarized. This work is concentrated on the structural stability, the phase compositions, and catalytic performance of perovskite-type oxides in oxygen/air electrodes. The main problems existing in the practical application of perovskite-type oxides as bifunctional electrocatalysts are pointed out and possible research directions in the future are recommended.
Key words: perovskite-type oxides; electrocatalysts; preparation; oxygen/air electrodes
Cite this article as: ZHUANG Shu-xin, HE Jia-yi, ZHANG Wei-peng, ZHOU Nan, LU Mi, LIAN Ji-qiong, SUN Jing-jing. Preparation and application of perovskite-type oxides for electrocatalysis in oxygen/air electrodes [J]. Journal of Central South University, 2019, 26(6): 1387-1401. DOI: https://doi.org/10.1007/s11771-019-4095-6.
1 Introduction
Pervoskite-type oxides, which are named after the Russian mineralogist Count Lev Aleksevich von Perovskit, have ABO3 type crystal structure. The ideal pervoskite ABO3 exhibits cubic structure similar to that of natural mineral CaTiO3, as shown in Figure 1 [1]. As seen, the cations with a large ionic radius have 12 coordinations to oxygen atoms and occupy A-sites, and cations with a smaller ionic radius have 6 coordinations and occupy B-sites. Generally, A and B are rare earth element/alkali earth metal and first row transition metal, respectively. Thus, vast elements can form wide variety of ideal or modified perovskites family, possessing extensively special physicochemical properties. And these properties can be tuned greatly depending on the elemental composition of A-site and B-site and their preparation methods.
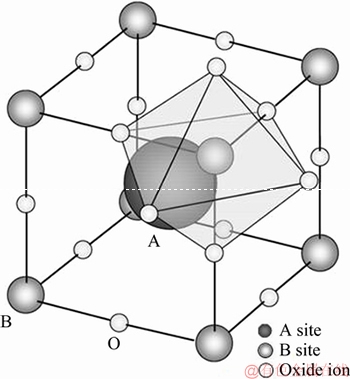
Figure 1 Structure of perovskite (ABO3)
In recent years, this wide variety of perovskite family is not only an important research material for ceramics industry, but also being developed as a new kind of functional materials. To date, a substantial amount of researches have been conducted on their catalytic activity, ferromagnetism and superconductivity, it is found that this kind of oxides is suitable for electrocatalyst of oxygen reduction reaction (ORR) and oxygen evolution reaction (OER), electrode materials of solid-oxide fuel cell, electronic material of electronic components, and high-temperature superconducting material. Preparation method is an important technique for obtaining materials with required structure, and physical/chemical properties. Different preparation methods produce different morphological oxides, which could be used in different fields of application. Presently, the common preparation methods for perovskite-type oxide include thermal decomposition method, solid state method, co-precipitation method, sol-gel method, hydrothermal method, reverse microemulsion method and template method. These preparation methods have both advantages and disadvantages. The choice of the preparation methods according to actual requirements should take into account different applications as well as the practicality of large-scale industrial production. Constitutionally, perovskite-type oxides are compounds that compose of more than two simple oxides with high melting points. Therefore, pure perovskite-type oxides must be synthesized under high temperature with long calcinations time, resulting in low specific surface area. In order to enlarge their surface area, perovskite-type oxides with various morphologies, including nanosheets [2, 3], nanofibers [4, 5], nanotubes [6, 7], nanoflowers [8] and nanocubes [9-11], have been extensively reported. And the morphologies and compositions of perovskite-type oxides can be regulated by different methods, which have significant influence on their physicochemical properties.
This review summarized different methods for preparing pervoskite-type oxides with different morphologies, and pointed out the existing dilemmas of perovskite-type oxides as electrocatalysts in oxygen/air electrodes.
2 Preparation of different morphological perovskite-type oxides
In principle, the preparation methods used to prepare solid compounds can be all used to synthesize perovskite-type oxides, including thermal decomposition, co-precipitation, solid state, sol-gel, reverse microemulsion, hydrothermal and template method.
2.1 Thermal decomposition method
Thermal decomposition method is to decompose the eutectic salts through heat treatment to obtain the homogeneous mixture of corresponding metal oxides, and then calcined at high temperature to form pervoskite structure. LAMMINEN et al [12] used La(NO3)3 and Ni(NO3)2 as starting materials, which were dissolved into deionized water in proportion, forming cocrystallization after evaporation of water, and then annealed in air at 900 °C for 24 h to obtain pure perovskite LiNiO3. XIA et al [13] adopted this method, in which La(NO3)3·6H2O, Co(C2H3O2)2·4H2O and Mn(C2H3O2)2·4H2O as source of metal were mixed stoichiometrically and then calcined at 850 °C for 6 h to form perovskite LaCoyMn1-yO3. WANG et al [14] used La(NO3)3·6H2O, CoSO4·7H2O and Na2C2O4 as raw materials, which were dried to form the 0.97/2La2(C2O4)3-CoC2O4·5·3H2O precursor, and a high-crystallized nanoparticles LaCoO3 were obtained by calcining the precursor at 877 °C for 2 h in air. FARHADI et al [15] successfully synthesized pure and single-phase nanoparticles of perovskite-type LaCoO3 via microwave-assisted thermal decomposition of La[Co(CN)6]·5H2O precursor at 650 °C within a very short reaction time of 10 min. However, the main shortcoming of this method is that organic salts and nitrates are used as starting materials, which will release harmful gases in the process of synthesis, such as NOx, CO and CO2 that can pollute the environment. Therefore, the fabrication should be conducted in the fume hood or in a well ventilated area.
2.2 Solid state method
The relevant metal oxides were stoichiometrically mixed, and then directly sintered for a long time at high temperature in air to obtain the pure phase perovskite oxide powder, known as high temperature solid method [16-18]. Compared to thermal deposition method, this method is more environmentally friendly due to the metal oxides without releasing toxic gas as starting materials. And simple preparation equipment and process make it more suitable for large-scale produce. However, the metal oxide precursors must be homogenized to react with each other thoroughly for fabricating pure pervoskite-type oxides. In this connection, the mixture of the corresponding oxides was treated by ball mill, and then calcined at 1100 °C for 4 h to obtain nearly single-phase and well crystallized La1-xSrxCoO3 [19]. Following the idea, WONG et al [20] used high purity CaO and TiO2 as raw materials, which were mixed by ball mill for 24 h and pressed into tablet followed by calcining at 1240 °C for 10 h to form pure prevoskite structure CaTiO3. In order to lower the calcination temperature, LIU et al [21] adopted a low-temperature solid-state reaction, in which the stoichiometrical oxides were dispersed uniformly in anhydrous alcohol by ball mill and then calcined at 600 °C for 2 h, to synthesize the monophase perovskite Na0.5K0.5NbO3. In order to increase the surface area, ABLAT et al [22] synthesized Bi1-xGdxFeO3 nanoparticles with high surface area via solid state method with secondary calcination, in which the ground powders were precalcined at 700 °C for 1 h, and then thoroughly ground again and further calcined at 820 °C for 2 h. Such prepared samples still possess larger particle size and lower specific surface area, and are usually used in the ceramic field where the good mechanical properties are required [23, 24].
2.3 Co-precipitation method
A stoichiometric amount of soluble metal salts are dissolved in solution, and a coprecipitant (such as OH- and CO32) is added to hydrolyze the metal cations forming insoluble hydroxide/carbonate mixtures that are co-precipitated out from the solution. The multi-component precipitates are then washed by deionized water to remove the unwanted original anions which become the precursor. Finally, the precursor is sintered at a certain temperature in air to form the perovskite-type oxides. NAVALE et al [25] successfully prepared ultrafine single phase LiNbO3 by a simple co-precipitation method, in which an aqueous mixture of ammonium carbonate and ammonium hydroxide was used to precipitate Li+ and Nb5+ cations as carbonate and hydroxide that were calcined at 700 °C for 6 h. Compared to the solid state method, the particle size of the samples was changed from micrometer to nanometer due to the lower calcination temperature. For further lowering the calcination temperature, JADHAV et al [26] employed freshly co-precipitated lanthanum and ferric (or cobalt) hydroxide mixtures as precursors, which were heated at 450 °C for 6 h to generate nano-crystalline LaFeO3 or LaCoO3. Whereafter, MA et al [27] applied co-precipitation method to synthesize La1-xCaxMnO3 nanoparticles and found that the co-precipitation method is easy to cause the aggregation of particles and the reduction of specific surface areas for long calcination time. The co-precipitation method overcomes the disadvantage of heterogeneous mixture in solid state methods as the metal ion components were mixed at molecular level that can accelerate the reaction between solid-phases during heat treatment.
2.4 Sol-gel method
The typical sol-gel method for the preparation of nanomaterials starts from a solution, which consists of the soluble metal salts (such as metal nitrate and acetate) as source of oxides, and then organic ligand (such as citric acid, malic acid lactic acid and ethylene glycol) is added as chelating agent. By controlling the reaction conditions such as temperature, pH value and aging time, hydroxyl ions become attached to the metal atom to form metal complexes, which undergo hydrolysis and polycondensation to produce sol at near room temperature, and then transform to gel after rapid evaporation of solvent. After drying and calcination, the oxides with nanostructure can be obtained. The sol-gel synthesis is an easy and convenient method for the preparation of a variety of advanced ceramics, catalysts and nanomaterials [28, 29]. The metal ions can be combined to a sol skeleton at the molecular level, in which polymers or fine particles are dispersed homogenously, so that the synthesized samples possess the advantages of small particle size, large specific surface area and uniform particles size distribution. Among the various sol-gel methods, the sol-gel method with nitrate as raw material and citric acid as ligand, namely citric-nitrate auto-combustion (CNA) method or Pechini method [30-32], is the main method for preparation of perovskite-type oxide nanoparticles. The advantages of CNA method are as follow. 1) Low calcination temperature and short calcination time make perovskite-type oxides not easy to agglomerate. Some researchers [33, 34] adopted the CNA method to successfully synthesize perovskite nanoparticles and found that the CNA gel precursor could spontaneously combusted between 300 and 400 °C, which would reduce the crystallization temperature of perovskite phase. DEGANELLO et al [35] systematically studied the effects of preparation conditions (such as fuel/oxidant ratios, citric acid/metal nitrate ratios and pH value) in CNA method on the morphologies, crystal structure and phase composition of pervskite-type oxides, and found that low fuel/oxidant, low citric acid/metal nitrates ratios and high pH values are favorable for preparing high-quality perovskites. In order to further reduce the formation temperature of the perovskite-type structure, JIANG et al [36] used glycin instead of citric acid as chelating reagent and found that the introduction of small molecule glycine could cause the release of large amounts of gas at 300 °C in the combustion process, which promotes the formation of nanoporous structure HoFeO3. 2) The combination of the metal atoms to form metal complexes facilitates controlling the phase composition of perovskite-type oxides accurately. BENALI et al [37] simultaneously adulterated Ca and Pb atoms to A-site of LaFeO3 oxides, and found the phase compositions of La0.8Ca0.2-xPbxFeO3 (x=0.00, 0.05, 0.10, 0.15 and 0.20) could be adjusted precisely by controlling the stoichiometric ratio of raw materials. JI et al [38] synthesized Ba0.5Sr0.5TiO3–MgO composite by a cirtrate gel in situ process, providing a new preparation route for perovskite oxide composites. Although the sol-gel method can produce the perovskite oxides in nanoscale, it has poor controllability over the morphologies of nanoparticles. Therefore, YANG et al [39] adopted a sol-gel-hydrothermal method to prepare momodisperse hollow perovskite BaTiO3 nanostuctues, and found that its morphology could be changed by controlling hydrothermal conditions.
2.5 Hydrothermal method
Hydrothermal synthesis is a thermochemical process involving thermal combination of corresponding precursors in hot compressed water, in which a series of complicated reactions induces changes in the physical properties of water (i.e., its density, solubility and dielectric constant) [40-44]. Through adjusting preparative conditions such as temperature, pressure, catalyst, and time to obtain the desired products. BOUKRIBA et al [45] prepared triangular prism-like of crystalline rhombohedral sodium niobate (r-NaNbO3) via a hydrothermal method at 180 °C for 6 h. ZHOU et al [46] successfully prepared Y1-xCaxMnO3 (x=0, 0.07, 0.55, 0.65) in one step via the mild hydrothermal synthesis, and found that the morphology of Y1-xCaxMnO3 strongly depended on the reaction temperature and alkalinity of the reaction system that evolve from irregular particles to 10 μm cubes. WANG et al [47] synthesized cube-shaped Sr-doped LaCrO3 crystalline with a narrow particle size distribution in the range of 1-2 μm under mild hydrothermal conditions. Afterward, MAKOVEC et al [48] adopted simple hydrothermal method without the use of surfactants to prepare single- crystalline dendrites of La1-xSrxMnO3. Subsequently, researchers adopted hydrothermal method to prepare perovskite-type oxides with different morphologies, such as rod shape [49, 50], nanowires [51] and nanotubes [52]. The size and shape of final products can be easily controlled by adjusting process parameters of the hydrothermal method such as reaction temperature and time, concentration and the molar ratios of starting materials. In most cases, it is necessary to further sinter the crystallized samples after hydrothermal process to obtain the target products with perovskite phase. XU et al [53] synthesized the single-crystal Pb2Ti2O6 pyrochlore precusor, which were calcined at 550 °C for 2 h to obtain the PbTiO3 perovskite without morphology change. CHOI et al [54] used hydrothermal method to synthesize the precursor followed by heat treatment at 1000 °C for 10 h to obtain Sr-doped LaCrO3 nanoparticles, exhibiting smaller particle size about 100 nm and more uniform size distribution than those synthesized by the conventional co-precipitation method. JI et al [55] prepared nanoporous LaFeO3 by hydrothermal- calcinations strategy, and found that the change in hydrothermal temperature from 110 to 200 °C gave rise to a great influence on the morphology, surface area, pore structure, and surface oxygen concentration of the final product. For shortening the hydrothermal reaction time, microwave- hydrothermal method was recently adopted to prepare perovskite-type oxides in which the reaction time can reduce to less than 2 h by microwave heating [56-58]. This method, which can facilely regulate the morphologies of products, has the advantages of simple operation, mild reaction conditions, and the as-prepared samples exhibit high purity, small particle size and uniform distribution.
2.6 Reverse microemulsion method
Microemulsions (ME) are multicomponent isotropic, optically transparent, thermodynamically stable nanosized dispersions, which are composed of three components: dispersed polar (water) and continuous non-polar (hydrocarbon) liquid phases, as well as a surfactant. A reverse ME is usually deemed as a system with a reverse micelle that provides additional flow or free water in the core. The formation of reverse micelles in ME is generally determined by hydrophobic interactions among polar surfactant groups. Reverse ME are homogeneous on macroscopic level and represent microheterogeneous systems on microscopic scales consisting of water-in-oil droplets (W/O), where the inorganic salts (reactants and precipitant) are dissolved in the water phase respectively. And these two reverse ME are mixed under agitation to facilitate the precipitation reaction to form precursors in the water phase of W/O droplets. By adjusting the proportion of W/O, selecting the type of emulsifier and regulating the rate of agitation, the reverse ME droplet size and shape can be tuned, giving rise to the controlling the morphology and particle size of the products. HE et al [59] compared the phase compositions of La0.8Ce0.2Cu0.4Mn0.6O3 (or La0.8Ce0.2Ag0.4Mn0.6O3) prepared by reverse ME method and prepared by sol-gel method, and found that no phase segregation occurred in the samples prepared by the reverse ME method. AMAN et al [60] employed a single-reverse ME process to synthesize pure cubic nanoparticles LaNiO3, and found that the single-reverse ME process resulted in a lower LaNiO3 crystallization temperature than other precipitation methods. MAI et al [61] used a facile multi-step reverse ME followed by a slow annealing method to fabricate hierarchical perovskite La0.5Sr0.5CoO3-δ mesoporous nanowires. SHOJAEI et al [62] fabricated CaSnO3 nanopowders via reverse ME method, and the results showed that the kind of surfactants (hexadecyltrimethylammonium bromide and polyoxyethylene octyl phenyl ether) has an important effect on the average size and morphology of the particles. ABAZARI et al [63] demonstrated a facile reverse ME synthesis of monodispersed LaFeO3 nanoparticles with size of 4-32 nm. In brief, the samples prepared by reverse ME method has the advantages of high purity, smaller particle size and uniform distribution and controllable morphology. However, the preparation process is too complicated to realize industrial production.
2.7 Template method
In the field of catalytic application, although the reduction of particle size could improve the contact area with the reactant and the surface-to- bulk atoms ratio, the utilization of surface area of nanosized pervskite-type oxides is still lower than expected. It will be beneficial to design porous perovskite-type oxides to further increase the specific surface area, because porous materials can increase the active reaction sites by adsorbing reactants on the surface and inside the pores, thus improving the catalytic performance [64]. The methods for preparing porous perovskite-type oxides generally include hard template method and soft template method. According to different templates, perovskite-type oxides with different structures can be produced. For hard template, porous silicas are considered as the most promising template due to their structural diversity (such as SBA-15 and KIT-6) and attracted intensive attention in recent years [65, 66]. For example, WANG et al [67] fabricated mesoporous LaCoO3 with high surface area (270 m2/g), which was replicated from the mesoporous SiO2 template (KIT-6). Typically, an equal mole of cobalt nitrate and lanthanum nitrate was first dissolved in a mixture consisting of deionized water, ethanol and citric acid, where the amount of citric acid was equal to the total moles of metal ions. The KIT-6 template was added to the above solution, which was stirred under 40 °C until it became sticky and then dried at 80 °C for 6 h, followed by calcinations at 700 °C for 4 h. Finally, the calcined sample was etched by 2 mol/L sodium hydroxide solution at room temperature to remove the silica frame, and the porous LaCoO3 was replicated. However, the residues of silica template in the samples were inevitable, and the structure of the oxides would be destroyed during the etching process that the severe condition was applied. Compared with the hard one, the soft template is more suitable for fabricating porous oxides, as the soft template can be thoroughly removed in the calcinations process, and the formation of pores and required oxides can occur simultaneously [68-70].
As a soft template for preparing porous perovskite-type oxides, polymeric materials, such as poly(methylmethacrylate) (PMMA) and polystyrene (PS), have also attracted extensive attention recently [71-73]. For example, PMMA microspheres were utilized as soft template for synthesizing three dimensionally ordered macroporous (3DOM) perovskite-type oxides and found that the PMMA microspheres were good template materials [74-77]. First prepared uncrosslinked, monodispersed PMMA microspheres and then added them to a mixture solution consisting of methanol, ethylene glycol and the required nitrate. Subsequently, the resulting solution was dried and calcined at a certain temperature to produce 3DOM perovskite-type oxides. However, the preparation and separation of soft template from the solution is more complex and difficult than that of hard template in most cases. Therefore, these two template methods have various advantages and disadvantages. The choice of hard or soft template depends on the purpose of the material. The main methods of bifunctional catalyst fabrication for ORR and OER are summarized in Table 1.
An analysis of catalyst preparation methods (Table 1) shows that the most popular method is the sol-gel method. However, it is difficult to compare the performance due to the different perovskite-type oxides used as catalysts. The longest cycling life of air electrode catalyzed by La0.6Ca0.4CoO3 achieved 300 h under 50 mA/cm2 current density.
In summary, different preparation methods, which could affect the properties and catalytic performances of the material, can be selected for different applications. Thermal decomposition and solid state methods trend to produce simple bulk perovskite-type oxides. The co-precipitation and sol-gel methods are good choices for preparing the complex composition perovskite-type oxides. The hydrothermal and reverse microemulsion methods are easy to fabricate nanosized perovskite-type oxides with various morphologies. The template method is a new process which can produce perovskite-type oxides with high specific surface area.
3 Applications in rechargeable metal-air/ oxygen batteries
Nowadays, mostly of the energy consumed globally is derived from traditional energy resources such as coal, oil and natural gas, causing severe environmental pollution and serious energy crisis [78, 79]. In recent years, renewable energy sources such as solar, wind, biomass and geothermal have been increasingly utilized. While, solar and wind power are constrained by climate conditions. Therefore, finding a reliable, efficient and safe way to store energy from these renewable and sustainable sources is an urgent need. Electrochemical conversion technologies, such as batteries with high power density, are deemed to be the promising power devices to solve those predicaments. Among various batteries, alkaline fuel cell [80], aluminum-air batteries [81-83], rechargeable metal-air batteries [84, 85] and air-metal hydride [86], in which ORR and OER are two pivotal processes happened at their cathodes, are attractive technologies for energy conversion and storage. Highly efficient bifunctional electrocatalysts are indispensable for accelerating electrochemical reactions so as to enhance the performance of these batteries. Currently, Pt/C and IrO2 present the best catalytic performance for ORR or OER. However, the two noble metal-based catalysts lack good bifunctional activities for both ORR and OER [87], the high-cost and scarcity limit their large-scale applications [88-90]. Among various non-noble metal-based catalysts, perovskite-type oxides have attracted tremendous attentions, due to their low-cost, excellent catalytic performance [91, 92].
Table 1 Electrochemical performance for typical perovskite bifunctional catalysts as air-electrodes

Perovskite-type oxides, which have the general formula ABO3, have been investigated extensively for their bifunctional catalytic abilities in alkaline electrolytes. Their properties can be greatly changed by partially replacing A and B cations with other metals. Generally speaking, A-site substitution mainly affects the ability of sorbed oxygen, whereas B-site substitution influences the activity of sorbed oxygen [93]. And their catalytic mechanism for ORR and OER was simply depicted in Figure 2.
As shown in Figure 2, there are two types of reaction mechanisms for ORR on perovskite-type oxides, corresponding to the direct 4e pathway and the 2e pathway (with generated peroxides), respectively. For the 4e pathway, the reactions are as follows:
O2+2H2O+2e→2OHads+2OH-
2OHads+2e→2OH-
Overall: O2+2H2O+4e→4OH-
For the 2e pathway, the reactions can be given as:
O2+2H2O+e→OH2,ads+OH-
OH2,ads+e→OH2-
Overall: O2+2H2O+2e→OH2-+OH-
And the OER on the oxide proceeds through the following mechanism:
Mz+OH-→Mz-OH+e
Mz-OH+OH-→Mz-H2O2+e
H2O2+OH-→HO2-+H2O
H2O2+HO2-→H2O+OH-+O2
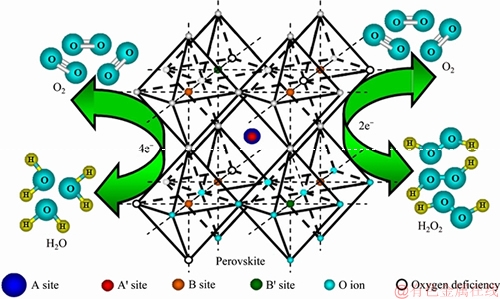
Figure 2 Illustration of O2 catalyzed by perovskite-type oxides in oxygen electrodes
where Mz is the transition-metal ion in the valence state (z+) at the surface of the perovskite. In order different perovskite type oxides with various replacements have been conducted by several groups as bifunctional catalysts [94-96].
Recently, SHAO-HORN et al [97] proposed that different doped perovskite-type oxides with low price could be used as bifunctional electrocatalysts and replace precious metals under alkaline conditions. Whereafter, ZHANG et al [98] and OHKUMA et al [99] applied La1-xCaxMO3 (M=Ni, Mn and Co) as electrocatalysts to the practical batteries, and found that their catalytic activities for ORR and OER was close to Pt/C. However, perovskites normally have rather low conductivity and low specific surface area, giving rise to relatively poor catalytic activity and hinder the industrial use. To date, there are two effective ways to improve its electronic conductivity. One of the effective strategies is to adulterate hetero element in ABO3 structures. In this aspect, CHANG et al [100-102] have successfully adulterated La0.6Ca0.4CoO3 with iridium by three different preparation methods and observed that the doping of iridium in the perovskite structure of La0.6Ca0.4CoO3 significantly enhanced its bifunctional abilities in alkaline medium, and their representative results are shown in Figure 3.
Another highly efficient way is to modify good conductive materials on the surface of perovskite- type oxides. ZHUANG et al [103] used a chemical reduction method to modify silver particles on La0.6Ca0.4CoO3 and found that the introduction of silver particles not only enhanced the electronic conductivity, but also increased the ORR and OER kinetics, the corresponding results as shown in Figure 4.
However, the adulteration and modification usually led to the increase of the volume of perovskite structure, which caused serious agglomeration of the catalysts, thus reduced their specific surface area. Compared with increasing the electronic conductivity, enlarging the specific surface area of pervoskite-type oxides is another difficulty.
Generally, there are two effective solutions to further enlarge its specific surface area as following. 1) Dispersing them on a substrate with high specific surface area. Mesoporous silica supported perovskites catalysts with a high specific surface area (250-300 m2/g) were prepared by DAI groups [104]. Nevertheless, the perovskite catalysts loaded on mesoporous silica are not suitable for the gas diffusion electrodes (GDEs), because the electronic conductivity and catalytic activity of GDEs will decrease when the insulated silica is introduced. 2) Nano-crystallizing pervoskite-type oxides. For instance, ZHAO et al [61] prepared a hierarchical mesoporous La0.5Sr0.5CoO2.91 nanowires that presented high-performance catalysts for the ORR with low peak-up potential and high limiting diffusion current. But, its low electronic conductivity still exists. In order to further increase their electronic conductivity and specific surface area simultaneously, HU et al [105] successfully fabricated La1-xCaxMnO3 anchored on the surface of graphene, and found that doping Ca into the composites can tune their catalytic activity for ORR/OER and sample prepared with x=0.4 possesses the highest electricatakytic activity, of which the electron transfer number is 3.6, indicating that the La0.6Ca0.4MnO3-graphene composites are potential air electrodes catalysts. Whereafter, PARK et al [106] developed a new class of hybrid bifunctional catalyst consisting of porous nanorod perovskite La0.5Sr0.5Co0.8Fe0.2O3 combined with nitrogen-doped reduced graphene oxide active towards both ORR and OER, presenting not only a comparable or superior performance to state-of- the-art Pt/C catalyst for ORR or OER, respectively, but also better durability. To date, LI et al [107,108] developed a novel class of carbonaceous materials hybrids and used as catalyst for ORR in Al-air batteries, delivering remarkable ORR catalytic activity with the direct 4e pathway. Following this idea, the new class of pervoskite-carbonaceous material hybrids will be promising bifunctional catalyst for metal-air battery applications.
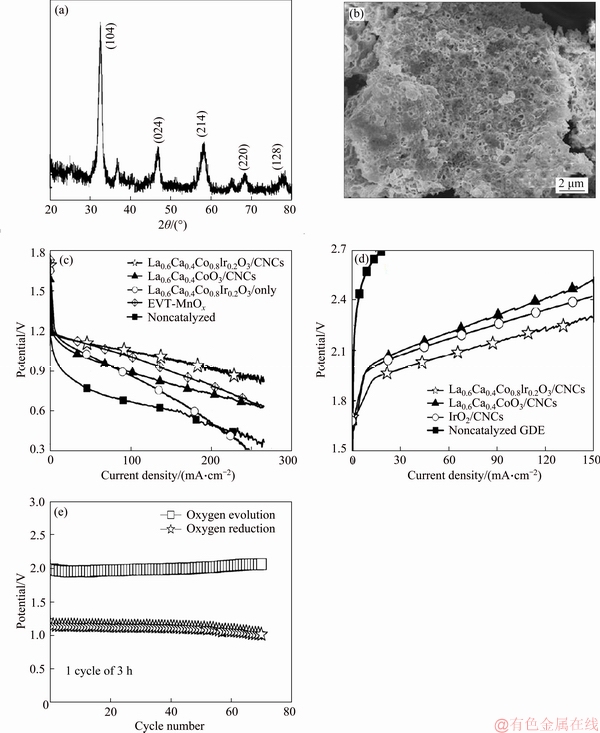
Figure 3 XRD pattern of La0.6Ca0.4Co0.8Ir0.2O3 (a), SEM image of La0.6Ca0.4Co0.8Ir0.2O3 (b), oxygen reduction i-V polarization curves of GDEs with various catalysts (c), oxygen evolution i-V polarization curves of GDEs with various catalysts (d), and cycle life performance of GDEs with La0.6Ca0.4Co0.8Ir0.2O3/CNCs (e) [102]

Figure 4 XRD patterns of silver-modified La0.6Ca0.4CoO3 samples (a), SEM image of silver-modified La0.6Ca0.4CoO3 samples (b), oxygen reduction i-V polarization curves of GDEs with various catalysts (c), oxygen evolution i-V polarization curves of GDEs with various catalysts (d), and cycle life performance of 0.03Ag-La0.6Ca0.4CoO3 catalyzed GDE (e) (“No IR corr” in Figures (c, d) means that none of the potential measurements was compensated for IR-drops) [103]
In summary, perovskite-type oxides are the promising electrocatalyst for ORR and OER in the application of rechargeable metal-batteries. Only improving their specific surface area and electronic conductivity simultaneously, perovskite-type oxides could truly replace the noble metal as electrocatalysts. In order to obtain highly active electrocatalysts, in situ growth of perovskite-type oxides on porous matrix with high conductivity should be the focus of future research.
4 Conclusions
A systematic investigation of the preparation methods of perovskite-type oxides with different morphologies and the applications of perovskite-type oxides in rechargeable metal-air batteries has been given. Perovskite-type oxides are compounds formed by the integration of more than two simple oxides at high calcinations temperature and long calcination time, so their specific surface areas are relative lower. The improvement of the surface area and surface properties of perovskite-type oxides is of vital importance for the surface electrocatalytic reactions. Various types of perovskite-type nanoparticles were prepared, such as nanocube, nanorod, nanosheet, nanofiber, mesoporous structure and nanotube, but relatively few of them are used as electrode materials.
With the continuous development of science and technology of materials, great academic achievements have been acquired in the preparation and application of perovskite-type oxides, it is still a big challenge to regulate their physicochemical properties such as the morphology, specific surface area, particle size and phase composition. The perovskite-type oxides prepared by traditional methods have not met the requirements in the electrode material field. How to fully develop the perovskite-type oxides with better performance has become an urgent issue to be solved by researchers. Current and future efforts should therefore focus on synthesizing a more effective catalyst and on learning about the surface poisoning of perovskite- type oxides in order to popularize these materials on a large scale.
References
[1] TANAKA H, MISONO M. Advances in designing perovskite catalysts [J]. Curr Opin Solid State Mater Sci, 2001, 5: 381-387. DOI: 10.1016/S1359-0286(01)00035-3.
[2] CHEN Xiao-hua, HU Jian-qiang, CHEN Zhi-wu, FENG Xiu-mei, LI Ai-qing. Nanoplated bismuth titanate sub- microspheres for protein immobilization and their corresponding direct electrochemistry and electrocatalysis [J]. Biosens Bioelectron, 2009, 24: 3448-3454. DOI: 10.1016/j.bios.2009.04.037.
[3] LI S, NECHACHE R, DAVALOS I A V, GOUPIL G, NIKOLOVA L, NICKLAUS M, LAVERDIERE J, RUEDIGER A, ROSEI F. Ultrafast microwave hydrothermal synthesis of BiFeO3 nanoplates [J]. J Am Ceram Soc, 2013, 96: 3155-3162. DOI: 10.1111/jace.12473.
[4] LENG Jing, LI Shuang, WANG Zhong-shan, XUE Yan-feng, XU Da-peng. Synthesis of ultrafine lanthanum ferrite (LaFeO3) fibers via electrospinning [J]. Mater Lett, 2010, 64: 1912-1914. DOI: 10.1016/j.matlet.2010.06.005.
[5] LI S, KATO R, WANG Q, YAMANAKA T, TAKEGUCHI T, UEDA W. Soot trapping and combustion on nanofibrous perovskite LaMnO3catalysts under a continuous flow of soot [J]. Appl Catal B: Environ, 2010, 93: 383-386. DOI: 10.10 16/j.apcatb.2009.10.012.
[6] ZHU Xin-hua, LIU Zhi-guo, MING Nai-ben. Perovskite oxide nanotubes: Synthesis, structural characterization, properties and applications [J]. J Mater Chem, 2010, 20: 4015-4030. DOI: 10.1039/b923119f.
[7] WANG J, MANIVANNAN A, WU N. Sol-gel derived La0.6Sr0.4CoO3nanoparticles, nanotubes, nanowires and thin films [J]. Thin Solid Films, 2008, 517: 582-587. DOI: 10.1016/j.tsf.2008.06.095.
[8] CHEN X, TANG Y, FANG L, ZHANG H, HU C, ZHOU H. Self-assembly growth of flower-like BiFeO3powders at low temperature [J]. J Mater Sci: Mater Electron, 2012, 23: 1500-1503. DOI: 10.1007/s10854-011-0617-1.
[9] WANG Wan-jun, BI Jin-hong, WU Ling, LI Zhao-Hui, FU Xian-zhi. Hydrothermal synthesis and catalytic performances of a new photocatalyst CaSnO3with microcube morphology [J]. Scripta Mater, 2009, 60: 186-189. DOI: 10.1016/ j.scriptamat.2008.10.001.
[10] DENG Ji-guang, ZHANG Lei, DAI Hong-xing, AU Chak- tong. A Study on the relationship between low-temperature reducibility and catalytic performance of single-crystalline La0.6Sr0.4MnO3+δmicrocubes for toluene combustion [J]. Catal Lett, 2009, 130: 622-629. DOI: 10.1007/s10562-009- 9901-6.
[11] NAKASHIMA K, KERA M, FUJII I, WADA S. A new approach for the preparation of SrTiO3nanocubes [J]. Ceram Int, 2013, 39: 3231-3234. DOI: 10.1016/j.ceramint.2012.10. 009.
[12] LAMMINEN J, KIVISAARI J, LAMPINEN M J, VIITANEN M,VUORISALO J. Preparation of air electrodes and long run tests [J]. J Electrochem Soc, 1991, 138: 905-908. DOI: 10.1149/1.2085745.
[13] XIA Xi, PAN Cun-xin. Peparation and characteristics of nanophase LaCoyMn1-YO3 by solid state reaction [J]. Chinese Journal of Applied Chemistry, 2001, 18: 96-99. http://yyhx. ciac.jl.cn/CN/article/downloadArticleFile.do?attachType=PD F&id=8799. (in Chinese)
[14] WANG Kai-tuo, WU Xue-hang, WU Wen-wei, LI Yong-ni, LIAO Sen. Synthesis of perovskite LaCoO3by thermal decomposition of oxalates: Phase evolution and kinetics of the thermal transformation of the precursor [J]. Ceram Int, 2014, 40: 5997. DOI: 10.1016/ j.ceramint.2013.11.048.
[15] FARHADI S, SEPAHVAND S. Microwave-assisted solid- state decomposition of La[Co(CN)6]·5H2O precursor: A simple and fast route for the synthesis of single-phase perovskite-type LaCoO3 nanoparticles [J]. J Alloy Compd, 2010, 489: 586-591. DOI: 10.1016/j.jallcom.2009.09.117.
[16] SCHAAK R E, MALLOUK T E. Perovskites by design:A toolbox of solid-state reactions [J]. Chem Mater, 2002, 14: 1455-1471. DOI: 10.1021/cm010689m.
[17] RUDSKAYA A G, PUSTOVAYA L E, KOFANOVA N B, KUPRIYANOV M F. Specific features of La1-xMnO3solid state synthesis [J] J Struct Chem, 2005, 46: 647-651. DOI: 10.1007/s10947-006-0183-1.
[18] BHELLA S S, KUTI L M, LI Q, THANGADURAI V. Electricaltransportproperties of In-doped Ce1-xInxO2-δ (x=0.1; 0.2) [J]. Dalton Trans, 2009: 9520-9528. DOI: 10.1039/ B910335J.
[19] ISUPOVA L A, ALIKINA G M, TSYBULYA S V, BOLDYREVA N N, KRYUKAVA G N, YAKAVLEVA I S, ISUPOV V P, SADYKOV V A. Real structure and catalytic activity of La1-xSrxCoO3 perovskites [J]. Int J Inorg Mater, 2001, 3: 559-562. DOI: 10.1016/S1466-6049(01)00062-9.
[20] WONG Y J, HASSAN J, HASHIM M. Dielectric properties, impedance analysis and modulus behavior of CaTiO3ceramic prepared by solid state reaction [J]. J Alloy Compd, 2013, 571: 138-144. DOI: 10.1016/j.jallcom.2013. 03.123.
[21] LIU Lai-jun, ZHENG Shao-ying, HUANG Rui-jing, SHI Dan-ping, HUANG Yan-min, WU Shuang-shuang, LI Yun-hua, FANG Liang, HU Chang-zheng. Na0.5K0.5NbO3and 0.9Na0.5K0.5NbO3–0.1Bi0.5Na0.5TiO3 nanocrystalline powders synthesized by low-temperature solid-state reaction [J]. Adv Powder Technol, 2013, 24: 908-912. DOI: 10.1016/j.apt. 2013.01. 001.
[22] ABLAT A, WU R, MAMAT M, LI J, MUHEMMED E, SI C, WU R, WANG J, QIAN H, IBRAHIM K. Structural analysis and magnetic properties of Gd doped BiFeO3ceramics [J]. Ceram Int, 2014, 40: 14083-14089. DOI: 10.1016/j.ceramin t.2014.05.137.
[23] BHALLA A S, GUO R, ROY R. The perovskite structure—A review of its role in ceramic science and technology [J]. Mater Res Innov, 2000, 4: 3-26. DOI: 10.1007/s100190000 062.
[24] ZHANG S, XIA R, SHROUT T R, ZANG G, WANG J. Piezoelectric properties in perovskite 0.948(K0.5Na0.5)NbO3– 0.052LiSbO3 lead-free ceramics [J]. J Appl Phys, 2006, 100: 104108-6. DOI: 10.1063/1.2382348.
[25] NAVALE S C, SAMUEL V, RAVI V. A coprecipitation technique to prepare LiNbO3powders [J]. Ceram Int, 2006, 32: 847-848. DOI: 10.1016/j.ceramint.2005.05.015.
[26] JADHAV A D, GAIKWAD A B, SAMUEL V, RAVI V. A low temperature route to prepare LaFeO3and LaCoO3 [J]. Mater Lett, 2007, 61: 2030-2032. DOI: 10.1016/j.matlet. 2006.08.009.
[27] MA J, THEINGI M, CHEN Q, WANG W, LIU X, ZHANG H. Influence of synthesis methods and calcination temperature on electrical properties of La1-xCaxMnO3 (x=0.33 and 0.28) ceramics [J]. Ceram Int, 2013, 39: 7839-7843. DOI: 10.1016/j.ceramint.2013.03.044.
[28] VEITH M, MATHUR S, LECERF N, HUCH V, DECKER T. Sol-gel synthesis of nano-scaled BaTiO3, BaZrO3 and BaTi0.5Zr0.5O3 oxides via single-source alkoxide precursors and semi-alkoxide routes [J]. J Sol-Gel Sci Technol, 2000, 15: 145-158. DOI: 10.1023/A:100879541.
[29] CHILIBON I, MARAT J N. Ferroelectric ceramics by sol–gel methods and applications: A review [J]. J Sol-Gel Sci Technol, 2012, 64: 571-611. DOI: 10.1007/s10971-012- 2891-7.
[30] SHIMIZU Y, UEMURA K, MATSUDA H, MIURA N, YAMAZOE N. Bi-functional oxygen electrode using large surface area La1-xCaxCoO3for rechargeable metal-air battery [J] J Electrochem Soc, 1990, 137: 3430-3433. DOI: 10. 11 49/1.208 6 234.
[31] ZHU Jun-jiang, YANG Xiang-guang, XU Xue-lian, WEI Ke-mei. Active site structure of NO decomposition on perovskite(-like) oxides:An investigation from experiment and density functional theory [J] J Phys Chem C, 2007, 111: 1487-1490. DOI: 10.1021/jp0662101.
[32] GRA A M P F, PREZAS P R, COSTA M M, VALENTE M A. Structural and dielectric characterization of LiNbO3 nano- size powders obtained by Pechini method [J]. J Sol-Gel Sci Technol, 2012, 64: 78-85. DOI: 10.1007/s10971-012- 2829-0.
A M P F, PREZAS P R, COSTA M M, VALENTE M A. Structural and dielectric characterization of LiNbO3 nano- size powders obtained by Pechini method [J]. J Sol-Gel Sci Technol, 2012, 64: 78-85. DOI: 10.1007/s10971-012- 2829-0.
[33] MARIN EK M, ZUPAN K, MA
EK M, ZUPAN K, MA EK J. Ni–YSZ cermet anodes prepared by citrate/nitrate combustion synthesis[J]. J Power Sources, 2002, 106: 178-188. DOI: 10.1016/S0378- 7753(01)01056-4.
EK J. Ni–YSZ cermet anodes prepared by citrate/nitrate combustion synthesis[J]. J Power Sources, 2002, 106: 178-188. DOI: 10.1016/S0378- 7753(01)01056-4.
[34] CHAKROBORTY A, DAS S A, MAITI B, MAITI H S. Preparation of low-temperature sinterable BaCe0.8Sm0.2O3 powder by autoignition technique [J]. Mater Lett, 2002, 57: 862-867. DOI: 10.1016/S0167-577X(02)00886-8.
[35] DEGANELLO F, MARC G, DEGANELLO G. Citrate– nitrate auto-combustion synthesis of perovskite-type nanopowders: A systematic approach [J]. J Eur Ceram Soc, 2009, 29: 439-450. DOI: 10.1016/j.jeurceramsoc.2008.06. 012.
G, DEGANELLO G. Citrate– nitrate auto-combustion synthesis of perovskite-type nanopowders: A systematic approach [J]. J Eur Ceram Soc, 2009, 29: 439-450. DOI: 10.1016/j.jeurceramsoc.2008.06. 012.
[36] JIANG L, LIU W, WU A, XU J, LIU Q, QIAN G, ZHANG H. Low-temperature combustion synthesis of nanocrystalline HoFeO3powders via a sol–gel method using glycin [J]. Ceram Int, 2012, 38: 3667-3672. DOI: 10.1016/j.ceramint. 2012.01.007.
[37] BENALI A, AZIZI S, BEJAR M, DHAHRI E, GRA A M F P. Structural, electrical and ethanol sensing properties of double-doping LaFeO3perovskite oxides [J]. Ceram Int, 2014, 40: 14367-14373. DOI: 10.1016/j.ceramint.2014.06. 029.
A M F P. Structural, electrical and ethanol sensing properties of double-doping LaFeO3perovskite oxides [J]. Ceram Int, 2014, 40: 14367-14373. DOI: 10.1016/j.ceramint.2014.06. 029.
[38] JI Lu-dong, ZHANG Jing-ji, GAO Yue-e, LI Yan-li, WANG Jiang-ying. Dielectric properties of Ba0.5Sr0.5TiO3–MgO composites synthesized by a citrate gel in situ process [J]. Ceram Int, 2014, 40: 11419- 11422. DOI: 10.1016/ j.ceramint.2014.03.084.
[39] YANG Xin, REN Zhao-hui, CHAO Chun-ying, JIANG Shan, DENG Shi-qi, SHEN Ge, WEI Xiao, HAN Gao-rong. Monodisperse hollow perovskite BaTiO3nanostructures prepared by a sol–gel–hydrothermal method [J]. Ceram Int, 2014, 40: 9663-9670. DOI: 10.1016/j.ceramint.2014.02.047.
[40] GUO H Y, LIN J G. Ferroelectric domain structure of highly textured BiFeO3 microcrystal films prepared by hydrothermal method [J]. J Cryst Growth, 2013, 364: 145-148. DOI: 10.1016/j.jcrysgro.2012.11.028.
[41] ZHANG Jing-ji, SHEN Bo, ZHAI Ji-wei, YAO Xi. Microwave dielectric properties and low sintering temperature of Ba0.5Sr0.5TiO3–Mg2TiO4 composites synthesized in situ by the hydrothermal method [J]. Ceram Int, 2013, 39: 5943-5948. DOI: 10.1016/j.ceramint.2012. 11.089.
[42] BASAVALINGU B, VIJAYA K M S, GIRISH H N, YODA S. Hydrothermal synthesis and characterization of rare earth doped yttrium aluminium perovskite-R: YAlO3(R=Nd, Eu and Er) [J]. J Alloy Compd, 2013, 552: 382-386. DOI: 10.1016/j.jallcom.2012. 10.091.
[43] SUN Zi-xiong, PU Yong-ping, DONG Zi-jing, HU Yao, LIU Xiao-yan, WANG Pei-kui, GE Meng. Dielectric and piezoelectric properties and PTC behavior of Ba0.9Ca0.1Ti0.9Zr0.1O3-xLa ceramics prepared by hydrothermal method [J]. Mater Lett, 2014, 118: 1-4. DOI: 10.1016/j.matlet.2013.12.043.
[44] FUENTES S, C SPEDES F, PADILLA-CAMPOS L, DIAZ- ROGUETT D E. Chemical and structural analysis related to defects in nanocrystalline Ba1-xSrxTiO3grown via hydrothermal sol–gel [J]. Ceram Int, 2014, 40: 4975-4984. DOI: /10.1016/j.ceramint.2013.09.134.
SPEDES F, PADILLA-CAMPOS L, DIAZ- ROGUETT D E. Chemical and structural analysis related to defects in nanocrystalline Ba1-xSrxTiO3grown via hydrothermal sol–gel [J]. Ceram Int, 2014, 40: 4975-4984. DOI: /10.1016/j.ceramint.2013.09.134.
[45] BOUKRIBA M, SEDIRI F, GHARBI N. Hydrothermal synthesis and electrical properties of NaNbO3 [J]. Mater Res Bull, 2013, 48: 574-580. DOI: 10.1016/j.materresbull.2012. 11.046.
[46] ZHOU Z, GUO L, YE F. Hydrothermal synthesis, magnetism and resistivity of orthorhombic perovskite manganates Y1-xCaxMnO3(x=0, 0.07, 0.55, 0.65) [J]. J Alloy Compd, 2013, 571: 123-131. DOI: 10.1016/j.jallcom.2013.03.220.
[47] WANG Shan, HUANG Ke-ke, ZHENG Bei-ning, ZHANG Jia-qi, FENG Shou-hua. Mild hydrothermal synthesis and physical property of perovskite Sr doped LaCrO3 [J]. Mater Lett, 2013, 101: 86-89. DOI: 10.1016/j.matlet.2013.03.083.
[48] MAKOVEC D, GOR AK T, ZUPAN K, LISJAK D. Hydrothermal synthesis of La1-xSrxMnO3dendrites [J]. J Cryst Growth, 2013, 375: 78-83. DOI: 10.1016/j.jcrysgro. 2013.04.019.
AK T, ZUPAN K, LISJAK D. Hydrothermal synthesis of La1-xSrxMnO3dendrites [J]. J Cryst Growth, 2013, 375: 78-83. DOI: 10.1016/j.jcrysgro. 2013.04.019.
[49] KUMAR R D, JAYAVEL R. Low temperature hydrothermal synthesis and magnetic studies of YMnO3 nanorods [J]. Mater Lett, 2013, 113: 210-213. DOI: 10.1016/j.matlet. 2013.09.070.
[50] ZHANG D, SHI F, CHENG J, YANG X, YAN E, CAO M. Preparation and characterization of orthorhombic NaNbO3 long bar [J]. Ceram Int, 2014, 40: 14279-14285. DOI: 10.1016/j.ceramint.2014.06.018.
[51] WANG J, DURUSSEL A, SANDU C S, SAHINI M G, HE Z, SETTER N. Mechanism of hydrothermal growth of ferroelectric PZT nanowires [J]. J Cryst Growth, 2012, 347: 1-6. DOI: 10.1016/j.jcrysgro.2012.03.022.
[52] AI Z, LU G, LEE S. Efficient photocatalytic removal of nitric oxide with hydrothermal synthesized Na0.5Bi0.5TiO3 nanotubes [J]. J Alloy Compd, 2014, 613: 260-266. DOI: 10.1016/j.jallcom.2014.06.039.
[53] XU Gang, ZHANG Yan-fang, HE Wan-bo, ZHAO Yan-gang, LIU Yong, SHEN Ge, HAN Gao-rong. Single-crystal lead titanate perovskite dendrites derived from single-crystal lead titanate pyrochlore dendrites by phase transition at elevated temperature [J]. J Cryst Growth, 2012, 346: 101-105. DOI: 10.1016/j. jcrysgro.2012.02.016.
[54] CHOI B H, PARK S, PARK B K, CHUN H H, KIMA Y, Controlled synthesis of La1-xSrxCrO3nanoparticles by hydrothermal method with nonionic surfactant and their ORR activity in alkaline medium [J]. Mater Res Bull, 2013, 48: 3651-3656. DOI: 10.1016/j.materresbull. 2013.04.084.
[55] JI K, DAI H, DENG J, SONG L, XIE S, HAN W. Glucose-assisted hydrothermal preparation and catalytic performance of porous LaFeO3for toluene combustion [J]. J Solid State Chem, 2013, 199: 164-170. DOI: 10.1016/j.jssc. 2012.12.017.
[56] WANG Z, ZHU J, XU W, SUI J, PENG H, TANG X. Microwave hydrothermal synthesis of perovskite BiFeO3 nanoparticles: An insight into the phase purity during the microwave heating process [J]. Mater Chem Phys, 2012, 135: 330-333. DOI: 10.1016/j.matchemphys.2012.04.053.
[57] PONZONI C, ROSA R, CANNIO M, BUSAGLIA V, FINOCCHIO E, NANNI P, LEONELLI C. Optimization of BFO microwave-hydrothermal synthesis: Influence of process parameters [J]. J Alloy Compd, 2013, 558: 150-159. DOI: 10.1016/j.jallcom.2013.01.039.
[58] L PEZ-JU
PEZ-JU REZ R, CASTA
REZ R, CASTA EDA-GUZM
EDA-GUZM N R, VILLAFUERTE-CASTREJ
N R, VILLAFUERTE-CASTREJ N M E. Fast synthesis of NaNbO3and K0.5Na0.5NbO3by microwave hydrothermal method [J]. Ceram Int, 2014, 40: 14757-14764. DOI: 10.1016/j.ceramint.2014.06.065.
N M E. Fast synthesis of NaNbO3and K0.5Na0.5NbO3by microwave hydrothermal method [J]. Ceram Int, 2014, 40: 14757-14764. DOI: 10.1016/j.ceramint.2014.06.065.
[59] HE H, LIU M, DAI H, QIU W, ZI X. An investigation of NO/CO reaction over perovskite-type oxide La0.8Ce0.2B0.4Mn0.6O3(B=Cu or Ag) catalysts synthesized by reverse microemulsion [J]. Catal Today, 2007, 126: 290-295. DOI: 10.1016/j.cattod.2007.06.004.
[60] AMAN D, ZAKI T, MIKHAIL S, SELIM S A. Synthesis of a perovskite LaNiO3nanocatalyst at a low temperature using single reverse microemulsion [J]. Catal Today, 2011, 164: 209-213. DOI: 10.1016/j.cattod.2010.11.034.
[61] ZHAO Yun-long, XU Lin, Mai Li-qiang, HAN Chun-hua, An Qin-you, XU Xu, LIU Xue, ZHANG Qing-jie. Hierarchical mesoporous perovskite La0.5Sr0.5CoO2.9 nanowires with ultrahigh capacity for Li-air batteries [J]. PNAS, 2012, 109: 19569-19574. DOI: 10.1073/ pnas.1210315109.
[62] SHOJAEI S, HASSANZADEH-TABRIZI S A, GHASHANG M. Reverse microemulsion synthesis and characterization of CaSnO3nanoparticles [J]. Ceram Int, 2014, 40: 9609-9613. DOI: 10.1016/j.ceramint.2014.02.037.
[63] ABAZARI R, SANATI S. Perovskite LaFeO3nanoparticles synthesized by the reverse microemulsion nanoreactors in the presence of aerosol-OT: Morphology, crystal structure, and their optical properties [J]. Superlattice Microst, 2013, 64: 148-157. DOI: 10.1016/j.spmi.2013.09.017.
[64] WANG Y, REN J, WANG Y, ZHANG F, LIU X, GUO Y, LU G. Nanocasted synthesis of mesoporous LaCoO3 perovskite with extremely high surface area and excellent activity in methane combustion [J]. J Phys Chem C, 2008, 112: 15293-15298. DOI: 10.1021/jp8048394.
[65] WANG N, YU X, WANG Y, CHU W, LIU M. A comparison study on methane dry reforming with carbon dioxide over LaNiO3perovskite catalysts supported on mesoporous SBA-15, MCM-41 and silica carrier [J]. Catal Today, 2013, 212: 98-107. DOI: 10.1016/j.cattod.2012.07.022.
[66] GAO Bao-zu, DENG Ji-guang, LIU Yu-xi, ZHAO Zhen-xuan, LI Xin-wei, WANG Yuan, DAI Hong-xing. Mesoporous LaFeO3catalysts for the oxidation of toluene and carbon monoxide [J]. Chinese J Catal, 2013, 34: 2223- 2229. DOI: 10.1016/S1872-2067(12)60689-5.
[67] WANG Yong-xia, CUI Xiang-zhi, LI Yong-sheng, SHU Zhu, CHEN Hang-rong, SHI Jian-lin. A simple co-nanocasting method to synthesize high surface area mesoporous LaCoO3oxides for CO and NO oxidations [J]. Micropor Mesopor Mat, 2013, 176: 8-15. DOI: 10.1016/j. micromeso.2013.03.033.
[68] XU Jun-feng, LIU Jian, ZHAO Zhen, ZHENG Jian-xiong, ZHANG Gui-zhen, DUAN Ai-jun, JIANG Gui-yuan. Three-dimensionally ordered macroporous LaCoxFe1-xO3 perovskite-type complex oxide catalysts for diesel soot combustion [J]. Catal Today, 2010, 153: 136-142. DOI: 10.1016/j.cattod.2010.01.063.
[69] SADAKANE M, HORIUCHI T, KATO N, SASAKI K, UEDA W. Preparation of three-dimensionally ordered macroporous perovskite-type lanthanum–iron-oxide LaFeO3 with tunable pore diameters: High porosity and photonic property [J]. J Solid State Chem, 2010, 183: 1365- 1371. DOI: 10.1016/j.jssc.2010.04.012.
[70] XIAO Ping, ZHU Jun-jiang, Li Hai-long, JIANG Wen, WANG Tao, ZHU Yu-jun, ZHAO Yan-xi, LI Jin-lin. Effect of textural structure on the catalytic performance of LaCoO3for CO oxidation [J]. Chem Cat Chem, 2014, 6: 1774-1781. DOI: 10.1002/cctc. 201402064.
[71] LIU Y, DAI H, DU Y, DENG J, ZHANG L, ZHAO Z, AU C T. Controlled preparation and high catalytic performance of three-dimensionally ordered macroporous LaMnO3with nanovoid skeletons for the combustion of toluene [J]. J Catal, 2012, 287: 149-160. DOI: 10.1016/j.jcat.2011.12.015.
[72] JI K, DAI H, DENG J, ZHANG L, WWANG F, JIANG H, AU C T. Three-dimensionally ordered macroporous SrFeO3-δ with high surface area: Active catalysts for the complete oxidation of toluene [J]. Appl Catal A: Gen, 2012, 425-426: 153-160. DOI: 10.1016/j.apcata.2012.03.013.
[73] LIU Yu-xi, DAI Hong-xing, DU Yu-cheng, DENG Ji-guang, ZHANG Lei, ZHAO Zhen-xuan. Lysine-aided PMMA- templating preparation and high performance of three- dimensionally ordered macroporous LaMnO3 with mesoporous walls for the catalytic combustion of toluene [J]. Appl Catal B: Environ, 2012, 119-120: 20-31. DOI: 10.1016/j.apcatb.2012.02.010.
[74] ZHAO Z, DAI H, DENG J, DU Y, LIU Y, ZHANG L. Three-dimensionally ordered macroporous La0.6Sr0.4FeO3-δ: High-efficiency catalysts for the oxidative removal of toluene [J]. Micropor Mesopor Mat, 2012, 163: 131-139. DOI: 10.1016/j.micromeso.2012.07.006.
[75] ARANDIYAN H, DAI H, DENG J, LIU Y, BAI B, WANG Y, LI X, XIE S, LI J. Three-dimensionally ordered macroporous La0.6Sr0.4MnO3 with high surface areas: Active catalysts for the combustion of methane [J]. J Catal, 2013, 307: 327-339. DOI: 10.1016/j.jcat.2013.07.013.
[76] ZHAO Zhen-xuan, DAI Hong-xing, DENG Ji-guang, DU Yu-cheng, LIU Yu-xi, ZHANG Lei. Preparation of three- dimensionally ordered macroporous La0.6Sr0.4Fe0.8Bi0.2O3-δ and their excellent catalytic performance for the combustion of toluene [J]. J Mol Catal A: Chem, 2013, 366: 116-125. DOI: 10.1016/j.molcata.2012.09. 014.
[77] LIU Y, DAI H, DENG J, LI X, WANG Y, ARANDIYAN H, XIE S, YANG H, GUO G. Au/3DOM La0.6Sr0.4MnO3: Highly active nanocatalysts for the oxidation of carbon monoxide and toluene [J]. J Catal, 2013, 305: 146-153. DOI: 10.1016/ j.jcat.2013.04.025.
[78] LI Wei, LIU Jun, ZHAO Dong-yuan. Mesoporous materials for energy conversion and storage devices [J]. Nature Reviews Materials, 2016, 1: 16023-16040. DOI: 10.1038/ natrevmats. 2016.23.
[79] SALANNE M, ROTENBERG B, NAOI K, KANEKO K, TABERNA P L, GREY C P, DUNN B, SIMON P. Efficient storage mechanisms for building better supercapacitors [J]. Nature Energy, 2016, 1: 16070-16080. DOI: 10.1038/ nenergy.2016.70.
[80] GEWIRTH A A, THORUM M S. Electroreduction of dioxygen for fuel-cell applications: Materials and Challenges [J]. Inorg Chem, 2010, 49: 3557-3566. DOI: 10.1021/ ic9022486.
[81] LI J, CHEN J, WANG H, REN Y, LIU K, TANG Y, SHAO M. Fe/N co-doped carbon materials with controllable structure as highly efficient electrocatalysts for oxygen reduction reaction in Al-air batteries [J]. Energy Storage Mater, 2017, 8: 49-58. DOI: 10.1016/j.ensm.2017.03.007.
[82] LI J, ZHOU Z, LIU K, LI F, PENG Z, TANG Y. Co3O4/Co-N-C modified ketjenblack carbon as an advanced electrocatalyst for Al-air batteries [J]. J Power Sources, 2017, 343: 30-38. DOI: 10.1016/j.jpowsour.2017.01.018.
[83] SONG J, REN Y, LI J, HUANG X, CHENG F, TANG Y, WANG H. Core-shell Co/CoNx@C nanoparticles enfolded by Co-N doped carbon nanosheets as a highly efficient electrocatalyst for oxygen reduction reaction [J]. Carbon, 2018, 138: 300-308.
[84] LI Yan-guang, GONG Ming, LIANG Yong-ge, FENG Ju, KIM Ji-Eun, WANG Hai-liang, HONG Guo-song, ZHANG Bo, DAI Hong-jie. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts [J]. Nat Commun, 2013, 4: 1805-1812. DOI: 10.1038/ncomms2812.
[85] LEE J S, KIM ST, CAO R, CHOI N S, LIU M, LEE K T, CHO J. Metal–air batteries with high energy density: Li–air versus Zn–air [J]. Adv Energy Mater, 2011, 1: 34-50. DOI: 10.1002/aenm.201000010.
[86] DONG H, KIROS Y, NOR US D. An air-metal hydride battery using MmNi3.6Mn0.4Al0.3Co0.7 in the anode and a perovskite in the cathode [J]. Inter J Hydrogen Energy, 2010, 35: 4336. DOI: 10.1016/j.ijhydene.2010.02.007.
US D. An air-metal hydride battery using MmNi3.6Mn0.4Al0.3Co0.7 in the anode and a perovskite in the cathode [J]. Inter J Hydrogen Energy, 2010, 35: 4336. DOI: 10.1016/j.ijhydene.2010.02.007.
[87] FU G, YAN X, CHEN Y, XU L, SUN D, LEE J M, TANG Y. Boosting bifunctional oxygen electrocatalysis with 3D graphene aerogel-supported Ni/MnO Particles [J]. Adv Mater, 2018, 30: 1704609. DOI: 10.1002/adma.201704609.
[88] WANG H, WANG W, GUI M, ASIF M, WANG Z, YU Y, XIAO J, LIU H. Uniform Fe3O4/Nitrogen-doped mesoporous carbon spheres derived from ferric citrate-bonded melamine resin as an efficient synergistic catalyst for oxygen reduction [J]. ACS Appl Mater Interfaces, 2017, 9: 335-344. DOI: 10.1021/acsami.6b11608.
[89] HUANG S F, HSU Y Y, CHANG C J, HSU C S, SUEN N T, CHAN T S, CHEN M H. Unraveling geometrical site confinement in highly efficient iron-doped electrocatalysts toward oxygen evolution reaction [J]. Adv Energy Mater, 2018, 8: 1701686. DOI: doi.org/10.1002/aenm.201701686.
[90] LI Y, YANG J, HUANG J, ZHOU Y, XU K, ZHAO N, CHENG X. Soft template-assisted method for synthesis of nitrogen and sulfur co-doped three-dimensional reduced graphene oxide as an efficient metal free catalyst for oxygen reduction reaction [J]. Carbon, 2017, 122: 237-246. DOI: 10. 1016/j.carbon.2017.06.046.
[91] BIRSELL M, PIRJAMALI M, KIROS Y. La0.6Ca0.4CoO3, La0.1Ca0.9MnO3 and LaNiO3 as bifunctional oxygen electrodes [J] . Electrochim Acta, 2002, 47: 1651-1660. DOI: 10.1016/S0013-4686(02)00002-6.
[92] NEBURCHILOV V, WANG H J, MARTIN J J, QU W. A review on air cathodes for zinc–air fuel cells [J]. J Power Sources, 2010, 195: 1271-1291. DOI: 10.1016/j.jpowsour. 2009.08.100.
[93] PE A M A, FIERRO J L G. Chemical structures and performance of perovskite oxides [J]. Chem Rev, 2001, 101: 1981-2018. DOI: 10.1021/cr980129f.
A M A, FIERRO J L G. Chemical structures and performance of perovskite oxides [J]. Chem Rev, 2001, 101: 1981-2018. DOI: 10.1021/cr980129f.
[94] SWETTE L, KACKLEY N, MCCATTY S A. Oxygen electrodes for rechargeable alkaline fuel cells. III [J]. J Power Sources, 1991, 36: 323-339. DOI: 10.1016/0378- 7753(91)87010-9.
[95] KANNAN A M, SHUKLA A K, SATHYANARAYANA S. Oxide-based bifunctional oxygen electrode for rechargeable metal/air batteries [J]. J Power Sources, 1989, 25: 141-150. DOI: 10.1016/0378-7753(89)85006-2.
[96] SWETTE L, KACKLEY N. Oxygen electrodes for rechargeable alkaline fuel cells–II [J]. J Power Sources, 1990, 29: 423-436. DOI: 10.1016/0378-7753(90)85015-5.
[97] SUNTIVICH J, GASTEIGER H A, YABUUCHI N, NAKANISHI H, GOODENOUGH J B, HORN Y S. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries [J]. Nature Chem, 2011, 3: 546-550. DOI: 10.1038/NCHEM.1069.
[98] ZHANG T, IMANISHI N, TAKEDA Y, YAMAMOTO O. Aqueous lithium/air rechargeable batteries [J]. Chem Lett, 2011, 40: 668-673. DOI: 10.1246/cl.2011.668.
[99] OHKUMA H, UECHI I, IMANISHI N, HIRANO A, TAKEDA Y, YAMAMOTO O. Carbon electrode with perovskite-oxide catalyst for aqueous electrolyte lithium-air secondary batteries [J]. J Power Sources, 2013, 223: 319-324. DOI: 10.1016/j.jpowsour.2012.09.028.
[100] CHANG Y M, WU P W, WU C Y, HSIEH Y F, CHEN J Y. Mechanical alloying preparation of La0.6Ca0.4CoIr0.25O3.5-δ as a bifunctional electrocatalyst in alkaline electrolyte [J]. Electrochem Solid State Lett, 2008, 11: B47-B50. DOI: 10.1149/1.2835200.
[101] CHANG Y M, HSIEH Y C, WU P W, LAI C H, CHANG T Y. Enhancement of bifunctional catalysis by Ir doping of La0.6Ca0.4CoO3 perovskites [J]. Mater Lett, 2008, 62: 4220-4222. DOI: 10.1016/j. matlet.2008.06.040.
[102] CHANG Y M, WU P W, WU C Y, HSIEN Y C. Synthesis of La0.6Ca0.4Co0.8Ir0.2O3perovskite for bi-functional catalysis in an alkaline electrolyte [J]. J Power Sources, 2009, 189: 1003-1007. DOI: 10.1016/j.jpowsour.2008.12.101.
[103] ZHUANG Shu-xin, HUANG Ke-long, HUANG Cheng-huan, HUANG Hong-xia, LIU Su-qin, FAN Min. Preparation of silver-modified La0.6Ca0.4CoO3 binary electrocatalyst for bi-functional air electrodes in alkaline medium [J]. J Power Sources, 2011, 196: 4019-4025. DOI: 10.1016/j.jpowsour. 2010.11.056.
[104] DENG J G, ZHANG L, DAI H X, AU C T. In situ hydrothermally synthesized mesoporous LaCoO3/SBA- 15 catalysts: High activity for the complete oxidation of toluene and ethyl acetate [J]. Appl Catal A, 2009, 352: 43-49. DOI: 10.1016/j.apcata.2008.09.037.
[105] HU Jie, WANG Li-na, SHI Li-na, HUANG Hao. Preparation of La1-xCaxMnO3perovskite-graphene composites as oxygen reduction reaction electrocatalyst in alkaline medium [J]. J Power Sources, 2014, 269: 144-151. DOI: 10.1016/j. jpowsour.2014.07.004.
[106] PARK H W, LEE D U, ZAMANI P, SEO M H, NAZAR L F, CHEN Z. Electrospun porous nanorod perovskite oxide/ nitrogen-doped graphene composite as a bi-functional catalyst for metal air batteries [J]. Nano Energy, 2014, 10: 192-200. DOI: 10.1016/j.nanoen.2014.09.009.
[107] LI J, ZHOU N, SONG J, FU L, YAN J, TANG Y, WANG H. Cu-MOF-derived Cu/Cu2O nanoparticles and CuNxCy species to boost oxygen reduction activity of Ketjenblack carbon in Al-air battery [J]. ACS Sustainable Chem Eng, 2018, 6: 413-421. DOI: 10.1021/acssuschemeng.7b02661.
[108] LI J, CHEN J, WAN H, XIAO J, TANG Y, LIM M, WANG H. Boosting oxygen reduction activity of Fe-N-C by partial copper substitution to iron in Al-air batteries [J]. Appl Catal B: Environ, 2019, 242: 209-217. DOI: 10.1016/j.apcatb. 2018.09.044.
(Edited by FANG Jing-hua)
中文导读
钙钛矿型氧化物的制备及其在氧/空气双功能电极中的应用
摘要:本文综述了近期钙钛矿型氧化物在氧/空气电极中作为氧还原和氧析出双功能电催化剂的制备方法。详细地介绍了各种制备方法并对其优缺点进行比较分析,发现不同的制备方法对钙钛矿型氧化物的形貌和物理化学性能影响很大。钙钛矿型氧化物作为双能电催化剂被广泛应用于金属-空气电池中,归纳了其制备方法与电催化性能之间的关系。在氧/空气电极应用中,重点讨论了影响钙钛矿型氧化物的结构稳定性、相组成和电催化活性的因素,指出了其作为双功能电催化剂在实际应用中存在的主要问题,并对今后的研究方向进行预测。
关键词:钙钛矿型氧化物;电催化剂;制备方法;氧/空气电极
Foundation item: Projects(51504212, 21573184, 51703061) supported by the National Natural Science Foundation of China; Project (2018J01521) supported by the Natural Science Foundation of Fujian Province, China; Project(fma2017202) supported by the Open Fund of Fujian Provincial Key Laboratory of Functional Materials and Applications (Xiamen University of Technology), China
Received date: 2018-10-23; Accepted date: 2019-01-25
Corresponding author: ZHUANG Shu-xin, PhD, Associate Professor; Tel: +86-592-6291337; E-mail: zsxtony@xmut.edu.cn; ORCID: 0000-0002-4946-1014

