Corrosion damage evolution and mechanical properties of carbon fiber reinforced aluminum laminate
来源期刊:中南大学学报(英文版)2021年第3期
论文作者:湛利华 吴欣桐 黄明辉 赵兴 王迅 赵国庆
文章页码:657 - 668
Key words:carbon fiber reinforced aluminum laminate; galvanic corrosion; electrochemistry; interlaminar shear strength; aluminum alloy
Abstract: Fiber metal laminates (FMLs), a kind of lightweight material with excellent comprehensive performance, have been successfully applied in aerospace. FMLs reinforced with carbon fiber have better mechanical properties than those with glass or aramid fiber. However, carbon fiber binding metal may lead to galvanic corrosion which limits its application. In this paper, electrochemical methods, optical microscope and scanning electron microscope were used to analyze the corrosion evolution of carbon fiber reinforced aluminum laminate (CARALL) in corrosive environment and explore anti-corrosion ways to protect CARALL. The results show that the connection between carbon fiber and aluminum alloy changes electric potential, causing galvanic corrosion. The galvanic corrosion will obviously accelerate CARALL corroded in solution, leading to a 72.1% decrease in interlaminar shear strength, and the crevice corrosion has a greater impact on CARALL resulting in delamination. The reduction of interlaminar shear strength has a similar linear relationship with the corrosion time. In addition, the adhesive layers between carbon fiber and aluminum alloy cannot protect CARALL, while side edge protection can effectively slow down corrosion rate. Therefore, the exposed edges should be coated with anti-corrosion painting. CARALL has the potential to be used for aerospace components.
Cite this article as: WU Xin-tong, ZHAN Li-hua, HUANG Ming-hui, ZHAO Xing, WANG Xun, ZHAO Guo-qing. Corrosion damage evolution and mechanical properties of carbon fiber reinforced aluminum laminate [J]. Journal of Central South University, 2021, 28(3): 657-668. DOI: https://doi.org/10.1007/s11771-021-4635-8.

J. Cent. South Univ. (2021) 28: 657-668
DOI: https://doi.org/10.1007/s11771-021-4635-8
WU Xin-tong(吴欣桐)1, 2, ZHAN Li-hua(湛利华)1, 2, HUANG Ming-hui(黄明辉)1, 2,ZHAO Xing(赵兴)1, 2, WANG Xun(王迅)1, 2, ZHAO Guo-qing(赵国庆)1, 2
1. State Key Laboratory of High-performance Complex Manufacturing, Central South University,Changsha 410083, China;
2. School of Mechanical and Electrical Engineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract: Fiber metal laminates (FMLs), a kind of lightweight material with excellent comprehensive performance, have been successfully applied in aerospace. FMLs reinforced with carbon fiber have better mechanical properties than those with glass or aramid fiber. However, carbon fiber binding metal may lead to galvanic corrosion which limits its application. In this paper, electrochemical methods, optical microscope and scanning electron microscope were used to analyze the corrosion evolution of carbon fiber reinforced aluminum laminate (CARALL) in corrosive environment and explore anti-corrosion ways to protect CARALL. The results show that the connection between carbon fiber and aluminum alloy changes electric potential, causing galvanic corrosion. The galvanic corrosion will obviously accelerate CARALL corroded in solution, leading to a 72.1% decrease in interlaminar shear strength, and the crevice corrosion has a greater impact on CARALL resulting in delamination. The reduction of interlaminar shear strength has a similar linear relationship with the corrosion time. In addition, the adhesive layers between carbon fiber and aluminum alloy cannot protect CARALL, while side edge protection can effectively slow down corrosion rate. Therefore, the exposed edges should be coated with anti-corrosion painting. CARALL has the potential to be used for aerospace components.
Key words: carbon fiber reinforced aluminum laminate; galvanic corrosion; electrochemistry; interlaminar shear strength; aluminum alloy
Cite this article as: WU Xin-tong, ZHAN Li-hua, HUANG Ming-hui, ZHAO Xing, WANG Xun, ZHAO Guo-qing. Corrosion damage evolution and mechanical properties of carbon fiber reinforced aluminum laminate [J]. Journal of Central South University, 2021, 28(3): 657-668. DOI: https://doi.org/10.1007/s11771-021-4635-8.
1 Introduction
Fiber metal laminates (FMLs) are hybrid materials based on thin sheets of metal and fiber reinforced composite layers [1]. This laminated structure which has excellent mechanical properties, impact resistance, damage tolerance characteristics and low density was developed at Delft University in the 1980s [2, 3]. During the last two decades, FMLs have been developed into three generations, namely aramid reinforced aluminum laminate (ARALL), glass fiber reinforced aluminum laminate (GLARE) and carbon fiber reinforced aluminum laminate (CARALL) [4, 5]. ARALL and GLARE are now used extensively in aircraft structures like fuselage panels, wing skin and tail skin due to their excellent properties. Recent years have seen an increase in studies on CARALL which is considered to be prospective in respect of comprehensive performance. JAKUBCZAK et al [6] and SHI et al [7] investigated the resistance to low velocity impact of CARALL, and DHALIWAL et al [8] employed the finite element method to predict the low velocity impact response of CARALL. Their results showed that the ply orientation in unidirectional carbon and the yield strength of aluminum alloy are of particular importance to the impact resistance. Fatigue behavior and life were noted by DADEJ et al [9] and GUPTA et al [10] in studies on CARALL under tension-compression stress. They examined fatigue crack of CARALL and tested residual fatigue life of CARALL. Moreover, ASGHAR et al [11] compared the fatigue crack growth rates of CARALL, ARALL and GLARE, indicating that CARALL exhibited the highest ultimate tensile strength and fracture toughness. Due to high stiffness, carbon fiber offers stronger bridging to aluminum alloy than aramid fiber and glass fiber, and CARALL provides excellent impact resistance property.
However, the application of CARALL is rarely reported due to galvanic corrosion [12]. Galvanic corrosion is known to occur when two dissimilar materials are in direct contact and a liquid electrolyte bridges the two materials. This corrosion phenomenon has caused wide concern with a tremendous rise in the application of carbon fiber reinforced plastic (CFRP) composite [13, 14]. In general, the CFRP acts as the cathode and the metallic materials (e.g., aluminum and steel) as the anode. The metallic materials which coupled to more noble material (CFRP) will experience accelerated corrosion compared to when the materials are used alone. LI et al [15] investigated the corrosion behavior of Al/CFRP joints after six-month exposure to a marine environment. They found severe corrosion on Al in the coupled regions due to galvanic corrosion. PENG et al [16] also found the same corrosion behavior of CFRP coupled to aluminum in a 3.5%NaCl solution, and they figured out the corrosion potential by employing the potentiodynamic polarization tests and zero resistance ammeter testing method. A study has been performed on damage types of the fuselages of Boeing 747 with an average life of 29500 flying hours. There are about 30% damages caused by corrosion [17].
To reduce the corrosion problems, the corrosion behavior of CARALL has to be studied. However, to the best of the author’s knowledge, very few research papers were focused on galvanic corrosion behavior of CARALL. VERMEEREN [12] first reported the galvanic corrosion researches of CARALL. He provided one way to prevent galvanic corrosion by isolating the aluminum from carbon fiber. WANG et al [18] and BIENIAS et al [19] once reported the galvanic corrosion analysis of CARALL on conference. They all agreed that it is necessary to conduct further investigation on the corrosion behavior of CARALL.
In order to further research the galvanic corrosion behavior of CARALL, as well as to explore the protection of CARALL from galvanic corrosion, the present study expands on previous researches by comparing the corrosion process of CARALL specimens protected in different ways. On one hand, electrochemical techniques including potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) were conducted to analyze the process and mechanism of galvanic corrosion. On the other hand, the morphology and residual mechanical properties of corroding specimens were investigated by optical microscopy (OM), scanning electron microscope (SEM) and interlaminar shear strength (ILSS) test.
2 Experiment
2.1 Materials and specimen preparation
Aluminum alloy 2024-T3 (AA2024) sheets with thickness of 0.3 mm (with 92%Al, 0.4% Fe, 0.2% Si, 4.7% Cu, 0.5% Mn, 1.7% Mg, 0.1% Cr, 0.25% Zn, 0.15% Ti), T800/X850 carbon fiber/epoxy prepreg with thickness of 0.2 mm supplied by CYTEC and 65% fiber volume fraction, all were the materials used for the manufacturing of CARALL specimens. And an adhesive layer (structural adhesive film J-271, Institute of Petrochemistry Heilongjiang Academy of Sciences) was included at each composite-metal interface according to the experiment need. In this study, phosphoric acid anodizing (PAA) treatment was used to make the surface of aluminum alloy sheet have multi-pore structures so as to increase the interlocking between the carbon fiber and aluminum alloy layers. A schematic illustration of CARALL specimen is shown in Figure 1(a), and the layup of CARALL is shown in Figure 1(b).

Figure 1 (a) Schematic of CARALL construction (3/2 laminate); (b) Layup of CARALL
Specimens were cured in a vacuum bag, and full vacuum (100 kPa) was maintained during whole cure. Layup method was employed to fabricate the specimens followed by using autoclave machine for curing. The cure cycle included a 180 min dwell at 180 °C with a 1.5 °C/min ramp rate and 0.6 MPa pressure applied on the layered system which was held for the duration of the temperature dwell. Autoclave vacuum press equipment was allowed to pressurize 0 to 1 MPa during the curing of composites. From our experience with CFRP laminates, it is assured that 0.6 MPa will be a good pressure for the consolidation of CARALL specimens. The autoclave curing cycle of CARALL is shown in Figure 2.
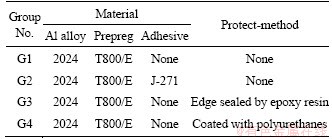
Each of the test specimens was 10 mm wide, 24 mm long and about 2 mm thick, cut out from the above CARALL specimens for the next tests. Four groups were set up in this study: group 1 (G1) was control group without any protection; group 2 (G2) had an adhesive J-271 at each composite-metal interface added; group 3 (G3) was insulated by using epoxy resin to mask their cut edges and back sides; group 4 (G4) was coated with polyurethanes in every surfaces, as shown in Table 1.

Figure 2 Autoclave curing cycle for CARALL
Table 1 Experimental testing details for each group
2.2 Electrochemical measurements
The electrochemical properties of specimens were characterized by electrochemical tests using a CHI660C electrochemical workstation (CH Instruments, China) with a three-electrode system. The electrochemical cell consisting of a saturated calomel electrode (SCE) was used as a reference electrode, Pt wire as a counter electrode, and specimen mounted in the substrate holder as working electrode. The area of the specimen exposed to the electrolyte was 1 cm2. Electrochemical corrosion measurements were investigated using potentiodynamic polarization and electrochemical impedance spectroscopy (EIS). All tests were carried out in 3.5 wt% NaCl solution at ambient temperature. Specimens were immersed for 30 min to ensure the steady-state prior to measurements; measurements were repeated at least three times. Potentiodynamic polarization measurements were performed from -1 to 0 V at a scan rate of 1 mV/s in order to obtain steady-state. The corrosion current density Icorr and potential Ecorr were obtained automatically from the Tafel plots using the CHI660C workstation analysis software. The EIS tests were carried out at open circuit potential in a frequency range from 100 kHz to 0.01 Hz with wide-amplitude of 10 mV. EIS analysis was performed by using a Zview analyzer.
2.3 Corrosion tests
There is no standard for corrosion of FMLs. Therefore, the accelerated exfoliation corrosion (EXCO) tests were chosen to investigate the corrosion behavior of CARALL specimens according to GB/T 22639-2008 recommendations. The EXCO tests were carried out in the immersion of specimens up to 96 h at room temperature (22-25°C) in a solution containing 4.0 mol/L NaCl+0.5 mol/L KNO3+0.1 mol/L HNO3 (pH=0.4). The ratio of solution volume to specimen surface area was 20 mL/cm2. The specimens were taken out and recorded after 24, 48, 72 and 96 h, respectively, and then cleaned with distilled water and dried. The specimens were visually quoted from pitting corrosion (P) to exfoliation A (EA), exfoliation B (EB), exfoliation C (EC) and exfoliation D (ED, very severe EFC) according to GB/T 22639-2008 recommendations.
2.4 Mechanical testing
Interlaminar shear strength (ILSS) of CARALL specimens after EXCO tests was evaluated via GB/T 1450.1-2005. Tests were performed on a mechanical testing device (MTS Systems Corporation, MN, USA) at a speed of 1 mm/min. Five specimens from each group were tested and their average values were reported. Corrosion surfaces of the specimens were analyzed with optical microscope (Keyence VHX5000, Japan) and TESCAN scanning electron microscope (Tescan Company, Brno, Czech), so as to study the corrosion behavior of CARALL.
3 Results and discussion
3.1 Polarization behavior of CARALL in 3.5% NaCl electrolyte
In potentiodynamic experiments, the current represents the reaction rate of anodic or cathodic reactions on the selected working electrodes. Thus, the current flowing through the electrode can be used as a reference for corrosion problems [20]. Figure 3 shows the polarization curves for 2024 aluminum alloy, Group 1, Group 2 and Group 3 in 3.5% NaCl electrolyte (the curves of Group 4 cannot be measured due to insulation). All curves show the effects of concentration polarization due to the oxygen reduction reaction (O2+2H2O+4e→ 4OH-) and activation polarization due to hydrogen generation (2H2O+2e→H2+2OH-) [21]. The corrosion potential (Ecorr), corrosion current density (Icorr), the polarization resistance (Rcorr) and other parameters obtained from the polarization curves by Tafel extrapolation method are given in Table 2. The Rcorr values were calculated using the relationship [22]:
 (1)
(1)
At the same time, according to Faraday’s law, the annual average corrosion depth velocity VL and the corrosion current density Icorr have the following relations:
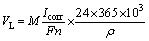 (2)
(2)
where M is the relative atomic mass of the metal; n is the valence number; and F is the Faraday’s constant.

Figure 3 Polarization curves for each group in 3.5 wt% NaCl at 30 °C (scan rate=1 mV/s)
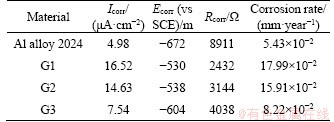
Corrosion potential (Ecorr) and corrosion current density (Icorr) are two important parameters which connect the electrochemistry and the practical corrosion behavior of metals [23]. The corrosion potential values can indicate the corrosion process occurring on surfaces while the corrosion current density values reflect the corrosion rate. Corrosion current density can be used as an important parameter to evaluate the corrosion resistance of the material. The lower the corrosion current, the better the corrosion resistance of material. From Table 2, the corrosion current density recorded of Group 1 and Group 2 is respectively 16.52 and 14.63 μA/cm2, both higher than Group 3, protected by edge sealed. The 2024 aluminum alloy (4.98 μA/cm2) possesses the lowest corrosion current (7.54 μA/cm2) among all groups. It is obvious that the corrosion current density of CARALL specimen increases compared to the 2024 aluminum alloy, and the potential of CARALL specimens is shifted toward positive direction relative to the potential of 2024 aluminum alloy, as shown in Figure 3. These results indicate that the carbon fiber composite greatly influenced the corrosion behavior of CARALL. In general, the potentials of aluminum alloys are much lower than the carbon fiber composite in the sodium chloride electrolyte, which indicates that aluminum alloys will be anodic with respect to the composite when they are galvanically coupled. The corrosion resistance in the 3.5% NaCl solution increases in the order of Group 1< Group 2< Group 3< AA2024. Compared with Group 1 and Group 2, the corrosion current density of Group 3 is tremendously decreased when the edges of specimens were sealed. But there is no significant difference in corrosion current density between Group 1 and Group 2, inferring that the bare parts of the edge are the place where major galvanic corrosion occurs instead of the interface between composite and metal. Thus, the polarization test results suggest that the corrosion susceptibility of CARALL is greater than the aluminum alloy in the 3.5% NaCl solution.
Table 2 Potentiodynamic polarization parameters of different Group in a 3.5% NaCl solution
3.2 Electrochemical impedance spectroscopy (EIS)
The EIS plots of CARALL specimens in 3.5% NaCl are shown in Figure 4(a). Group 4 which appears as straight lines perpendicular to solid axis is not shown in the complex plane impedance diagram due to the protection of polyurethanes. The spots of the three groups are similar, and their electrode behaviors are controlled by double layer capacitance. Nyquist plots reveal two regions which are composed of a semicircle at high-frequency region and an approximate straight-line at low frequency region. The large capacitive loop at high frequency is due to the resistance of solution and epoxy. Al loses its electrons and forms Al3+ on interface between epoxy, which leads to the formation of hydroxyl and oxide ions [24]. And the line indicates that there is a diffusion behavior at low frequencies. The electrolyte solution reacts with the surface of Al, and then the charge intermediates or corrosion products adsorb on the metal or composites. It is more likely that the corrosion products will diffuse to the solution side [25]. 3.30 version of ZSimpWin software was used for circuit fitment. The equivalent circuit (Figure 4(b)) employed is similar to that for a coated metal exposed to electrolyte [26]. It consists of these parameters: solution and epoxy polarization resistance (Rs and Rc, respectively), coating capacitance (Cc), double layer charging (Qdl), charge transfer resistance (Rct), and constant phase element (Cw) in parallel with a diffusion resistance (Rw) and L representing the diffusion processes in the interface between composite metals. The electrochemical parameters obtained from circuit fitment are given in Table 3.
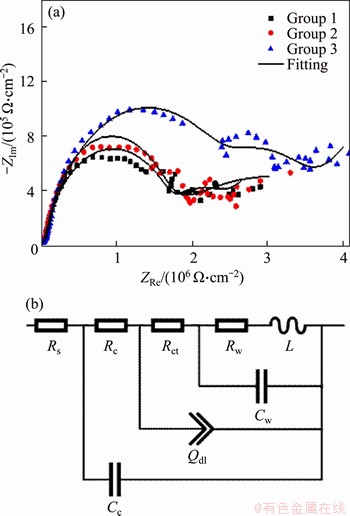
Figure 4 EIS complex plane plots of each group in 3.5% NaCl and fitting curves (a) and circuit used to fit experimental EIS data (b)
Table 3 Data fitting of CARALL specimens
According to the fitting results and Figure 4, the capacitance of CARALL specimens decreased by different protection. The decrease in capacitance indicates a decrease in water absorption, which increases resistance to corrosion. As for resistance, the test solution was identical and, hence, the difference of Rs could be ignored. The impedance value can be expressed by Rct. The higher the Rct, the better the corrosion resistance. It can be seen that Rct of Group 3 is the highest, indicating that it has the best corrosion resistance. Generally, a semicircle with larger radius of curvature in the high frequency region is more resistant to corrosion. As shown in Figure 4(a), it is further confirmed that the specimens in Group 3 show better resistance to corrosion than those in Group 1 and Group 2.
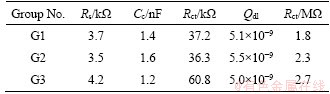
Compared to the Nyquist plot, Bode diagram shown in Figure 5 gives a clear explanation to how the electrochemical system behaves depending upon the frequency and how it reduces the experimental data dispersion. The |Z| Bode plot shows a nearly straight line which corresponds to a capacitor. This kind of capacitive extends to the low frequency end of the spectrum, indicating that the charge transfer between metal and composites has very low kinetics. Generally, the anticorrosion capacity is corresponding to the impedance peak at low frequencies, and thus the CARALL of Group 1 had the worst corrosion performance. Figure 5(b) also shows that the phase angle was monotonically increasing with frequency at low frequency but is not monotonic at a higher frequency. And the differences in Bode plots become greater between Group 3 and Group 1 (Group 2), which is consistent with the other testing results mentioned.
3.3 Exfoliation corrosion characterization
The corrosion behavior of CARALL specimens treated with various protections were evaluated by the standard EXCO tests, and the images of specimens after different immersion time are shown in Figure 6. The specimens are sampled in the rolling direction and vertical rolling direction. As the specimens sank into solution, there were a lot of bubbles around the specimens of Group 1 and Group 2, and only a small portion of bubbling lied on the surface of Group 3, while no significant response was observed in Group 4. After 24 h, pitting corrosion was evident on the surfaces of Group 1 and Group 2, and corrosion products which exuded out of the surfaces showed a dark gray. After 96 h of exposure, more severe exfoliation could appear on the surfaces of Group 1 and Group 2 which were covered with thick reddish corrosion products with pits and lifting of metal [27]. It can be found that the corrosion ranks for Group 1 and Group 2 change from pitting corrosion B (PB) to EC after being exposed for 24-96 h, and some specimens even have delamination failure on the interface between composite and metal. While for Group 3, the corrosion rating degrades from pitting corrosion A (PA) to EA during test. There is no obvious change in Group 4 by visual evaluation based on corrosion morphologies due to the protection of polyurethanes. According to this ranking, the specimens in Group 1 had the most rapidly corrosion rate and the susceptibility can be ranked in the order: Group 1>Group 2>Group 3> Group 4 [28]. It is clear that the resistance of the specimens to EXCO was remarkably reduced due to the reduction of protective measures.
Figure 5 Bode plots for CARALL specimens in 3.5% NaCl solution

Figure 6 Macrostructure of each group after exfoliation corrosion:
The metallographic cross-section of specimens after immersing in the EXCO solution for 96 h and the average exfoliation corrosion depth are shown in Figures 7 and 8, respectively. The specimens of Group 1 and Group 2 have the most severe exfoliation corrosion, and the corrosion occurs at the interface between composite and metal which extended along the edge to the central part. Specimens of Group 3 are affected less by the edge protection, of which pitting corrosion and exfoliation corrosion occurs on the aluminum alloy surface. Specimens of Group 4 are maintained in good condition due to the protection of polyurethanes. The CARALL specimens in Group 1 are very susceptible to exfoliation during test, and the specimens of Group 2 also have sensitivity comparable to Group 1. While the extent of corrosion of specimens in Group 3 is obviously reduced compared to that in Group 1 or Group 2. This indicates that the interface between metal and composites may not be the main cause for the galvanic corrosion. Furthermore, although specimens in Group 1, Group 2 and Group 3 have been corroded, the corrosion morphology shows a significant difference. The specimens of Group 3 show absolutely no progression of the corrosion cracks, whereas the delamination and cracks take place in specimens of Group 1 and Group 2. The delamination and cracks for specimens of Group 1 and Group 2 are the evidence that the corrosion occurs not only at the surface but also at the interface. This corrosion type is undoubtedly fatal for the layered structure of CARALL. Moreover, it should be noted, in Figure 8, that the rate of corrosion is not constant with time. For Group 1 and Group 2, it shows a rapid corrosion rate with a change of about 4.2 μm/h and then slows to about 2.5 μm/h. The average exfoliation rating of CARALL specimens is obviously higher than that of aluminum alloy [29, 30]. Again, this is in agreement with the observations of the surface ratings. Further analysis was conducted on the surface of aluminum alloy (Group 1) by scanning electron microscope (SEM). The image of surface (Figure 9(a)) exposed to solution shows that the alloy has been seriously corroded and the characteristic of intergranular corrosion can be observed. Pitting corrosion first appears; then several large pits are located at the grain boundaries; exfoliation corrosion finally occurs along with the large pit [31, 32]. This process with the influence of galvanic corrosion accelerates alloy’s corrosion. The SEM image obtained in the bonding surface (Figure 9(b)) shows a heterogeneous layer of products and cracked surface appears. Elements H and O are detected by EDS analysis. It is possible that an insoluble compound of aluminum, Al(OH)3, may form and deposit in the pits, which forms mud-cracks after being dried.

Figure 7 Cross-sectional views of FMLs after exfoliation corrosion:

Figure 8 Average exfoliation depth of different groups
During the exfoliation corrosion process, the surface of CARALL shows pitting corrosion and exfoliation corrosion on aluminum alloy with a large number of cracks and pits along the grain boundaries [33]. On the bonding interface, with the aluminum alloy corroded, crevice corrosion takes place and extends to central part. Corrosion products OH- in Al(OH)3 can be replaced by Cl- to form soluble AlCl3 on the surface [34]. But on the bonding interface, corrosion products mainly distribute along the grain boundaries in the corroded area due to a narrow environment at the interface, which increases interfacial stress and accelerates delamination. The crevice corrosion will directly lead to delamination and greatly destroy the sandwich structure, compared to pitting corrosion and exfoliation corrosion. As shown in Figure 10, the bare carbon fiber on the edge is the main cause of galvanic corrosion, and then induces the crevice corrosion at the interface of metal and composites. Corrosion occurs at the side edge expanding to the center region, which makes the top and bottom surfaces of metal layer corrode simultaneously. Besides, the corrosion products build expanding force on the interface, which will further accelerate delamination at the side edge. Consequently, the crevice corrosion on the bonding interface can greatly affect the CARALL during corrosion process. Crevice corrosion may make a greater impact on CARALL than galvanic corrosion.
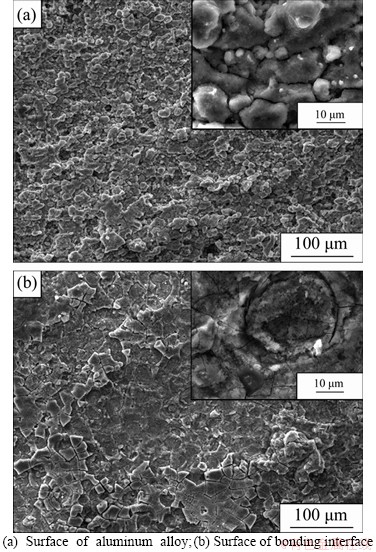
Figure 9 Microstructure of Group 1 after 96 h exfoliation corrosion:
3.4 Interlaminar shear strength
To directly investigate the effects of corrosion on CARALL, the residual interlaminar shear strength of specimens immersed in EXCO solution after 96 h was conducted. As shown in Figure 11, there is a remarkable decrease in ILSS of specimens in Group 1, Group 2 and Group 3 which were subjected to EXCO solution. Interlaminar shear strength of original material is (86.2±3.4) MPa. ILSSs of these groups are respectively measured as (23.5±2.5) MPa and (22.1±1.6) MPa, which have a decrease of more than 70% compared to original material. ILSS value of Group 3 is (42.2±2.4) MPa. For Group 4, the specimens are little affected from corrosion due to the protection of the polyurethanes, having (84.6±3.1) MPa interlaminar shear strength. It is known that interlaminar shear failure mode of FML is the result of the combined action of fiber fracture, metal fracture and interlaminar shear cracking [35]. During corrosion process, the surfaces of metal appear pitting corrosion and exfoliation corrosion, and the solution penetrates to the interior of CARALL along the composite metal interface. Besides, the composites may be affected by hygroscopic stress. It can be seen that the ILSS of specimens in Group 4 is less affected by corrosion. But the ILSSs of Group 1, Group 2 and Group 3 decrease by more than 50%. This indicates that the galvanic corrosion has quite an influence on the structure and properties of CARALL, and the side edge exposed to the solution intensifies these effects. It can be therefore assumed that the crevice corrosion is more severe than other corrosion effects.
In order to better understand the degradation law of residual ILSS (IL) of the CARALL after EXCO test, the ILSS and reduction proportion evolution were obtained as shown in Figure 12. The ILSS reduction(IR) was calculated by the following relationship:
 (3)
(3)
where S0 and Sx are the average ILSS of the specimens in Group 1 after immersing for 0 and x h. It can be observed that after immersing for 24, 48, 72 and 96 h strength of about 30.3%, 53.6%, 64.9% and 72.1% was lost, respectively. In the initial 48 h immersion, the ILSS decreases rapidly due to the pitting corrosion on the surface of aluminum alloy and crevice corrosion between composite and metal interface. In the last 48 h immersion, the ILSS decreases slowly. As discussed above, crevice corrosion has seriously reduced the interlaminar shear strength of CARALL at initial stage of corrosion process. But when the process lasts for a period of time, the dissolved oxygen in the crevice is blocked by corrosion products, reducing corrosion rate. The crevice corrosion behavior of CARALL in solution could be divided into three stages: an incubation period, a rapid development stage and a stable development stage. To simplify the model, the ILSS of specimens is regarded as a linear decrease with the increasing of immersing time. Consequently, the ILSS and ILSS reduction evolution was fitted, respectively:
IL=-0.597t+77.16 (4)
IR=0.0075t+0.0394 (5)
where t represents the immersing time. The results can provide a reference for the CARALL exposed in the serious environmental conditions.

Figure 10 Crevice corrosion of CARALL in electrolyte solution
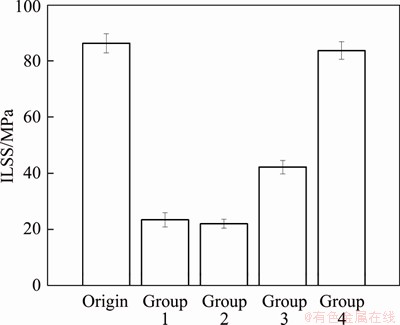
Figure 11 Residual ILSS of each group after 96 h exfoliation corrosion

Figure 12 Residual ILSS of G1 every 24 h and fitting curves
4 Conclusions
Experimental measurements were conducted on CARALL specimens to observe and analyze the corrosion behavior. Important findings from the current study are summarized as follows:
1) According to the electrochemical analysis, the corrosion potential can be affected by galvanic corrosion, and the corrosion current decreases due to the protection. When CARALL specimens were immersed in solution, the anodic polarization reaction is Al=Al3++3e, and the cathodic polarization reaction is O2+2H2O+4e=4OH-. The EIS results show that protect treatments have a positive effect on reducing corrosion. The exfoliation corrosion tests show that the surface of aluminum alloy suffers from pitting corrosion and exfoliation corrosion and the bonding interface is affected by crevice corrosion during immersion process.
2) The interlaminar shear strength of CARALL specimens is affected obviously by galvanic corrosion with a 72.1% decrease after 96 h immersion. The crevice corrosion has a greater impact on the ILSS properties of CARALL according to the results from the images analysis and ILSS tests. The adhesive layer cannot improve the corrosion resistance, but it is necessary to improve the comprehensive performance of CARALL. To protect the CARALL from galvanic corrosion, the main method is isolating the contact of carbon fiber and aluminum alloy as much as possible, especially at the side edge.
3) In addition, few electrochemical analyses were conducted in this study. More research needs to be done to improve the corrosion resistance of CARALL. And further research needs to be conducted to explore the corrosion behavior of CARALL in long-term corrosive environment by theory and experiment. Furthermore, to apply CARALL to reality, variety problems need to be investigated in addition to the galvanic corrosion problem.
Contributors
The overarching research goals were developed by WU Xin-tong, ZHAN Li-hua and HUANG Ming-hui. WU Xin-tong and ZHAO Guo-qing measured and analyzed the data. The initial draft of the manuscript was written by WU Xin-tong and WANG Xun. ZHAO Xing edited the draft of manuscript. All authors replied to reviewers’ comments and revised the final version.
Conflict of interest
WU Xin-tong, ZHAN Li-hua, HUANG Ming-hui, ZHAO Xing, WANG Xun and ZHAO Guo-qing declare that they have no conflict of interest.
References
[1] VLOT A, GUNNINK J W. Fibre metal laminates [M]. Netherlands: Kluwer Academic Publishers, 2001. ISBN: 978-1-4020-0038-6.
[2] SADIGHI M, ALDERLIESTEN R C, BENEDICTUS R. Impact resistance of fiber-metal laminates: A review [J]. International Journal of Impact Engineering, 2012, 49: 77-90. DOI: 10.1016/j.ijimpeng.2012.05.006.
[3] DADEJ K, BIENIAS J, SUROWSKA B. On the effect of glass and carbon fiber hybridization in fiber metal laminates: Analytical, numerical and experimental investigation [J]. Composite Structures, 2019, 220: 250-260. DOI: 10.1016/ j.compstruct.2019.03.051.
[4] CHEN Y, WANG Y, WANG H. Research progress on interlaminar failure behavior of fiber metal laminates [J]. Advances in Polymer Technology, 2020, 4(5): 1-20. DOI: 10.1155/2020/ 3097839.
[5] CHANDRASEKAR M, ISHAK M R, JAWAID M, LEMAN Z, SAPUAN S M. An experimental review on the mechanical properties and hygrothermal behaviour of fibre metal laminates [J]. Journal of Reinforced Plastics and Composites, 2016, 36(1): 72-82. DOI: 10.1177/073168441 6668260.
[6] JAKUBCZAK P, BIENIAS J, SUROWSKA B. The influence of fibre orientation in aluminium-carbon laminates on low-velocity impact resistance [J]. Journal of Composite Materials, 2018, 52(8): 1005-1016. DOI: 10.1177/00219983 17719569.
[7] SHI Y, PINNA C, SOUTIS C. Impact damage characteristics of carbon fibre metal laminates: Experiments and simulation [J]. Applied Composite Materials, 2020, 27(5): 511-531. DOI: 10.1007/s10443-020-09800-y.
[8] DHALIWAL G S, NEWAZ G M. Modeling low velocity impact response of carbon fiber reinforced aluminum laminates (CARALL) [J]. Journal of Dynamic Behavior of Materials, 2016, 2(2): 181-193. DOI: 10.1007/s40870-016- 0057-3.
[9] DADEJ K, BIENIAS J, SUROWSKA B. Residual fatigue life of carbon fibre aluminium laminates [J]. International Journal of Fatigue, 2017, 100: 94-104. DOI: 10.1016/ j.ijfatigue.2017.03. 026.
[10] GUPTA R, MAHATO A, BHATTACHARYA A. Strength and failure behavior of carbon fiber reinforced aluminum laminates under flexural loading [J]. Mechanics of Advanced Materials and Structures, 2020. DOI: 10.1080/15376494. 2020.1786754.
[11] ASGHAR W, NASIR M A, QAYYUM F, SHAH M, AZEEM M, NAUMAN S, KHUSHNOOD S. Investigation of fatigue crack growth rate in CARALL, ARALL and GLARE [J]. Fatigue & Fracture of Engineering Materials & Structures, 2017, 40(7): 1086-1100. DOI: 10.1111/ffe.12566.
[12] VERMEEREN C A J R. The application of carbon fibres in ARALL laminates [D]. Delft: Delft University of Technology, 1991: 1-62. https://repository.tudelft.nl/islandora/object/uuid: e146 63a0-6fe6-499d-b5dd-22e56ac8f127.
[13] SHAN M, GUO K, GOU G, FU Z, YANG B, LU W. Effect of anodizing on galvanic corrosion behavior of T300 CFRP/5083P-O Al bolted joints [J]. Materials and Corrosion, 2020, 71(3): 409-418. DOI: 10.1002/maco.201911235.
[14] HAKANSSON E, HOFFMAN J, PREDECKI P, KUMOSA M. The role of corrosion product deposition in galvanic corrosion of aluminum/carbon systems [J]. Corrosion Science, 2016, 114: 10-16. DOI: 10.1016/j.corsci.2016.10. 011.
[15] LI Sheng-xi, KHAN H A, HIHARA L H, CONG Hong-bo, LI Jing-jing. Corrosion behavior of friction stir blind riveted Al/CFRP and Mg/CFRP joints exposed to a marine environment [J]. Corrosion Science, 2018, 132: 300-309. DOI: 10.1016/j.corsci.2018.01.005.
[16] PENG Z, NIE X. Galvanic corrosion property of contacts between carbon fiber cloth materials and typical metal alloys in an aggressive environment [J]. Surface & Coatings Technology, 2013, 215(4): 85-89. DOI: 10.1016/j.surfcoat. 2012.08.098.
[17] VOGELESANG L B, VLOT A. Development of fibre metal laminates for advanced aerospace structures [J]. Journal of Materials Processing Technology, 2000, 103(1): 1-5. DOI: 10.1016/s0924-0136(00)00411-8.
[18] WANG Wen-xue, TAKAO Y, MATSUBARA T. Galvanic corrosion-resistant carbon fiber metal laminates [C]// The 16th International Conference on Composite Material. 2007: 1-10. http://iccmcentral.org/Proceedings/ICCM16proceedi ngs/contents/pdf/WedK/WeKM105ge_wangw224701p.pdf.
[19] BIENIAS J, ANTOLAK C, JAKUBCZK P, MAJERSKI K, SUROWSKA B. Corrosion studies of selected fiber meatal laminates with carbon and glass fibers [C]// The 19th International Conference on Composite Materials. 2013: 1-2. http://confsys.encs.concordia.ca/ICCM19/AllPapers/FinalVersion/SUR81670.pdf.
[20] MONDAL J, MARQUES A, AARIK L, KOZLOVA J, SIMOE A, SAMMELSELG V. Development of a thin ceramic-graphene nanolaminate coating for corrosion protection of stainless steel [J]. Corrosion Science, 2016, 105: 161-169. DOI: 10.1016/j.corsci.2016.01.013.
[21] WANG Yun-yan, LUO Yong-jian, XU Hui, XIAO Hai-juan. Corrosion behavior and electrochemical property of Q235A steel in treated water containing halide ions (F-, Cl- ) from nonferrous industry [J]. Journal of Central South University, 2020, 27(4): 1224-1234. DOI: 10.1007 /s11771-020-4362-6.
[22] ZHANG P, NIE X, NORTHWOOD D O. Influence of coating thickness on the galvanic corrosion properties of mg oxide in an engine coolant [J]. Surface and Coatings Technology, 2009, 203(20): 3271-3277. DOI: 10.1016/ j.surfcoat.2009.04.012.
[23] HE Jia-jia, YAN Hong, ZOU Yong-cheng, YU Bao-biao, HU Zhi. Microstructure and corrosion behavior of as-cast ADC12 alloy with rare earth Yb addition and hot extrusion [J]. Journal of Central South University, 2020, 27(6): 1654-1665. DOI: 10.1007/s11771-020-4397-8.
[24] CHARITHA B P, RAO P. Pullulan as a potent green inhibitor for corrosion mitigation of aluminum composite: Electrochemical and surface studies [J]. International Journal of Biological Macromolecules, 2018, 112: 461-472. DOI: 10.1016/j.ijbiomac.2018.01.218.
[25] SEE S C, ZHANG Z Y, RICHARDSON M. A study of water absorption characteristics of a novel nano-gelcoat for marine application [J]. Progress in Organic Coatings, 2009, 65(2): 169-174. DOI: 10.1016/j.porgcoat.2008.11.004.
[26] SCHEM M, SCHMIDT T, GERWANN J, WITTMAR M, VEITH M, THOMPSON G E, MOLCHAN I S, HASHIMOTO T, SKELDON P, PHANI A R, SANTUCCI S, ZHELUDKEVICH M L. Ceo-filled sol–gel coatings for corrosion protection of AA2024-T3 aluminium alloy [J]. Corrosion Science, 2009, 51(10): 2304-2315. DOI: 10.1016/ j.corsci.2009.06.007.
[27] WANG Xue-hui, WANG Ji-hui, YUE Xin, GAO Yun. Effect of aging treatment on the exfoliation corrosion and stress corrosion cracking behaviors of 2195 Al–Li alloy [J]. Materials and Design, 2015, 67: 596-605. DOI: 10.1016/ j.matdes.2014.11.007.
[28] LI B, PAN Q L, ZHANG Z Y, LI C. Research on intercrystalline corrosion, exfoliation corrosion, and stress corrosion cracking of Al–Zn–Mg–Sc–Zr alloy [J]. Materials & Corrosion, 2014, 64(7): 592-598. DOI: 10.1002/maco. 201206727.
[29] LI Shuai, GUO Dan, DONG Hong-gang. Effect of flame rectification on corrosion property of Al–Zn–Mg alloy [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(2): 250-257. DOI: 10.1016/S1003-6326(17)60029-3.
[30] ZHANG Jun-long, LI Jing-yuan, TIAN Shao-kun, LV Dan. Effects of solution treatment on microstructure transformation, tensile and exfoliation corrosion properties of 7136 aluminum alloy [J]. Journal of Materials Engineering and Performance, 2019, 28: 1312-1323. DOI: 10.1007/ s11665-019-03893-8.
[31] LIU Sheng-dan, LIAO Wen-bo, TANG Jian-guo, ZHANG Xin-ming, LIU Xin-yu. Influence of exfoliation corrosion on tensile properties of a high strength Al-Zn-Mg-Cu alloy [J]. Journal of Central South University, 2013, 20(1): 1-6. DOI: 10.1007/s11771-013-1451-9.
[32] ZHAO Jing-wei, LUO Bing-hui, HE Ke-jian, BAI Zhen-hai, LI Bin, CHEN Wei. Effects of minor Zn content on microstructure and corrosion properties of Al-Mg alloy [J]. Journal of Central South University, 2016, 23(12): 3051-3059. DOI: 10.1007/s11771-016-3368-6.
[33] ZHANG Teng, HE Yu-ting, CUI Rong-hong, AN Tao. Long-term atmospheric corrosion of aluminum alloy 2024-T4 in a coastal environment [J]. Journal of Materials Engineering and Performance, 2015, 24(7): 2764-2773. DOI: 10.1007/s11665-015-1541-y.
[34] COMEZ N, DURMUS H. Corrosion behavior and mechanical properties of cold metal transfer welded dissimilar AA7075-AA5754 alloys [J]. Journal of Central South University, 2020, 27(1): 18-26. DOI: 10.1007/ s11771-020-4274-5.
[35] ANDRZEJ K, TOMASZ T, MARIUSZ K, MAREK H, MACIEJ P. The influence of temperature gradient thermal shock cycles on the interlaminar shear strength of fibre metal laminate composite determined by the short beam test [J]. Composites Part B: Engineering, 2019, 176: 1-7. DOI: 10.1016/j. compositesb.2019.107217.
(Edited by YANG Hua)
中文导读
碳纤维增强铝合金层板的腐蚀损伤演变与力学性能
摘要:纤维金属层板是一种综合性能优异的轻量化材料,已经成功地应用于航空航天领域。其中,使用碳纤维增强的纤维金属层板比采用玻璃纤维或芳纶纤维的层板具有更好的力学性能。然而,碳纤维与金属的结合容易引起电偶腐蚀,极大地限制了其应用。本文采用电化学方法、光学显微镜和扫描电子显微镜等分析了碳纤维增强铝合金层板在腐蚀环境中的腐蚀演变,探讨了防止碳纤维增强铝合金层板发生腐蚀的保护方法。结果表明,碳纤维与铝合金的连接改变了电位从而引起电偶腐蚀,电偶腐蚀会明显导致碳纤维增强铝合金层板的腐蚀速度变快,导致层间剪切强度下降达到72.1%;同时,缝隙腐蚀也对层板的影响很大从而引起分层破坏。层间剪切强度的下降与腐蚀时间呈现出类似线性的关系。此外,碳纤维与铝合金之间的粘接层并不能起到保护作用,而侧边保护却可以有效地减缓层板腐蚀速度。因此,碳纤维增强铝合金层板应该在裸露的边部涂上防腐涂料。该材料有潜力用于航空航天部件。
关键词:碳纤维增强铝合金层板;电偶腐蚀;电化学测试;层间剪切强度;铝合金
Foundation item: Project(51675538) supported by the National Natural Science Foundation of China
Received date: 2020-07-17; Accepted date: 2021-01-07
Corresponding author: ZHAN Li-hua, PhD, Professor; E-mail: yjs-cast@csu.edu.cn; ORCID: https://orcid.org/ 0000-0001-9419-4149

