
Effect of Cr doping on secondary phases and electrical properties of zinc oxide ceramic thick film varistors
JIANG Sheng-lin(姜胜林), ZHANG Hai-bo(张海波), XIE Tian-tian(谢甜甜), FAN Mao-yan(范茂彦),
ZENG Yi-ke(曾亦可), L? Wen-zhong(吕文中)
Engineering Research Centre for Functional Ceramics, Department of Electrical Science and Technology, Huazhong University of Science and Technology, Wuhan 430074, China
Received 15 July 2007; accepted 10 September 2007
Abstract: In order to get high-performance low voltage varistors, Cr2O3 doped ZnO ceramic thick films were fabricated by modified sol-gel process. The precursors were fabricated by dispersing doped-ZnO ceramic nano-powders in the sols, which were prepared by dissolving zinc acetate dihydrate into 2-methoxyethanol and stabilized by diethanolamine and glacial acetic acid and doped with a concentrated solution of bismuth nitrate, phenylstibonic acid, cobalt nitrate, manganese acetate and chromium nitrate. The results show that ZnCr2O4 phase can form in ZnO based ceramic films doped 1.0% (mole fraction) Cr2O3. Three secondary phases, such as Bi2O3, Zn7Sb2O12, and ZnCr2O4 phases, are detected in the thick films. The Raman spectra show that the intensity and the position of Raman bands of Zn7Sb2O12 and ZnCr2O4 phases change obviously with increasing Cr2O3 doping. The nonlinearity coefficient α of ZnO thick films is 7.0, the nonlinear voltage is 6 V, and the leakage current density is 0.7 ?A/mm2.
Key words: sol-gel processs; thick films; ZnO; secondary phases; low voltage varistors
1 Introduction
ZnO ceramic thick films have great potentials and advantages of fabricating low-power low voltage varistors[1-3]. The effects of dopants on the properties of ZnO bulk ceramics and thin films have been widely studied, Bi2O3 and other glass materials accelerate the formation of the grain boundary, improve the density of the ceramics[4]. Sb2O3 can lower the leakage current density, and enhance the nonlinear coefficient α[5-7]. Al2O3 and Ga2O3 added as the donor can enhance the carrier density, decrease the resistivity, and improve the electrical properties of the film varistors in the large current rising region[8-9], while the doping of alkali metals such as K2O, Li2O, Na2O can form acceptor barrier in the ZnO grain boundary, and increase the nonlinear coefficient of the films[10-11]. The transition metal such as Co, Mn, and Ni can also improve the nonlinear coefficient[12].
However, to the best of my knowledge few researchers have investigated the influences of dopants on the low voltage nonlinear I—φ characteristics of the ZnO thick films, which are different from the previous ZnO bulk ceramics. The annealing temperature of ZnO-based ceramic films by sol-gel processing is lower than 850 ℃. The formation of the grain boundaries, and the secondary phases are different from those of ZnO bulk ceramics. The differences will affect the electrical properties of ZnO ceramic thick films. The electrical properties of ZnO film varistors can be influenced by many factors such as electrode materials[13-14], dopants[15], annealing temperature and film thickness. We have studied the electrical properties of the thick films at different annealing temperatures[16].
The electrical properties are affected strongly by the secondary phases, and the types of secondary phases formed depend on the amount and the type of additives. Therefore, the present work focuses on the effects of the dopants (especially Cr2O3) on secondary phases and the nonlinear I—φ characteristics of low voltage ZnO-based ceramic films. This will contribute to developing high-performance low-power low voltage ZnO ceramic thick film varistors.
2 Experimental
ZnO ceramic thick films were deposited on the Au/SiO2/Si substrates by a modified sol-gel process. The sols were prepared by zinc acetate dihydrate (Zn(CH3COO)2?2H2O)(chemical purity), dopants such as Bi(NO3)3?5H2O, Mn(CH3COO)2?4H2O, Co(NO3)2?6H2O, Cr(NO3)3?9H2O, Sb2O3 and the solvents, such as 2-methoxyethanol. Zn(CH3COO)2?2H2O and the dopants were first dissolved in 2-methoxyethanol by addition of diethanolamine (MEA) and glacial acetic acid at 60 ℃, respectively. The resultant solution was stirred at room temperature for more than 24 h to yield a clear, stable and homogeneous sol. The precursors were fabricated by dispersing the ZnO nano-powders in the sols. The films with 10-20 layers were deposited on Au/SiO2/Si by spining at 2 000 r/min for 30 s, and annealed at 550- 950 ℃ in air for 2 h. Then, part of the 5 μm thick films was corroded to reveal part of the lower electrodes. After the upper electrodes had been deposited by sputtering, the thick films were cut to several samples. The detailed processing has been reported in Ref.[17].
The I—φ characteristics of low voltage ZnO ceramic thick film varistors were measured by transistor characteristics tester, the nonlinear voltage ( ) and the leakage currents were measured by MY-4C varistors synthesize parameter-testing instrument. The phases of the samples were analyzed by RIGAKU D/max-3B X-ray diffractometer with Cu Kα radiation (30 kV, 30 mA). Raman spectra of the films were obtained by means of Renishaw System RM-1000. Raman spectra were excited with the 514.5 nm line of an Ar+ laser at an incident power of 20 mW and obtained in the range of 100-2 000 cm-1.
) and the leakage currents were measured by MY-4C varistors synthesize parameter-testing instrument. The phases of the samples were analyzed by RIGAKU D/max-3B X-ray diffractometer with Cu Kα radiation (30 kV, 30 mA). Raman spectra of the films were obtained by means of Renishaw System RM-1000. Raman spectra were excited with the 514.5 nm line of an Ar+ laser at an incident power of 20 mW and obtained in the range of 100-2 000 cm-1.
3 Results and discussion
Fig.1 shows the XRD patterns of the ZnO ceramic films doped with 0.5% Cr2O3 annealed at 750 ℃, the α-spinel and ZnCr2O4 phase is observed. In ZnO bulk ceramics, the formation of ZnCr2O4 phase is determinated by the content of Cr2O3 whether Sb2O3 exists or not. When the amount of Cr2O3 is no more than 1.0%, ZnCr2O4 phase can not form in the ZnO ceramics doped with Sb2O3[18], and only the amount of Cr2O3 is more than 5.0%, the ZnCr2O4 phase can be observed[19]. In ZnO thick films, ZnCr2O4 phase can form in the condition of a little amount of Cr2O3 doping. This indicates that Cr3+ and Zn2+ distribute in molecular level, and lead to the formation of ZnCr2O4 phase, which distributes at the grain boundaries, inhibiting the growth of ZnO grains, and reducing the concentration of Cr3+ in spinel phases. This suggests that the effect of Cr2O3 on the carrier density and barrier height at grain boundaries of ZnO thick films are lower than that of ZnO bulk ceramics.
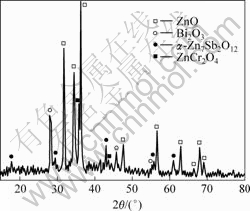
Fig.1 XRD patterns of ZnO films doped with 0.5% Cr2O3
Fig.2 shows the evolution nonlinear voltages nonlinear coefficient α, and the leakage current density of thick film varistors with Cr2O3 content. Cr2O3 doped in ZnO ceramic thick films plays an important role in the two ways during the annealing, one is stabilizing spinel phases, the other is inhibiting the growth of ZnO grains. β-spinel phase has a good stability during sintering but poor stability on cooling, and would transform to pyrochlore phase. Cr2O3 has the effect of inhibiting the formation of β-spinel phase. When ZnO thick films are sintered at high temperature, Cr3+ cations dissolve into Bi enrichment phases and α-spinel phases. The α-spinel phases involve with Cr3+, having a good stability not only in sintering procedure, but also in the cooling procedure[18]. Therefore, Cr2O3 has the effect of stabilizing spinel phases. The composition and stability of spinel phases has a notable effect on the electrical properties of the film varistors. The stability of spinel

Fig.2 Nonlinear voltages, coefficient and leakage current density of film varistors changed with Cr2O3 Content
phases lead to the diffusion and redistribution of dopants in thick film. These could change the features of ZnO grain and intergranular phases, and finally affect the electrical properties of ZnO ceramic thick films[20]. In addition, Cr2O3 inhibits the growth of ZnO grains, causing the refining of the ZnO and spinel grains[18], and leading to the increase of nonlinear voltage. Cr3+ ion dissolving in the spinel phases partly diffuses into ZnO lattice as donor, and increases the carrier density in ZnO thick films[21], decreases the barrier height of grain boundaries, thus leading to the increase of leakage current. Consequently, when the additive Cr2O3 is no more than 0.5%, the leakage current of the films increases but notably decreases with the increase of Cr2O3.
Fig.3 shows the Raman spectra of ZnO thick films with different dopants. The proportion of spinel phase formed in the films reaches the theoretical value when the films is only doped with Bi2O3 and Sb2O3 as seen in Fig.3(a), and the broad Raman peaks, 710.7 cm-1 and 715 cm-1 appear. The spinel phase peaks become stronger and sharper but the secondary peaks disappear with increasing MnO doped into the films, as seen in Fig.3(b).With Co2O3 and Cr2O3 continually doping into ZnO films, the spectra peaks move to low wave number side. When Co2O3 is added in the films, the spinel peak moves to 708 cm-1 and a subordinate peak appears at 716 cm-1. When Cr2O3 is added in the films, the spinel peak moves to 709 cm-1 and becomes stronger and sharper, indicating that the Zn7Sb2O12 spinel structure is notably affected by the dopants especially the trivalent cations. Because the trivalent cations enter the spinel octahedral structure and replace Zn2+, the spinel structure changes remarkably, which further proves the previous XRD results.

Fig.3 Raman spectra of Zn7Sb2O12 phase when ZnO based films doped with Bi2O3 and Sb2O3(a); Bi2O3, Sb2O3 and MnO(b); Bi2O3, Sb2O3, MnO and Co2O3(c); and Bi2O3, Sb2O3, MnO and Cr2O3(d)
Fig.4 shows the Raman spectra of ZnO based films doped with different contents of Cr2O3, the primary ZnCr2O4 peaks appear at 825 cm-1 and 826 cm-1. With increasing amount of Cr2O3, ZnCr2O4 peaks position shifts 1 cm-1, and the peaks become stronger and sharper, which indicates that the increasing Cr2O3 doping leads to ZnCr2O4 phase growing larger. In all of the samples doped with different oxides, the lattice of the films doped with Cr2O3 is the smallest, which indicates that the ZnO films are mainly affected by Cr3+ cations. Because Cr3+ cations have higher octahedral activation energy than the other dopants cations, and are very easy to substitute Zn2+ cations. The peak at 709 cm-1 is ascribed to Zn7Sb2O12 and shifts to 713 cm-1.
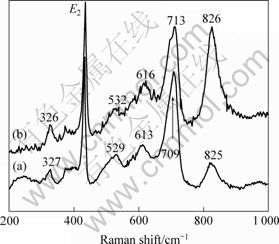
Fig.4 Raman spectra of ZnO based films doped with 0.25% Cr2O3(a) and 0.5% Cr2O3(b)
The dopant cations form intergranular phases at ZnO grain boundary and diffuse to adjacent ZnO lattice to substitute part of the Zn2+. The substitution would cause the distortion of the lattice and results in increasing carrier density. Because the diffusion of additive cations almost occurs at adjacent of grain boundary, the donor density at grain boundary is much higher than that in grain. The Zn7Sb2O12 and ZnCr2O4 secondary phases form quickly at lower annealing temperature, which leads to the formation of stable grain boundaries. In this procedure, the other cantions such as Mn2+, Mn4+ and Cr3+ diffused into the secondary phase lattices due to substitution and diffustion, which would cause the lattice distortion. Therefore, besides the distortion of the lattice caused by the substitution of the dopant cations, the surface lattice distortion of the ZnO lattice caused by the formation of secondary phases in grains is considered to be the main origin of the properties changes of ZnO based thick films.
4 Conclusions
1) Thanks to dopants mixing in molecular level by modified sol-gel process, the formation of ZnCr2O4 phase of the films doped with little Cr2O3 is very easy and reduces the amount of Cr3+ dissolved into the spinel phases.
2) Cr3+ diffuses into ZnO grains and decreased the barrier height of grain boundaries, and leads to increase of leakage current, consequently, the Cr2O3 content is no more than 0.5%.
3) Raman spectra indicate three secondary phases, such as Bi2O3, Zn7Sb2O12 spinel, and ZnCr2O4 phases, appear in ZnO thick films. The formation of ZnCr2O4 phase in the films at low temperature is of great advantage to stabilize the Zn7Sb2O12 spinel phase, and improve ZnO grain boundary features as well. The peak of 709 cm-1 is ascribed to Zn7Sb2O12 and shifts to 713 cm-1 with Cr2O3 doping.
4) When the amounts of additives Bi2O3, Sb2O3, MnO and Cr2O3 are 0.5%, 1.5%, 0.5% and 0.75%, respectively, the nonlinearity coefficient α of ZnO thick films is 7.0, the nonlinear voltage is 6 V, and the leakage current density is 0.7 ?A/mm2.
References
[1] SUZUOKI Y, OHKI A, MIZUTANI T. Electrical properties of ZnO-Bi2O3 thin-film varistors[J]. J Phys D, 1987, 20(4): 511-517.
[2] HORIO N, HIRAMATSU M, NAWATA M, LMAEDA K, TORII T. Preparation of zinc oxide/metal oxide multilayered thin films for low-voltage varistors[J]. Vacuum, 1998, 51(4): 719-722.
[3] DE LA RUBIA LOPEZ M A, PEITEADO M, FERNANDEZ J F, CABALLERO A C, HOLC J, DRNOVSEK S, KUSCER D, MACEK S, KOSEC M. Thick film ZnO based varistors prepared by screen printing[J]. J Eur Ceram Soc, 2006, 26(14): 2985-2989.
[4] GUPTA T K. Application of zinc oxide varistors[J]. J Am Ceram Soc, 1990, 73(7): 1817-1840.
[5] BERNIK S, DANEU N, RECNIK A. Inversion boundary induced grain growth in TiO2 or Sb2O3 doped ZnO-based varistor ceramics[J]. J Eur Ceram Soc, 2004, 24(15/16): 3703-3708.
[6] KIM J, KIMURA T, YAMAGUCHI T. Sintering of zinc oxide doped with antimony oxide and bismuth oxide[J]. J Am Ceram Soc, 1989, 72(8): 1390-1395.
[7] KIM J, KIMURA T, YAMAGUCHI T. Sintering of zinc oxide doped with antimony oxide and bismuth oxide[J]. J Am Ceram Soc, 1989, 72(8): 1390-1395.
[8] NORTON D P, HEO Y W, IVILL M P, IP K, PEARTON S J, CHISHOLM M F, STEINER T. ZnO: Growth, doping and processing[J]. Mater Today, 2004, 7(6): 34-40.
[9] GILBERT I, FREER R. Donor and acceptor doping of zinc oxide varistors[J]. J Phys: Condens Matter, 2002, 14(4): 945-954.
[10] GUPTA T K, MILLER A C. Improved stability of the ZnO varistor via donor and acceptor doping at the grain boundary[J]. J Mater Res, 1988, 3(4): 745-754.
[11] WATARI T, BRADT R C. Grain growth of sintered ZnO with alkali oxide additions[J]. J Ceram Soc Jpn, 1993, 101(1178): 1085-1089.
[12] BERNIK S, ZUPANCIC P, KOLAR D. Influence of Bi2O3/TiO2, Sb2O3 and Cr2O3 doping on low-voltage varistor ceramics[J]. J Eur Ceram Soc, 1999, 19(6/7): 709-713.
[13] LAVROV R I, IVON A I, CHERNENKO I M. Comparative characteristics of silver and copper electrodes on ZnO varistor ceramics[J]. J Eur Ceram Soc, 2004, 24(9): 2591-2595.
[14] IP K, GILA B P, ONSTINE A H, LAMBERS E S , HEO Y W, BAIK K H, NORTON D P, PEARTON S J, KIM S, LAROCHE J R, REN F. Improved Pt/Au and W/Pt/Au Schottky contacts on n-type ZnO using ozone cleaning[J]. Appl Phys Lett, 2004, 84(25): 5133-5135.
[15] PEARTON S J, NORTON D P, IP K, HEO Y W, STEINER T. Recent progress in processing and properties of ZnO[J]. Prog Mater Sci, 2005, 50 (3): 293-340.
[16] JIANG S L, ZHANG H B, HUANG Y Q, LIU M D, LIN R Z. Effect of annealing temperature of a modified sol-gel process on the electrical properties of low voltage ZnO-based ceramic films[J]. Mater Sci Eng B, 2005, 117(3): 317-320.
[17] HUANG Y Q, LIU M D, ZENG Y K, LI C R, XIA D L, LIU S B. Preparation and properties of ZnO-based ceramic films for low-voltage varistors by modified sol-gel process[J]. Mater Sci Eng B, 2001, 86(3): 232-236.
[18] CHO S G, LEE H, KIM H S. Effect of chromium on the phase evolution and microstructure of ZnO doped with bismuth and antimony[J]. J Mater Sci, 1997, 32(16): 4283-4287.
[19] SHIMIZU Y, LIN F C, TAKAO Y, EGASHIRA M. Zinc oxide varistor gas sensors: Ⅱ. Effect of chromium(Ⅲ) oxide and yttrium oxide additives on the hydrogen-sensing properties[J]. J Am Ceram Soc, 1998, 81(6): 1633-1643.
[20] BRANKOVI? Z, BRANKOVI? G, POLETI D, VARELA J A. Structural and electrical properties of ZnO varistors containing different spinel phases[J]. Ceram. International, 2001, 27(1): 115-122.
[21] KIM Y H, KAWAMURA H, NAWATA M. Effect of Cr2O3 additive on the electrical properties of ZnO varistor[J]. J Mater Sci, 1997, 32(6): 1665-1670.
(Edited by LONG Huai-zhong)
Foundation item: Project(2004CB619300) supported by the Basic Research Development Program of China; Project(NCET-04-0703) supported by the Program for New Century Excellent Talents in University
Corresponding author: ZHANG Hai-bo; Tel: +86-27-87542693; E-mail: hbzhang@smail.hust.edu.cn