利用Cyphos IL101从废液晶显示器中回收铟的湿法冶金工艺的开发
来源期刊:中国有色金属学报(英文版)2020年第9期
论文作者:Sumitra NAYAK Niharbala DEVI
文章页码:2556 - 2567
关键词:铟;萃取;分离;Cyphos IL 101;液晶面板
Key words:indium; extraction; separation; Cyphos IL 101; liquid crystal display panel
摘 要:本研究的主要目的是从废液晶显示面板中回收铟。为此,研究一种离子液体Cyphos IL101。首先,考察平衡时间、酸浓度、氯离子浓度、氢离子浓度等萃取参数对Cyphos IL 101萃取In(III)萃取效率的影响。用0.005 mol/L Cyphos IL 101在2.0 mol/L HCl中定量萃取铟,并用1.0 mol/L H2SO4定量反萃。用Job法确定萃合物组成,为R3R''''PInCl4(R=C6H13; R′=C14H29)。基于多金属(萃取)研究的结果,Cyphos IL 101进一步用于从废液晶显示器浸出液中去除铟、锡和铜。获得从废液晶显示器中回收铟的优化条件。为了分离金属离子铟、锡和铜,根据McCabe-Thiele分析,进行逆流萃取和选择性反萃。为了从进料中完全除去锡,在O/A比为1:3的条件下进行两段萃取,并用0.1 mol/L H2SO4进行铟和锡的选择性反萃。提出从废液晶显示器中分离铟的工艺流程。
Abstract: The main goal of this study was to recover indium from the waste liquid crystal display (LCD) panel. In this context, an ionic liquid Cyphos IL 101 was explored. The extraction parameters such as equilibration period, acid concentration, chloride ion concentration, hydrogen ion concentration were examined on the extraction efficiency of Cyphos IL 101 towards In(III). Quantitative extraction of indium was found at 2.0 mol/L HCl using 0.005 mol/L Cyphos IL 101 and quantitative stripping with 1.0 mol/L H2SO4. Job’s method was used to determine the extracted species and R3R''''PInCl4 (R=C6H13; R′=C14H29) was proposed. Based on the observations on multi-metal studies, Cyphos IL 101 was further employed for the removal of indium, tin and copper from the leach liquors of waste LCDs. Optimized conditions were generated for the recovery of indium from waste LCDs. McCabe-Thiele diagram analysis, counter-current extraction and selective stripping were carried out to separate the metal ions, i.e., indium, tin and copper. Two stages at O/A ratio of 1:3 were required for complete removal of tin from the feed and selective stripping of In and Sn was achieved using 0.1 mol/L H2SO4. A scheme for separating indium from the waste LCDs was proposed.
Trans. Nonferrous Met. Soc. China 30(2020) 2556-2567
Sumitra NAYAK1, Niharbala DEVI1,2
1. Department of Chemistry, Institute of Technical Education and Research, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar 751030, India;
2. Biofuels and Bioprocessing Research Center, Institute of Technical Education and Research, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar 751030, India
Received 3 January 2020; accepted 18 May 2020
Abstract: The main goal of this study was to recover indium from the waste liquid crystal display (LCD) panel. In this context, an ionic liquid Cyphos IL 101 was explored. The extraction parameters such as equilibration period, acid concentration, chloride ion concentration, hydrogen ion concentration were examined on the extraction efficiency of Cyphos IL 101 towards In(III). Quantitative extraction of indium was found at 2.0 mol/L HCl using 0.005 mol/L Cyphos IL 101 and quantitative stripping with 1.0 mol/L H2SO4. Job’s method was used to determine the extracted species and R3R'PInCl4 (R=C6H13; R′=C14H29) was proposed. Based on the observations on multi-metal studies, Cyphos IL 101 was further employed for the removal of indium, tin and copper from the leach liquors of waste LCDs. Optimized conditions were generated for the recovery of indium from waste LCDs. McCabe-Thiele diagram analysis, counter-current extraction and selective stripping were carried out to separate the metal ions, i.e., indium, tin and copper. Two stages at O/A ratio of 1:3 were required for complete removal of tin from the feed and selective stripping of In and Sn was achieved using 0.1 mol/L H2SO4. A scheme for separating indium from the waste LCDs was proposed.
Key words: indium; extraction; separation; Cyphos IL 101; liquid crystal display panel
1 Introduction
In the world scenario, the fastest growing waste stream is waste electronic and electrical equipment (WEEE). It is growing day by day in huge amount across the world for which it is considered as a real secondary mine known as urban mine. Base metals, rare earth elements, precious metals and critical elements can be reclaimed from the above waste. One critical element indium is present in the liquid crystal displays (LCDs) in the form of indium tin oxide (ITO) which belongs to WEEE and widely used in TV screens, desktops, laptops, mobile phones, signboards and any type of display screen boards. The lifetime of the above-mentioned goods is 6-7 years generating a large amount of waste. ITO is a mixture of 80%-90% of In2O3 and 10%-20% of SnO2 [1,2]. Around 65% of the world’s indium production has been used for making ITO films which are used in LCDs [3]. Indium has no primary source and it is derived as a by-product from other metallurgical processes or secondary raw materials. It is generally found in sphalerite, ores of copper, lead and tin [4,5]. Approximately 250 μg/g of indium is present in LCD screens while in sphalerite it is within 2-100 μg/g [6]. LCD screens may be considered as a potential source of indium. Various reports have been stated for indium recovery from ITO through various routes [7-11]. But the most versatile and flexible technology is the hydrometallurgical route because of low cost and low energy consumption where solvent extraction plays a key role.
The review of scientific literature about the recovery of indium from LCDs following solvent extraction processes has been discussed here. YANG et al [6] reported the separation of indium from HCl or H2SO4 solution using DEHPA (di-2-ethylhexyl phosphoric acid), TBP (tri-butyl phosphate), Cyanex 272 (2,4,4-trimethylpentyl phosphinic acid) and Cyanex 923 (mixture of four trialkyl-phosphine oxides). On extraction with 0.1 mol/L DEHPA and stripping with 1 mol/L HCl, indium recovery was 99% with 93% purity. Another study reported the extraction of indium and tin from LCD panels in H2SO4, HNO3 and HCl media using 1 mol/L D2EHPA, 1 mol/L TBP and a mixture of 0.2 mol/L D2EHPA and 0.8 mol/L TBP [12]. In HNO3 leaching solution, the separation of tin and indium was not noticed, and the separation factor increased with increase in HCl concentration; however, 20% co-extraction of tin was obtained. The flow-sheet was developed with H2SO4 leaching solution. The recovery of indium and yttrium from HCl-leached flat panel display waste using Cyanex 923 and DEHPA was studied. It was reported that indium was separated from the leachate by extraction with 0.25 mol/L Cyanex 923 in kerosene followed by stripping with 1 mol/L HNO3. In the meantime, yttrium was recovered upon extraction with 0.2 mol/L DEHPA in kerosene followed by stripping with 2 mol/L HCl. The purities of the final products of indium and yttrium were 95% [13]. Similarly, the extraction of indium from the etching solution of indium tin oxide using 0.3 mol/L D2EHPA was studied and reported that the extraction efficiency of indium reached 92% with 8.62 g/L of indium in the organic phase at an O/A ratio (volume ratio) of 1:1 and quantitative stripping was conducted at O/A ratio of 1:2 using 2 mol/L HCl [14]. Indium separation from other impurities was optimized by varying O/A ratio, pH and extractant concentration. Satisfactory separation of aluminium, cerium and iron from indium was achieved in the pH range of 1-1.5 at room temperature using 0.3 mol/L D2EHPA. D2EHPA was also used as an extractant to recover indium from the leaching solution of LCD panels of discarded mobile phones [15]. The best condition reported for the process was A/O ratio of 2:5, 20 min contact time with solution of pH 0.5 and the D2EHPA extractant concentration of 30% (volume fraction). Individual extraction of In(III) using various extractants was also studied [16-21].
A class of extractants called ionic liquids (ILs) have been widely reported; however, few reports are available on indium extraction by ILs [22]. These ILs have exclusive features like low vapour pressure, nonflammability, high thermal stability and low melting point [23] and are made up of a cation and an anion [24,25] and it is also possible to tune the physicochemical properties of ILs [26]. Extractions of many metal ions were reported using different ILs [27-39]. Different forms of Aliquat 336 diluted with kerosene were applied for the extraction and separation of Co(II) and Ni(II) from acidic sulfate solutions. The extraction rates of metal ions were found to increase with increasing pH and extractant concentration. The stripping of the two metal ions from the organic phase was also investigated. The optimized condition was employed for the separation and recovery of Co(II), Ni(II) and In(III) from Ni-MH battery which was leached by 2.0 mol/L H2SO4. A flow sheet was developed from the experimental observations [35]. Also, bifunctional IL (trioctylmethy lammonium/ 2,4,4-trimethylpentyl phosphinate, A336/Cy272) in kerosene was used to extract and separate copper from other base metal ions as investigated by DEVI [37]. The study concluded that, the IL could extract copper more efficiently than the individual extractant Aliquat 336 or Cyanex 272. 0.1 mol/L H2SO4 was found as the stripping agent for complete removal of copper from the loaded organic phase. The extraction rates of metal ions from a synthetic solution containing copper with other metal impurities followed an order Fe>Zn> Cu>Cd>Co>Ni. Another study on the separation and recovery of V(V) and Cr(III) from acidic sulfate leaching liquors of ilmenite using 0.4 mol/L Aliquat 336 in kerosene revealed that the extraction process was pH dependant [39]. The metal ions V(V) and Cr(III) were precipitated at different pH ranges, forming ammonium metavanadate (pH 7.5-8.0) and chromium hydroxide (pH 9.0-10.0). On separation and calcination of both precipitates at 500 °C, the chemical compositions of corresponding oxides, i.e. V2O5 and Cr2O3 were reported to be 97.34% V2O5, 0.59% Cr2O3, 0.25% Al2O3 and ≤1.82% of other impurities, and 98.03% Cr2O3, 1.29% Al2O3 and ≤0.68% of other impurities, respectively. Phosphonium ILs under the trademark of Cyphos ILs are available by Cytec industries namely Cyphos IL 101, Cyphos IL 104 and Cyphos IL 109. These ILs do not release fluorinated anion to the aqueous phase unlike ILs containing fluorinated anions and possess high thermal and chemical stability, less toxicity than ammonium-based ILs [40]. Trihexyl (tetradecyl) phosphonium chloride (Cyphos IL 101) and trihexyl (tetradecyl) phosphonium bis-2,4,4-(trimethylpentyl) phosphinate (Cyphos IL 104) were used for the extraction of various transition metals [41-46], rare earth metals [47,48] and rare metals [49,50]. CIESZYNSKA and WISNIEWSKI [41] studied the extraction of Pd(II) from HCl solution using Cyphos IL 101 diluted in toluene and reported that the extraction was fast, efficient and dependent upon HCl concentration. The extraction rate decreased from 97% to 54% while changing HCl concentration from 1.0 to 3.0 mol/L. 0.5 mol/L NH4OH was the stripping agent used for back- extraction. The extraction of rare earth metals, i.e. trivalent lanthanides (La(III), Nd(III), Gd(III), and Lu(III)) was studied by KUMARI et al [48] using Cyphos IL 104 diluted in kerosene from chloride medium and the result indicated that very high extractability of Cyphos IL 104 towards trivalent lanthanides was obtained, also the stripping of loaded organic phase was good in dilute HCl. Further, the extraction of rare metal Ga(III) was investigated using Cyphos IL 104 diluted in toluene from chloride medium [49]. Results as reported by the authors suggested that Cyphos IL 104 was an efficient extractant for extraction of Ga(III) and 99.8% extraction rate of Ga(III) was observed with 3 mol/L HCl. The proposed extracted complex was found to be 1:1 (metal:ionic liquid complex, molar ratio) and the extracted species was reported as R3R′PGaCl4.
In the present work, an attempt has been made to extract indium from the liquid crystal display (LCD) panel using Cyphos IL 101. Initially, the extraction behavior of Cyphos IL 101 with In(III) from acidic chloride medium was investigated where the effects of various parameters like equilibration period, acid concentration, extractant concentration, metal concentration, chloride ion concentration, hydrogen ion concentration, Job’s method, temperature and presence of other metals were studied. Upon finding the optimum conditions, it was extended to recover indium present in the LCD panel with selective extraction and separation of tin, indium and copper. McCabe-Thiele plot, counter-current extraction and selective stripping were studied and reported. Each step was represented in a flow-sheet.
2 Experimental
2.1 Reagents and material
The stock solution of indium (III) (In(III)) was prepared by dissolving indium chloride (>99% purity, Sigma Aldrich) in double-distilled water. 5 mL of concentrated hydrochloric acid was added to prevent hydrolysis. Ionic liquid trihexyl (tetradecyl) phosphonium chloride (Cyphos IL 101) was provided by Cytec Industries Inc., Canada (Now Cytec Solvay). Kerosene was used as the diluent to get the desired sample concentrations. Analytical grade chemicals were used without further purification in the experiments. Waste LCD which is the part of a discarded computer used for the study was collected from the IT shop at Bhubaneswar. The collected computers were dismantled physically and the LCD screen containing indium was used for the experimental work.
2.2 Methodology and measurement
In a separating funnel, 10 mL of the organic and aqueous phases were taken and vigorously shaken for 10 min except for shaking time variation study. All the experiments were carried out at room temperature (27±1) °C except for temperature variation study. The aqueous phase after extraction named as raffinate was collected after phase disengagement and the metal ion concentration was analyzed by inductively coupled plasma mass spectroscopy (ICP-MS) of Perkin Elmer model- NexION 300X. Using mass balance, the concentration of the metal ion in the organic phase was calculated. The average values of the triplicate experiments were reported for result and discussion. The FTIR spectra of Cyphos IL 101 and indium loaded Cyphos IL 101 were recorded using FTIR spectrometer (Model-Cary-660) of Agilent Technologies.
3 Results and discussion
3.1 Extraction of indium using Cyphos IL 101
3.1.1 Determination of equilibration period
The time required to attain equilibrium was an important factor in liquid-liquid extraction. To find out this, the extraction of 0.001 mol/L In(III) was carried out from 1 mol/L HCl using 0.005 mol/L Cyphos IL 101 in kerosene at different time intervals. It was found that the extraction rate increased up to 5 min and then remained constant afterwards, which indicated that equilibrium was achieved within 5 min for In(III) with 0.005 mol/L Cyphos IL 101 (Fig. 1). So, in each of the experiments, the shaking time of 10 min was kept constant to presume complete equilibrium.
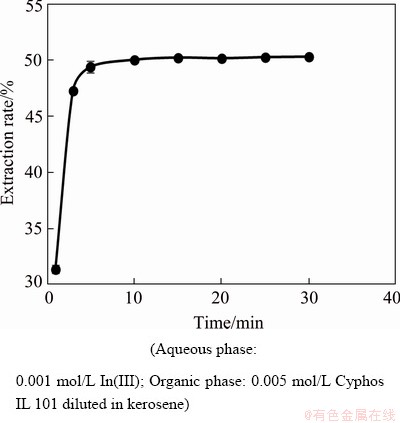
Fig. 1 Effect of time on extraction rate of In(III) using 0.005 mol/L Cyphos IL 101 in kerosene
3.1.2 Influence of HCl concentration
The extraction rate of the extractant with the metal ion sometimes depends on the concentration of acid used in the aqueous phase. For substantiation, experiments were carried out using organic phase concentration of 0.005 mol/L and an aqueous solution containing 0.001 mol/L In(III) and the HCl concentration was varied from 0.001 to 5.0 mol/L (Fig. 2). From the plot of extraction rate versus lg [HCl] (Fig. 2), it was observed that the extraction rate of indium increased with increase in HCl acid concentration up to 2 mol/L with a extraction rate of 97.2% and remained constant thereafter up to 5 mol/L HCl.
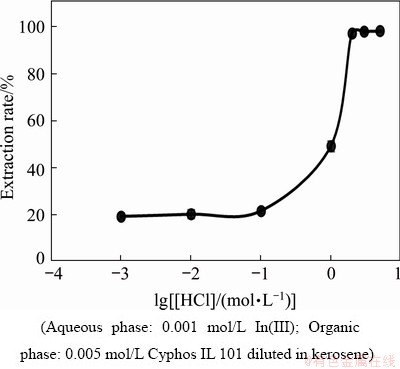
Fig. 2 Effect of HCl concentration on extraction rate of In(III)
3.1.3 Prediction of extracted species
The composition of the extracted species was studied by many researchers using several stoichio- metric coefficients obtained by the distribution data from various experimental parameters. In this case, the Job’s method was explored to predict the nature of the extracted indium species. One set of experiment was carried out where the concentrations of indium and Cyphos IL 101 were varied, keeping the total concentration fixed at 0.01 mol/L with 2.0 mol/L HCl concentration in the aqueous phase. It can be seen from the plot of indium extracted into the organic phase (in mg/L) versus mole fraction of Cyphos IL 101 (Fig. 3) that an extracted complex with molar ratio of metal/ionic liquid complex being 1:1 was formed with indium and Cyphos IL 101 extractant [51]. Then, two sets of experiments were carried out to verify this. One was the loading experiment which was performed keeping the extractant concentration at 0.005 mol/L and varying the indium concentration from 0.001 to 0.01 mol/L and maintaining the aqueous acidity at 2.0 mol/L. Figure 4 represents the plot of the ratio of initial extractant concentration [Cyphos IL 101]i and indium concentration extracted to the organic phase [In(III)]org versus initial indium concentration, and it was observed that the concentration ratio gradually decreased and reached 1.0 with increase in concentration of indium which supported the formation of the complex with molar ratio of metal/ligand being 1:1. Then, the extractant concentration variation study was carried out where the metal ion concentration was kept at 0.001 mol/L and the Cyphos IL 101 concentration in the range from 0.001 to 0.01 mol/L. The plot of lg D (D is the distribution coefficient) vs lg [Cyphos IL 101] gave a slope of 0.91, supporting the participation of 1 mol of ionic liquid corresponding to 1 mol of indium in the extraction process (Fig. 5).
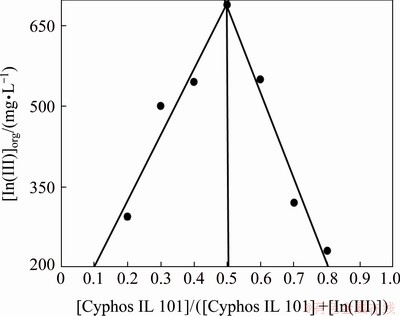
Fig. 3 Job’s plot for In(III) ions ([Cyphos IL 101] + [In(III)]=0.01 mol/L)
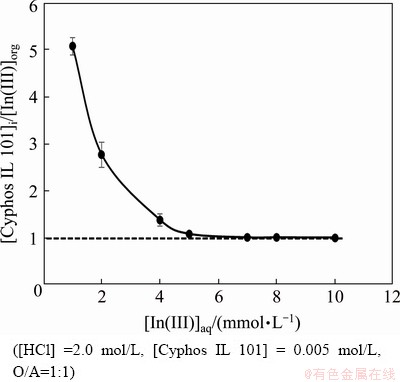
Fig. 4 Loading test results for In(III) on Cyphos IL 101
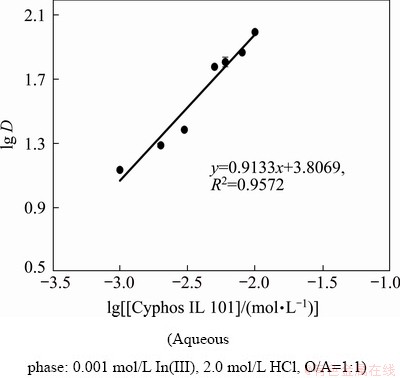
Fig. 5 Plot of lg D vs lg [Cyphos IL 101]
3.1.4 Effect of chloride ion concentration
The effect of chloride ion concentration on indium extraction was studied using 0.005 mol/L Cyphos IL 101 in the chloride concentration range of 1.0 (only HCl) to 3.0 mol/L (with addition of NaCl). It was found that the extraction rate of indium increased with increase of chloride concentration from 47.2% to 96.0%. lg-lg plot of distribution coefficient and chloride ion concentration from both hydrochloric acid and sodium chloride yielded a slope of 3.01 (Fig. 6), indicating participation of three chloride ions in the extraction process.
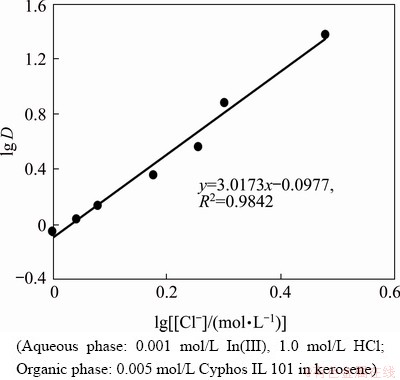
Fig. 6 Plot of lg D vs lg [Cl-] for indium extraction
3.1.5 Effect of hydrogen ion concentration
The influence of hydrogen ion concentration on the extraction of indium was studied in the manner where, the hydrogen ion concentration was varied from 0.001 to 2.0 mol/L whilst the chloride ion was kept constant by adding required amount of sodium chloride. Figure 7 represents the plot of lg D vs lg [H+]. The obtained slope value confirmed that H+ was not involved during the extraction of indium with Cyphos IL 101.
3.1.6 Extraction mechanism
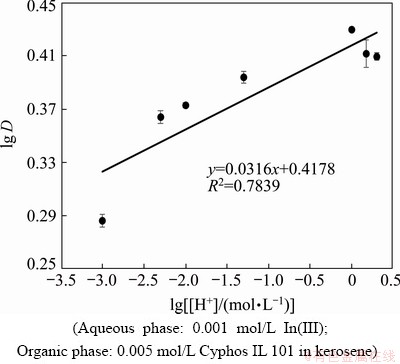
Fig. 7 Plot of lg D vs lg [H+] for indium extraction with Cyphos IL 101
In the extraction of In(III) from HCl acid and chloride medium using acidic and solvating type of extractants, In3+, InCl2+ and InCl3 were considered to be the predominant species in the weak and moderate chloride media [18,20,52,53]. The extraction with long chain amines from chloride medium suggested the presence of species containing 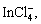
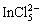 in the organic solution [54-56]. Our results in terms of slope values obtained from the Job’s plot, extractant, chloride ion and hydrogen ion concentrations studies proposed the following extraction process for indium extraction with Cyphos IL 101.
in the organic solution [54-56]. Our results in terms of slope values obtained from the Job’s plot, extractant, chloride ion and hydrogen ion concentrations studies proposed the following extraction process for indium extraction with Cyphos IL 101.
In3+aq+3Cl-aq+[R3R′PCl]org [R3R′PInCl4]org (1)
[R3R′PInCl4]org (1)
From the FTIR spectra of neat Cyphos IL 101 and In-loaded Cyphos IL 101 (Fig. 8), it was found that the peaks pertaining to P—C stretching in Cyphos IL 101 at 1034, 1095 and 1446 cm-1, were shifted to 1063, 1088 and 1442 cm-1 in case of In-loaded Cyphos IL 101, respectively. All these observations suggested that the complex R3R′PInCl4 was formed in the organic phase.
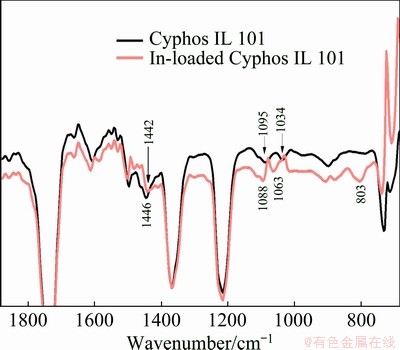
Fig. 8 FTIR spectra of 0.05 mol/L Cyphos IL 101 and 0.007 mol/L In-loaded Cyphos IL 101
3.1.7 Effect of temperature
The variation of temperature has a considerable effect on the extraction of metal ions. Ionic liquids are high molecular mass compounds and highly viscous. As viscosity is inversely proportional to temperature, the increase in temperature decreases the viscosity of the ionic liquid and favors mass transfer [57,58]. Experiments were carried out varying the temperature from 27 to 60 °C using 0.005 mol/L Cyphos IL 101 with 0.001 mol/L InCl3 from 1 mol/L HCl. The distribution of indium with experimental conditions is shown in Fig. 9. It was found that the indium extraction was enhanced with increase in temperature. For example, the D value was 1.06 at 27 °C (300 K) and increased to 1.78 at 60 °C (333 K). Using the slope and intercept values of Fig. 9, the thermodynamic parameters ΔHΘ and ΔSΘ were calculated using Eq. (2) [42] and found to be 12.71 kJ/mol and 87.33 J/(K·mol), respectively.
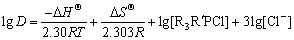 (2)
(2)
Endothermic and entropy driven process has been envisaged from the positive value of ΔHΘ and ΔSΘ, respectively.
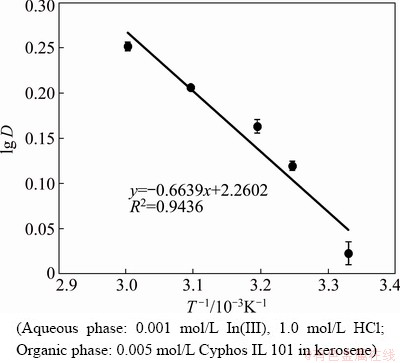
Fig. 9 Effect of temperature on indium extraction
3.1.8 Effect of stripping agents and stability of Cyphos IL 101
Table 1 Competencies of various stripping agents on stripping of In(III) from loaded Cyphos IL 101
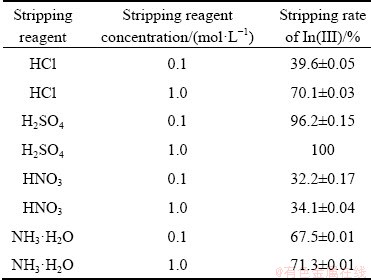
The reuse of extractants has been very important in solvent extraction as it minimizes the cost of the plant operation and also reduces the pollution. Various stripping agents were engaged for re-extraction of indium from the loaded organic phase containing 556 mg/L of indium. From Table 1, it was found that (96.2±0.15)% and 100% stripping rates were obtained with 0.1 and 1.0 mol/L H2SO4, respectively. Nearly 70% stripping rates were found with 1.0 mol/L HCl and 1.0 mol/L NH3·H2O, respectively. The stability of Cyphos IL 101 using 1.0 mol/L H2SO4 was studied in the following manner. 10 mL of 0.005 mol/L Cyphos IL 101 was contacted with 10 mL fresh 1.0 mol/L H2SO4 up to 10 times and the dissolved phosphorus was analyzed by means of ICP-MS. From Fig. 10, it could be seen that the solubility of the phosphonium cation was low, i.e. 0.31 mg/L,through ten fresh contacts, which suggested that the loss of extractant to the raffinate was minimal after one conditioning step.

Fig. 10 Stability of 0.005 mol/L Cyphos IL 101 in 1 mol/L H2SO4 at O/A of 1:1
3.1.9 Separation of binary metal ions
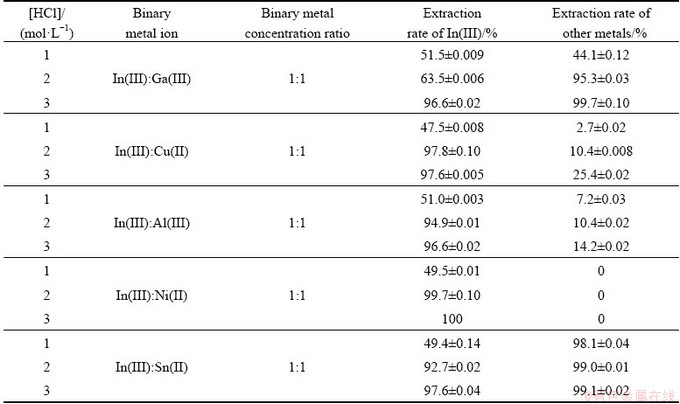
For an ore or any secondary sources, where the desired metal was present, other impurities were also allied with it. The extraction behavior of In(III) in the presence of other commonly associated metal ions from binary-metal solutions was investigated and the binary separation data were presented in Table 2. It was found from Table 2 that the preference of extraction of In(III) over Al(III), Ni(II) and Cu(II) was more and reverse in the case of Ga(III) and Sn(II).
Table 2 Effect of HCl concentration on extraction rate of indium from binary mixtures (Aqueous phase: 0.001 mol/L In(III), 0.001 mol/L Ga(III)/Cu(II)/Al(III)/Ni(II)/Sn(II); Organic phase: 0.005 mol/L Cyphos IL 101 in kerosene)
3.2 Recovery of In(III) from LCD panel using Cyphos IL 101
3.2.1 Collection of LCD waste and leaching
LCDs are the widespread components used in the electronic industry, but the life span of an LCD is typically 5-6 years [59]. Indium tin oxide is the main part of the LCD used as a secondary source of indium recovery from the LCD wastes [60]. The discarded computer having the LCD panel was collected from the local market. The collected discarded computer was dismantled into different components and LCD panel having indium tin oxide coating was segregated. The collected LCD panel was cut into small pieces (5 cm × 5 cm) using a glass cutter. Different concentrations of HCl were mol taken for leaching of metal ions. The waste LCD panel was immersed in 1, 3, 6 and 12.27 (concentrated) mol/L HCl (each volume of 50 mL) overnight for better dissolution of the metal ions. After 24 h of leaching, the leachate was collected and stored for further separation and analysis. The analysis report revealed that indium, copper and tin were the major elements present in the leach liquor (Table 3). From Table 3, it was also observed that with an increase in HCl concentration the copper dissolution increased but the dissolution of indium and tin was not affected obviously. Hence, 1 mol/L HCl was taken as the leachant concentration and the leach liquor was generated for separation and recovery of indium.
3.2.2 Optimization of Cyphos IL 101 concentration
It was observed from binary metal studies that the extraction order of tin, indium and copper was Sn(II)>In(III)>Cu(II). For optimization of Cyphos IL 101 concentration, the leach liquor was equilibrated with three different concentrations of Cyphos IL 101 (0.002, 0.003 and 0.005 mol/L). Further investigation was carried out using Cyphos IL 101 concentration of 0.003 mol/L which pertained 99.4% extraction rate of tin along with 22.7% extraction rate of indium and zero extraction rate of other metals (Fig. 11).
3.2.3 McCabe-Thiele diagram and counter-current extraction
For removal of tin from the leach liquor, the McCabe-Thiele plot was constructed by equilibrating the leach liquor with 0.003 mol/L Cyphos IL 101 at different phase ratios. The aqueous to organic ratio was varied from 1:5 to 5:1, overall keeping the total volume constant. Figure 12 shows McCabe-Thiele plot for Sn(II) extraction. It was apparent in Fig. 12 that complete removal of tin from the leach liquor was possible in two-stage with organic to aqueous phase ratio of 1:3. Then, the prediction of McCabe-Thiele plot was confirmed by performing two-stage counter-current extraction at O/A ratio of 1:3. The resulting loaded organic contained 60.3 mg/L Sn(II) and 49.8 mg/L In and the corresponding raffinate concentrations were 0.001 mg/L Sn(II), 33.7 mg/L Cu(II) and 76.8 mg/L In(III). Since the extraction was carried out at O/A ratio of 1:3, the organic phase was enriched thrice with tin and indium concentrations.
3.2.4 Selective stripping of tin and indium
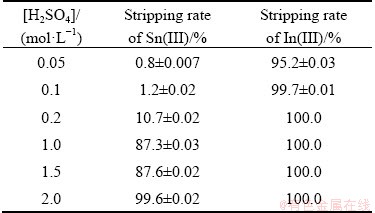
To recover tin and indium from the loaded organic phase, different sulfuric acid concentrations were used for selective stripping of indium and tin. Complete stripping of indium was observed at low acid concentrations with very little contamination of tin (0.472 mg). Therefore, the loaded organic phase was treated with 0.1 mol/L H2SO4 to back extract indium. The purity of indium in the stripped solution was found to be 99.1%. After removal of indium using 0.1 mol/L H2SO4, the loaded organic phase containing tin was stripped with 2.0 mol/L sulfuric acid having 99.7% purity (Table 4).
Table 3 Compositions of leaching liquor of LCD panel
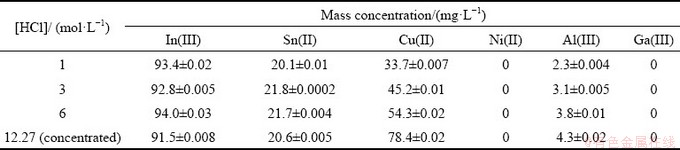
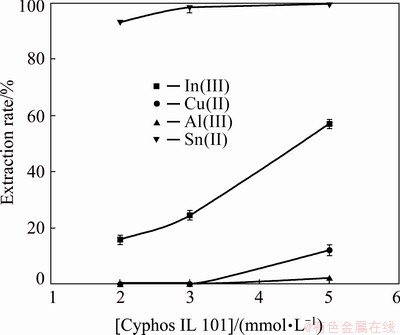
Fig. 11 Effect of Cyphos IL 101 on extraction of metal ions
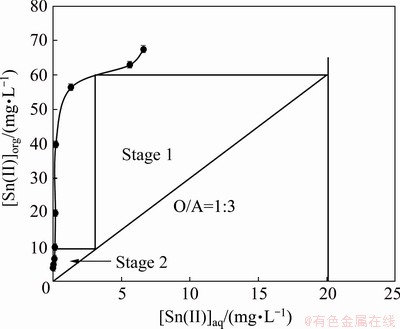
Fig. 12 McCabe-Thiele plot for Sn(II) extraction
Table 4 Selective stripping of Sn(II) and In(III)
3.3 Recovery of indium and copper from raffinate
From the initial experiments, it has been explored that quantitative extraction of indium was observed at and above 2.0 mol/L HCl. So, the acid concentration of the raffinate containing 76.8 mg/L In(III) and 33.7 mg/L Cu(II) was measured and the acidity was increased to 2.0 mol/L by adding calculated amount of concentrated HCl. Then, it was extracted with 0.003 mol/L Cyphos IL 101. The results showed that all the indium was extracted along with 1.5% copper co-extraction. Then, the loaded organic was stripped with 0.1 mol/L H2SO4 and the stripped solution containing 99.3% pure indium was mixed with the previous stripping solution of indium. The total process is represented in a flow-sheet in Fig. 13.
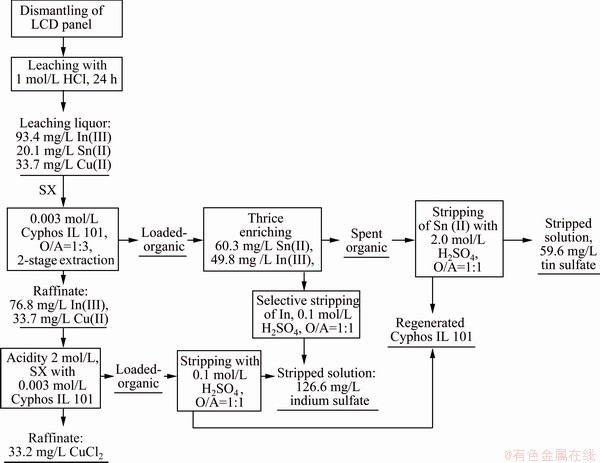
Fig. 13 Tentative flow-sheet for recovery of Sn(II), In(III) and Cu(II) from LCD panel
From the above flow-sheet, the products obtained were tin sulfate, indium sulfate and copper chloride. Quantification of indium as well as tin was found and the ionic liquid was successfully regenerated and could be reused for the extraction of the studied metal ions.
4 Conclusions
(1) Indium extraction using Cyphos IL 101 was investigated and explored with various extraction parameters. Quantitative extraction of In(III) was found with 2.0 mol/L HCl and 0.005 mol/L extractant. Job’s method was used to determine the extracted species and proposed as R3R'PInCl4. The extraction of In(III) has been found to be endothermic, spontaneous and entropy driven from the temperature variation study.
(2) Binary separation in the presence of Ga(III), Sn(II), Cu(II), Ni(II) and Al(III) was carried out and the extraction rate of indium over Al(III), Cu(II) and Ni(II) was more and reverse in the case of Sn(II) and Ga(III). 100% stripping rate was observed using 1.0 mol/L H2SO4.
(3) 1 mol/L HCl was used for leaching of indium and associated metal ions from liquid crystal displays. Three times enriched tin and indium extraction (60.3 mg/L Sn(II) and 49.8 mg/L of In(III) was found at two-stage of extraction with O/A ratio of 1:3 using 0.003 mol/L Cyphos IL 101.
(4) Selective stripping method was used for separation of tin and indium from the loaded organic phase where 99.1% and 99.7% of indium and tin were recovered. Indium from the raffinate has been separated from copper with increasing the aqueous phase acidity to 2.0 mol/L and then extracting it with 0.003 mol/L Cyphos IL 101.
(5) A complete flow-sheet was developed for the whole process.
Acknowledgments
Our thanks to Cytec Solvay, Canada for the gift sample of Cyphos IL 101 and Prof (Dr.) M. R. Nayak, President, SOA (Deemed to be University), for continuous encouragement to carry out the research work.
References
[1] SILVEIRA A V M, FUCHS M S, PINHEIRO D K, TANABE E H, BERTUOL D A. Recovery of indium from LCD screens of discarded cell phones [J]. Waste Manage, 2015, 45: 334-342.
[2] FONTANA D, FORTE F, de CAROLIS R, GROSSO M. Materials recovery from waste liquid crystal displays: A focus on indium [J]. Waste Manage, 2015, 45: 325-333.
[3] ZHANG K, WU Y, WANG W, LI B, ZHANG Y, ZUO T. Recycling indium from waste LCDs: A review [J]. Resources, Conservation and Recycling, 2015, 104(A): 276-290.
[4] BAILAR J C Jr, EMELEUS H J, NYHOLM S R, TROTMAN-DICKENSON A F. Comprehensive inorganic chemistry [M]. Oxford: Pergamon Press, 1973.
[5] ALFANTAZI A M, MOSKALYK R R. Processing of indium: A review [J]. Minerals Engineering, 2003, 16: 687-694.
[6] YANG J, RETEGAN T, EKBERG C. Indium recovery from discarded LCD panel glass by solvent extraction [J]. Hydrometallurgy, 2013, 137: 68-77.
[7] PARK J C. The recovery of indium metal from ITO-scrap using hydrothermal reaction in alkaline solution [J]. Bulletin of the Korean Chemical Society, 2011, 32(10): 3796-3798.
[8] ROCCHETTI L, AMATO A, FONTI V, VEGLIO F, BEOLCHINI F. Innovative method to extract indium from LCD panels [J]. Chemical Engineering Transactions, 2015, 43: 1987-1992.
[9] LI Y, LIU Z, LI Q, LIU Z, ZENG L. Recovery of indium from used indium–tin oxide (ITO) targets [J]. Hydrometallurgy, 2011, 105: 207-212.
[10] WILLNER J, FORNALCZYK A, BERNADETA GAJDA B, SATERNUS M. Bioleaching of indium and tin from used LCD panels [J]. Physicochemical Problems of Mineral Processing, 2018, 54(3): 639-645.
[11] LI R D, YUAN T C, FAN W B, QIU Z L, SU W J, ZHONG N Q. Recovery of indium by acid leaching waste ITO target based on neural network [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(1): 257-262.
[12] VIROLAINEN S, IBANA D, PAATERO E. Recovery of indium from indium tin oxide by solvent extraction [J]. Hydrometallurgy, 2011, 107: 56-61.
[13] YANG J, RETEGAN T, STEENARI B M, EKBERG C. Recovery of indium and yttrium from flat panel display waste using solvent extraction [J]. Separation and Purification Technology, 2016, 166: 117-124.
[14] CHOU W S, SHEN Y H, YANG S J, HSIAO T C, HUANGE L F. Recovery of indium from the etching solution of indium tin oxide by solvent extraction [J]. Environmental Progress & Sustainable Energy, 2016, 35(3): 758-763.
[15] PEREIRA E B, SULIMAN A L, TANABE E H, BERTUOL D A. Recovery of indium from liquid crystal displays of discarded mobile phones using solvent extraction [J]. Minerals Engineering, 2018, 119: 67-72.
[16] LEE M S, AHN J G, LEE E C. Solvent extraction separation of indium and gallium from sulphate solutions using D2EHPA [J]. Hydrometallurgy, 2002, 63(3): 269-276.
[17] ALGUACIL F J. Solvent extraction of indium (III) by LIX 973N [J]. Hydrometallurgy, 1999, 51(1): 97-102.
[18] GUPTA B, MUDHAR N, SINGH I. Separations and recovery of indium and gallium using bis(2,4,4- trimethylpentyl)phosphinic acid (Cyanex 272) [J]. Separation and Purification Technology, 2007, 57: 294-303.
[19] HASEGAWA Y, SHIMADA T, NIITSU M. Solvent extraction of 3B group metal ions from hydrochloric acid with trioctylphosphine oxide [J]. Journal of Inorganic and Nuclear Chemistry, 1980, 42: 1487-1489.
[20] SATO T, YASUMURA H, MIZUNO Y, NISHIMURA T. Solvent extraction of trivalent gallium, indium and thallium from hydrochloric acid solutions by TOPO and TBP [C]//Proceedings of the International Solvent Extraction Conference. Melbourne (Australia), 1996: 559-564.
[21] AVILA RODRIGUEZ M, COTE G, BAUER D. Recovery of Indium (III) from mixed hydrochloric acid-sulphuric acid media by solvent extraction with Cyanex 301 [J]. Solvent Extraction and Ion Exchange, 1992, 10: 811-827.
[22] DEFERM C, van de VOORDE M, LUYTEN J, OOSTERHOF H, FRANSAER J, BINNEMANS K. Purification of indium by solvent extraction with undiluted ionic liquids [J]. Green Chemistry, 2016, 18: 4116-4127.
[23] KHUPSE N D, KUMAR A. Ionic liquids: New materials with wide applications [J]. Indian Journal of Chemistry—Section A, 2010, 49: 635-648.
[24] PLECHKOVA N V, SEDDON K R. Applications of ionic liquids in the chemical industry [J]. Chemical Society Reviews, 2008, 37: 123-150.
[25] WELTON T. Room-temperature ionic liquids: Solvents for synthesis and catalysis [J]. Chemical Reviews, 1999, 99(8): 2071-2083.
[26] ROGERS R D, SEDDON K R. Chemistry. Ionic liquids— Solvents of the future? [J]. Science, 2003, 302(5646): 792-793.
[27] JENSEN M P, NEUEFEIND J, BEITZ J V, SKANTHAKUMAR S, SODERHOLM L. Mechanisms of metal ion transfer into room-temperature ionic liquids: The role of anion exchange [J]. Journal of the American Chemical Society, 2003, 125: 15466-15473.
[28] MEHDI H, BINNEMANS K, HECKE K V, MEERVELT L V, NOCKEMANN P. Hydrophobic ionic liquids with strongly coordinating anions [J]. Chemical Communications, 2010, 46: 234-236.
[29] MESSADI A, MOHAMADOU A, BOUDESOCQUE S, DUPONT L, GUILLON E. Task-specific ionic liquid with coordinating anion for heavy metal ion extraction: Cation exchange versus ion-pair extraction [J]. Separation and Purification Technology, 2013, 107: 172-178.
[30] de LOS RIOS A P, HERNANDEZ-FERNANDEZ F J, ALGUACIL F J, LOZANO L J, GINESTA A, GARCIA- DIAZ I, SANCHEZ-SEGADO S, LOPEZ F A, GODINEZ C. On the use of imidazolium and ammonium-based ionic liquids as green solvents for the selective recovery of Zn(II), Cd(II), Cu(II) and Fe(III) from hydrochloride aqueous solutions [J]. Separation and Purification Technology, 2012, 97: 150-157.
[31] MISHRA R K, ROUT P C, SARANGI K, NATHSARMA K C. Solvent extraction of Fe(III) from the chloride leach liquor of low grade iron ore tailings using Aliquat 336 [J]. Hydrometallurgy, 2011, 108: 93-99.
[32] de MENDONCA FABREGA F, MANSUR M B. Liquid–liquid extraction of mercury (II) from hydrochloric acid solutions by Aliquat 336 [J]. Hydrometallurgy, 2007, 87: 83-90.
[33] EL-NADI Y A, AWWAD N S, NAYL A A. A comparative study of vanadium extraction by Aliquat 336 from acidic and alkaline media with application to spent catalyst [J]. International Journal of Mineral Processing, 2009, 92: 115-120.
[34] WIONCZYK B, APOSTOLUK W. Equilibria of extraction of chromium(III) from alkaline solutions with trioctyl- methylammonium chloride (Aliquat 336) [J]. Hydrometallurgy, 2005, 78: 116-128.
[35] NAYL A A. Extraction and separation of Co(II) and Ni(II) from acidic sulfate solutions using Aliquat 336 [J]. Journal of Hazardous Materials, 2010, 173: 223-230.
[36] TIAN G C, LI J, HUA Y X. Application of ionic liquids in hydrometallurgy of nonferrous metals [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(3): 513-520.
[37] DEVI N. Solvent extraction and separation of copper from base metals using bifunctional ionic liquid from sulfate medium [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(3): 874-881.
[38] YANG H L, CHEN J, ZHANG D L, WANG W, CUI H M, LIU Y. Kinetics of cerium(IV) and fluoride extraction from sulfuric solutions using bifunctional ionic liquid extractant (Bif-ILE) [A336][P204] [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(6): 1937-1945.
[39] NAYL A A, ALY H F. Solvent extraction of V(V) and Cr(III) from acidic leach liquors of ilmenite using Aliquat 336 [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(12): 4183-4191.
[40] ULLAH Z, BUSTAM M A, MAN Z, KHAN A S. Phosphonium-based ionic liquids and their application in separation of dye from aqueous solution [J]. ARPN: Journal of Engineering and Applied Sciences, 2016, 11: 1653-1659.
[41] CIESZYNSKA A, WISNIEWSKI M. Extraction of palladium(II) from chloride solutions with Cyphos IL 101/toluene mixtures as novel extractant [J]. Separation and Purification Technology, 2010, 73: 202-207.
[42] RYBKA P, REGEL-ROSOCKA M. Nickel(II) and cobalt(II) extraction from chloride solutions with quaternary phosphonium salts [J]. Separation Science and Technology, 2012, 47: 1296-1302.
[43] ZHU Z, TULPATOWICZ K, PRANOLO Y, CHENG C Y. Solvent extraction of molybdenum and vanadium from sulphate solutions with Cyphos IL 101 [J]. Hydrometallurgy, 2015, 154: 72-77.
[44] POSPIECH B. Studies on extraction and permeation of cadmium(II) using Cyphos IL 104 as selective extractant and ion carrier [J]. Hydrometallurgy, 2015, 154: 88-94.
[45] CIESZYNSKA A, WISNIEWSKI M. Extractive recovery of palladium(II) from hydrochloric acid solutions with Cyphos IL 104 [J]. Hydrometallurgy, 2012, 113-114: 79-85.
[46] SWAIN S S, NAYAK B, DEVI N, DAS S, SWAIN N. Liquid–liquid extraction of cadmium(II) from sulfate medium using phosphonium and ammonium based ionic liquids diluted in kerosene [J]. Hydrometallurgy, 2016, 162: 63-70.
[47] MAHANDRA H, SINGH R, GUPTA B. Liquid-liquid extraction studies on Zn(II) and Cd(II) using phosphonium ionic liquid (Cyphos IL 104) and recovery of zinc from zinc plating mud [J]. Separation and Purification Technology, 2017, 177: 281-292.
[48] KUMARI A, SINHA M K, SAHU S K, PANDEY B D. Solvent extraction and separation of trivalent lanthanides using cyphos IL 104—A novel phosphonium ionic liquid as extractant [J]. Solvent Extraction and Ion Exchange, 2016, 34: 469-484.
[49] NAYAK S, DEVI N. Studies on extraction of gallium(III) from chloride solution using Cyphos IL 104 and its removal from photodiodes and red mud [J]. Hydrometallurgy, 2017, 171: 191-197.
[50] NAYAK S, DEVI N. Separation and recovery of gallium(III) ions from aqueous phase by liquid-liquid extraction using a novel extractant, Cyphos IL 101 [J]. Turkish Journal of Chemistry, 2017, 41: 892-903.
[51] OHTO K, FUJIMOTO Y, INOUE K. Stepwise extraction of two lead ions with a single molecule of calix[4]arene tetracarboxylic acid [J]. Analytica Chimica Acta, 1999, 387: 61-69.
[52] SATO T, SATO K. Liquid-liquid extraction of indium(III) from aqueous acid solutions by acid organophosphorus compounds [J]. Hydrometallurgy, 1992, 30: 367-383.
[53] BRUNETTE J P, TAHERI M, GOETZ-GRANDMONT G, LEROY M J F. Extraction of indium(III) from chloride medium with 1-phenyl-3-methyl-4-acylpyrazol-5-ones: Synergic effect with high molecular weight ammonium salts [J]. Polyhedron, 1982, 1: 457-460.
[54] GOOD M L, HOLLAND F F. Extraction of In(III) and Ga(III) from aqueous chloride media by long chain alkyl amines and quaternary salts [J]. Journal of Inorganic and Nuclear Chemistry, 1964, 26: 321-327.
[55] NELSON A D, FASCHING J L, MCDONALD R L. Extraction of Fe(III) and In(III) from aqueous HCl by tri-n-octylamine in nitrobenzene [J]. Journal of Inorganic and Nuclear Chemistry, 1965, 27: 439-447.
[56] FISCHER C, WAGNER H, BAGREEV V V, STOJANOV E S. On the extraction of In(III) with tri-n-octylamine from HCl solutions [J]. Journal of Inorganic and Nuclear Chemistry, 1977, 39: 513-517.
[57] GHATEE M H, ZARE M, MOOSAVI F, ZOLGHADR A R. Temperature-dependent density and viscosity of the ionic liquids 1-alkyl-3-methylimidazolium iodides: Experiment and molecular dynamics simulation [J]. Journal of Chemical & Engineering Data, 2010, 55(9): 3084-3088.
[58] HOOGERSTRAETE T V, ONGHENA B, BINNEMANS K. Homogeneous liquid–liquid extraction of metal ions with a functionalized ionic liquid [J]. The Journal of Physical Chemistry Letters, 2013, 4(10): 1659-1663.
[59] ZHUANG X, HE W, LI G, HUANG J, YE Y. Materials separation from waste liquid crystal displays using combined physical methods [J]. Polish Journal of Environmental Studies, 2012, 21: 1921-1927.
[60] WANG X, LU X, ZHANG S. Study on the waste liquid crystal display treatment: Focus on the resource recovery [J]. Journal of Hazardous Materials, 2013, 244-245: 342-347.
Sumitra NAYAK1, Niharbala DEVI1,2
1. Department of Chemistry, Institute of Technical Education and Research, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar 751030, India;
2. Biofuels and Bioprocessing Research Center, Institute of Technical Education and Research, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar 751030, India
摘 要:本研究的主要目的是从废液晶显示面板中回收铟。为此,研究一种离子液体Cyphos IL101。首先,考察平衡时间、酸浓度、氯离子浓度、氢离子浓度等萃取参数对Cyphos IL 101萃取In(III)萃取效率的影响。用0.005 mol/L Cyphos IL 101在2.0 mol/L HCl中定量萃取铟,并用1.0 mol/L H2SO4定量反萃。用Job法确定萃合物组成,为R3R'PInCl4(R=C6H13; R′=C14H29)。基于多金属(萃取)研究的结果,Cyphos IL 101进一步用于从废液晶显示器浸出液中去除铟、锡和铜。获得从废液晶显示器中回收铟的优化条件。为了分离金属离子铟、锡和铜,根据McCabe-Thiele分析,进行逆流萃取和选择性反萃。为了从进料中完全除去锡,在O/A比为1:3的条件下进行两段萃取,并用0.1 mol/L H2SO4进行铟和锡的选择性反萃。提出从废液晶显示器中分离铟的工艺流程。
关键词:铟;萃取;分离;Cyphos IL 101;液晶面板
(Edited by Wei-ping CHEN)
Corresponding author: Niharbala DEVI; Tel: +91-674-2351777; E-mail: niharbaladevi@soa.ac.in
DOI: 10.1016/S1003-6326(20)65401-2

