
Electroless Ni-P plating on Mg-Li alloy by two-step method
LUO Hong-jie, SONG Bin-na, LIU Yi-han, YAO Guang-chun
School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China
Received 30 October 2010; accepted 27 May 2011
Abstract: Pretreated Mg-Li alloy sheets were pre-plated in a NiCO3·2Ni(OH)2·4H2O solution to form a thin Ni-P alloy film and then plating in a NiSO4·6H2O solution was carried out to obtain a protective coating. The surface morphology, structure and corrosion resistance of the coating were studied. The results showed that a flat, bright and compact plating layer, which was integrated into the matrix metal, was obtained. The P content of the Ni-P coating reached 13.56% (mass fraction). The hardness value of the Ni-P coating was about HV 549. The polarization curve showed that the corrosion potential of the Ni-P coating reached -0.249 V (vs SCE). A long passivation region was found on the polarization curve, and this phenomenon indicated that the coating has an excellent anti-corrosion property.
Key words: Mg-Li alloy; pretreatment; electroless plating; Ni-P coating; corrosion resistance
1 Introduction
Mg-Li alloys are among the lowest dense metallic materials with densities ranging from 1.3 to 1.4 g/cm3 [1]. Mg-Li alloys have many excellent properties such as high specific strength, formability, impact resistance, and damping capacity, and they offer resistance to energetic particle penetration [2]. These properties are useful for aerospace and military applications. However, the corrosion resistance of Mg-Li alloys is worse than that of other magnesium alloys because of the addition of active lithium [3]. Therefore, various anticorrosive strategies need to be developed to improve the practical use of Mg-Li alloys.
Many methods to improve the corrosion resistance of magnesium alloys have been developed, including electroless plating, coating conversion, anodizing, ion implantation, gas-phase deposition processes and organic coatings [4-5]. But, research on the corrosion protection of Mg-Li alloys is relatively scarce [6-8]. Among these surface techniques, the coating prepared by electroless nickel plating has excellent properties, such as high corrosion resistance, high wear resistance, good lubricity, high hardness and acceptable ductility [9]. Traditionally, nickel-plating baths are acidic with nickel sulfate as the main salt and these baths are more efficient than basic nickel carbonate baths [10]. However, magnesium and its alloys corrode more easily in a nickel sulfate bath than in a nickel carbonate bath while nickel carbonate has low solubility. Plenty of hydrofluoric acid, which is harmful to the environment, is needed to dissolve nickel carbonate [11]. Additionally, the process of electroless nickel plating in a basic nickel carbonate bath requires careful control. Therefore, the basic nickel carbonate bath is expensive both in operation and in price.
The direct electroless nickel plating specifically consists of a simple procedure but has similar process problems as the basic methods. The electroless nickel plating on Mg-Li alloys is not established yet. A two-step electroless nickel plating process, which was successfully used to protect the alloys, is reported in this paper. This was done to avoid corrosion by sulfate ions, to improve the binding capacity between the coating and the matrix metal and to reduce the cost of using basic nickel carbonate as the only main salt.
2 Experimental
The substrate material used was Mg-9.3%Li-2.0% Zn (mass fraction) with dimensions of 20 mm×30 mm× 2 mm, and was produced in-house [12]. All reagents including nickel sulfate and basic nickel carbonate etc were analytically pure and purchased from Sinopharm Chemical Reagent Co., Ltd. Sample preparation was divided into three steps. First, the Mg-Li alloy sheet was pretreated by alkaline degreasing, acid cleaning and activation. The aim of alkaline degreasing was to remove the grease and sewage. The aim of acid cleaning was to remove the oxide film, oxide skin, corrosion products on the alloy surface, loosely attached metal scrap generated during cold-working and dirt embedded in the samples. The purpose of activation was to further remove oxides and hydroxides and to completely expose the matrix texture so that a thinner Ni-P alloy film could form on the surface. Secondly, an electroless Ni-P plating was done on the pretreated Mg-Li alloy sheet to form a thinner film, and NiCO3·2Ni(OH)2·4H2O was used as the main salt. Finally, a second electroless Ni-P plating on the previously mentioned Mg-Li alloy sheet was carried out to obtain an antiseptic coating and NiSO4·6H2O was used as the main salt. The detailed procedure is shown in Fig. 1.
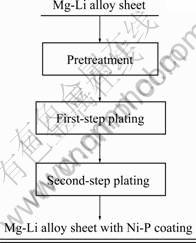
Fig. 1 Process diagram for electroless Ni-P plating on Mg-Li alloy sheet
A morphological analysis of the Ni-P plating layer was carried out with a SSX-550 scanning electron microscope (SEM). Components in the coating were determined using an energy dispersive X-ray spectrometer (EDS) coupled to the SEM. A file test and a cross cut test were used to study the adhesion between the coating and the matrix. Potentiodynamic polarization curves were used to study the corrosion potential and the corrosive environment was a 3.5% (mass fraction) NaCl solution at room temperature. A Vickers hardness tester was used to evaluate the rigidity of the coating.
3 Results and discussion
Mg-Li alloys have high chemical activity. When being exposed to the atmosphere or aqueous solutions, oxide and hydroxide films form easily on their surfaces [13]. It is, therefore, necessary to remove all contamination from their surfaces to get a good protective coating. Table 1 shows the compositions of the pretreatment solutions for the Mg-Li alloy sheets and their corresponding operation conditions. All solutions used were freshly prepared from analytical grade reagents and deionized water. The effectiveness of alkaline degreasing was checked by the water-film break way. The loss of mass was used to judge the degree of etching after acid cleaning. The effectiveness of the activation was evaluated by SEM observations. Detailed operating conditions can be found in Ref. [14]. A clean Mg-Li alloy sheet that could support the Ni-P alloy was obtained by the given pretreatment methods.
The obtained solution compositions for the first step of electroless Ni-P plating and the corresponding technical conditions are listed in Table 2. The plating solution compositions and the corresponding technical conditions were determined using a single factor test, and the deposition rate was used as the evaluation criterion. Major factors such as the temperature, pH, main salt concentration, reductant concentration and the amount of lactic acid were investigated in detail.
Table 1 Compositions and operating conditions for pretreatment of Mg-Li alloy sheets

Table 2 Compositions of plating solution and conditions for the first electroless plating step

The first electroless Ni-P plating step was done to avoid corrosion of the Mg-Li alloy matrix by SO42-. It may also be referred to be pre-plating. The purpose of pre-plating is to lay a foundation for the next electroless plating as a thin layer of Ni-P alloy is formed on the surface of the sample. To reduce the thickness of the coating during pre-plating, the layer should be controlled to be as thin as possible. Determining the pre-plating time is very important because the deposition rate increases with increasing the reaction time. Figure 2 shows the surface morphologies of the obtained samples at different times. The pre-plating time was 5 min, 10 min and 15 min and the corresponding photographs are shown in Figs. 2(a), (b) and (c), respectively. Figure 2(a) shows that many small Ni-P nodules are present on the sample surface but they do not cover the sample surface completely. Compact Ni-P nodules are formed as shown in Figs. 2(b) and 2(c), but the obtained Ni-P nodules are small and the thickness of plating layer is thin in Fig. 2(b). Therefore, the selected pre-plating time is 10 min. The samples pre-plated for 10 min can fulfill the requirements for the next electroless plating in the sulfuric acid system.

Fig. 2 Surface morphologies of samples after pre-plating for different time: (a) 5 min; (b) 10 min; (c) 15 min
It is obvious that the corrosion resistance of the electroless Ni-P coating in the nickel sulfate bath is better than that in the basic nickel carbonate bath. To obtain a better corrosion resistant coating, further electroless Ni-P plating was carried out in a nickel sulfate bath. Table 3 lists the plating solution compositions and the plating conditions. The plating solution compositions and technological conditions were determined by an orthogonal test wherein we used the deposition rate and the corrosion potential as evaluation criteria.
Table 3 Compositions of plating solution and conditions for the second electroless plating step
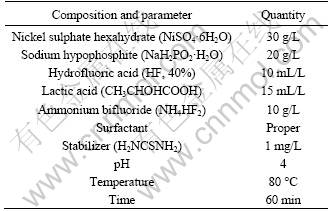
Figure 3 shows SEM photographs of the sample prepared by the second step of the electroless plating process and its EDS spectrum. The surface morphology of the Ni-P coating is shown in Fig. 3(a). The surface of the substrate is fully covered and shows a typical nodular structure. Furthermore, the Ni-P coating is composed of compact nodules with relatively uniform size and distribution. Figure 3(b) shows the cross-section morphology of the Ni-P coating. The adhesion between the coating and the matrix is good as no gaps and inclusion are present. The thickness of the whole Ni-P coating is about 20 μm. The thickness of the pre-plated Ni-P coating is more than 4 μm and the thickness of the second step plated Ni-P coating is about 16 μm. Figure 3(b) also shows that the pre-plated Ni-P coating and the later Ni-P coating are well integrated, and this results in an excellent adhesion. Figure 3(c) shows an EDS spectrum of the second step plated Ni-P coating. The Ni content is 86.44% (mass fraction) and the P content is 13.56% which is a high phosphorus content coating. The difference of phosphorus content in Ni-P coatings exhibits different electrochemical properties [15]. It is generally believed that the corrosion potential φcorr of a Ni-P coating will increase as the phosphorus content increases [16].
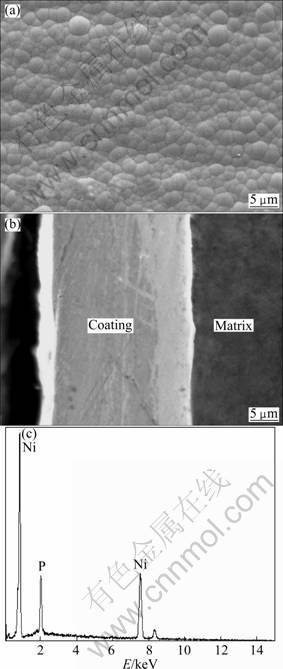
Fig. 3 Surface and cross-section morphologies of Ni-P coating and its EDS spectrum: (a) Surface morphology; (b) Cross- section morphology; (c) EDS spectrum
The cross-section morphology of the Ni-P coating, as detected by SEM, and the corresponding EDS spectra are shown in Fig. 4. Figure 4(a) shows a cross-section SEM photograph of the Ni-P coating. Figure 4 (b) gives the P element distribution in the Ni-P coating along the line shown in Fig. 4(a). This shows that the P content changes very little from 0 to 15 μm and then declines slightly. This phenomenon indicates that the P content of the coating in the sulfate system is higher than that in the carbonate system. Different coating colors may result from different P contents. The P content in the coating depends on the bath temperature, the plating solution composition and the other operating conditions [17]. However, the Ni content in the coating is relatively uniform and its fluctuation is small, as shown in Fig. 4(c).

Fig. 4 Cross-section morphology of Ni-P coating and corresponding EDS spectra: (a) SEM image; (b), (c) EDS spectra scanned from coating surface to substrate along line shown in Fig. 4(a)
Figure 5 shows the potentiodynamic polarization curves for the Mg-Li alloy matrix and for the matrix covered by a Ni-P coating. The cathode reaction in the polarization curves corresponds to the evolution of hydrogen and the anodic polarization curve is the most important feature that relates to the corrosion resistance [18]. As seen in Fig. 5, the corrosion potential φcorr of the Mg-Li alloy matrix is about -1.65 V. After the addition of the Ni-P coating, the corrosion potential φcorr increases to -0.249 V, which is a 1400 mV positive shift compared with the matrix.

Fig. 5 Polarization curves of Mg-Li alloy sheet before and after electroless plating
The corrosion current density Jcorr is also significantly lower than that of the matrix. There is an obvious passivation region in curve (b) where the width of the passivation region is more than 700 mV. These results show that the corrosion resistance of the Mg-Li alloy matrix has been greatly improved.
In addition, the fail test and cross-cut test indicated that the binding force between the Ni-P coating and the matrix was good as no peeling or abscission phenomena were apparent. A microhardness test showed that the average Vickers hardness of the Ni-P coating was HV549.
4 Conclusions
1) A compact and protective coating was obtained by a two-step electroless Ni-P plating on a Mg-Li alloy sheet. The coating was found to be well integrated with the matrix and was a high phosphorus coating because the P content reached 13.56%.
2) Potentiodynamic polarization curves revealed that the corrosion potential of the Mg-Li alloy sheet covered by a Ni-P coating rose to -0.249 V and shifted 1400 mV in the positive direction. A significant passivation region existed in the polarization curve of the Mg-Li alloy sheet after electroless plating.
3) The average Vickers hardness of the Ni-P coating is HV 549.
References
[1] SHARMA A K, UMA RANI R, MAYANNA S M. Thermal studies on electrodeposited black oxide coatingon magnesium alloys [J]. Thermochimica Acta, 2001, 376(1): 67-75.
[2] SONG J M, WEN T X, WANG J Y. Vibration fracture properties of a lightweight Mg-Li-Zn alloy [J]. Scripta Mater, 2007, 56(6): 529-532.
[3] ZHANG C H, HUANG X M, ZHANG M L, GAO L L, WU R Z. Electrochemical characterization of the corrosion of a Mg-Li alloy [J]. Mater Lett, 2008, 62(14): 2177-2180.
[4] SATHIYANARAYANAN S, SYED A S, VENKATACHARI G. Corrosion resistant properties of polyaniline–acrylic coating on magnesium alloy [J]. Appl Surf Sci, 2006, 253(4): 2113-2117.
[5] ZHU X M, YANG H G, LEI M K. Corrosion resistance of Al ion implanted AZ31 magnesium alloy at elevated temperature [J]. Surf Coat Tech, 2007, 201(15): 6663-6666.
[6] LI J F, ZHENG Z Q, LI S C, REN W D, ZHANG Z. Preparation and galvanic anodizing of a Mg-Li alloy [J]. Mater Sci Eng A, 2006, 433(1-2): 233-240.
[7] YAMAUCHI N, UEDA N, OKAMOTO A, SONE T, TSUJIKAWA M, OKI S. DLC coating on Mg-Li alloy [J]. Surf Coat Tech, 2007, 201(9-11): 4913-4918.
[8] ZHANG H, YAO G C, WANG S L, LIU Y H, LUO H J. A chrome-free conversion coating for magnesium-lithium alloy by a phosphate-permanganate solution [J]. Surf Coat Tech, 2008, 202(9): 1825-1930.
[9] YAN Hong. New techniques in electroless Ni and composite plating [M]. Beijing: National Defence Industry Press, 1999: 1-3. (in Chinese)
[10] ZHANG W X, HUANG N, HE J G, JIANG Z H, JIANG Q, LIAN J S. Electroless deposition of Ni-W-P coating on AZ91D magnesium alloy [J]. Applied Surface Science, 2007, 253 (11): 5116-5121.
[11] AMBAT R, ZHOU W. Electroless nickel-plating on AZ91D magnesium alloy: effect of substrate microstructure and plating parameters [J]. Surf Coat Tech, 2004, 179(2-3): 124-134.
[12] LI H B, YAO G C, GUO Z Q, LIU Y H, YU H J, JI H B. Microstructure and mechanical properties of Mg-Li alloy with Ca addition [J]. Acta Metallurgica Sinica, 2006, 19(5): 355-361.
[13] SHARMA A K, UMA RANI R, MALEK A, ACHARYA K S N, MUDDU M, KUMAR S. Black anodizing of a magnesium-lithium alloy [J]. Metal Finishing, 1996, 94(4): 16-22.
[14] LUO H J, LIU Y H, SONG B N, ZHEN L. Electroless Ni-P plating on magnesium-lithium alloy [C]//NYBERG E A. Magnesium Technology 2009. San Francisco: TMS, 2009: 351-354.
[15] ZHANG W X, JIANG Z H, LI G Y, JIANG Q, LIAN J S. Electroless Ni-P/Ni-B duplex coatings for improving the hardness and the corrosion resistance of AZ91D magnesium alloy [J]. Applied Surface Science, 2008, 254(16): 4949-4955.
[16] BAI A, CHUANG P Y, HU C C. The corrosion behavior of Ni-P deposits with high phosphorous contents in brine media [J]. Mater Chem Phys, 2003, 82(1): 93-100.
[17] EL MAHALLAWY N, BAKKAR A, SHOEIB M, PALKOWSKI H, NEUBERT V. Electroless Ni-P coating of different magnesium alloys [J]. Surf Coat Tech, 2008, 202(21): 5151-5157.
[18] GU C D, LIAN J S, HE J G, JIANG Z H, JIANG Q. High corrosion-resistance nanocrystalline Ni coating on AZ91D magnesium alloy [J]. Surf Coat Tech, 2006, 200(18-19): 5413-5418.
镁锂合金表面两步法化学镀镍工艺
罗洪杰,宋滨娜,刘宜汉,姚广春
东北大学 材料与冶金学院,沈阳 110819
摘 要:首先,将预处理后的合金样品在碱式碳酸镍溶液中进行预镀,目的是在镁锂合金表面形成一层Ni-P合金薄膜;然后,在硫酸镍溶液中进行二次镀覆,获得具有保护作用的镀层。对获得的镀层的表面形貌、结构和抗腐蚀能力进行研究。结果表明:采用该方法能够在镁锂合金表面形成平整、光亮、致密的镀层,镀层与基体结合良好。镀层中磷含量达到13.56%(质量分数),镀层的维氏硬度约为HV549。极化曲线测试表明,Ni-P镀层的腐蚀电位升高至-0.249 V(vs SCE),并有一个很宽的钝化区,这种现象显示该镀层具有良好的抗腐蚀能力。
关键词:镁锂合金;预处理;化学镀;Ni-P镀层;抗腐蚀性能
(Edited by YANG Hua)
Foundation item: Projects (50974114, 51174060) supported by National Natural Science Foundation of China; Project (2008AA03Z512) supported by High-tech Research and Development Program of China; Project (20070145049) supported by PhD Programs Foundation of Ministry of Education of China
Corresponding author: LUO Hong-jie; Tel: +86-24-83686462; E-mail: luohj@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(11)60999-0