Trans. Nonferrous Met. Soc. China 24(2014) 868-875
Kinetics and equilibrium adsorption of copper(II) and nickel(II) ions from aqueous solution using sawdust xanthate modified with ethanediamine
Lu XIA1,2,3, Yi-xu HU1, Bo-han ZHANG1
1. School of Chemistry and Chemical Engineering, Guangxi University for Nationalities, Nanning 530008, China;
2. Guangxi Key Laboratory of Chemistry and Engineering of Forest Products, Nanning 530008, China;
3. Key Laboratory of Chemical and Biological Transforming Process of Guangxi Higher Education Institutes, Nanning 530008, China
Received 25 March 2013; accepted 8 July 2013
Abstract: Sawdust xanthate modified with ethanediamine was used for the removal of Cu(II) and Ni(II) from aqueous solution. The influence of various operating parameters such temperature and adsorbent dosage on the adsorption isotherms of modified sawdust was investigated. Thermodynamic parameters, namely Gibbs free energy (△GΘ), enthalpy (△HΘ) and entropy (△SΘ) of Cu(II) and Ni(II) adsorption process were calculated, showing that the adsorption is a spontaneous and exothermic process. The modified extended Langmuir equation approaches provide excellent prediction of the binary adsorption. In single and binary systems, the overall adsorption data were best described by the pseudo-second order kinetic model, then the calculated values of activation energy of Cu(II) and Ni(II) adsorption process were 59.12 and 55.92 kJ/mol respectively. The results show that the affinity of each metal ion onto the modified sawdust surface is influenced by the presence of the other one.
Key words: copper; nickel; adsorption; modified sawdust
1 Introduction
Heavy metal pollution has become one of the most serious environmental problems today, for its difficulty in degrading into harmless under natural condition [1], especially in aquatic environments. It is known that the industrial effluent is discharged into environment directly or indirectly, such as metallurgical, electrical and electronics, mining and smelting, metal plating industry, resulting in extensive damages to the ecosystem [2].
Heavy metal ions removal from aqueous solutions has been commonly carried out by several processes: 1) chemical treatment, such as electrochemical and chemical precipitation; 2) physico-chemical treatment, for instance solvent extraction, ion-exchange, and membrane filtration; 3) biological treatment, such as biomaterial adsorption [3]. Studies on the treatment of toxic metals containing effluents showed that adsorption process is an effective technique for the heavy metal removal from dilute solutions.
According to the result by BAILEY et al [4], an adsorbent can be considered low-cost if it’s abundant in nature, required little processing, and a by-product of industry. Most of the adsorption studies have been focused on the untreated plant wastes, such as chitosan, starch, calcium alginate, orange peel, sawdust, Eichhornia crassipes, sugarcane bagasse, cellulose, konjac [5-8]. However, its performance to remove heavy metal ions cannot be obvious without chemical treatment. For example, the adsorption capacities of Cu(II) and Ni(II) were obtained as 0.057 mmol/g and 0.055 mmol/g on nature sawdust, respectively. These chemical treated biosorbents can effectively sequester dissolved metal ions out of dilute complex solutions in a short time with high efficiency.
The aim of this work is to evaluate the adsorption capacity of modified sawdust as well as to investigate the adsorption equilibrium and kinetics of Cu(II) and Ni(II) divalent ions in both single and binary component systems. And the modified extended Langmuir model depicted the complexity of the adsorption process, allowing fitting well the multi-component adsorption data in a 3D representation. The kinetic parameters, such as the reaction order and rate constants, are able to be obtained by calculating the adsorption data, and then can evaluate the adsorption efficiency of the modified sawdust.
2 Experimental
2.1 Chemicals
The chemicals used were of analytical reagent grade without further purification. Distilled water was also used as dilution media. Sawdust was collected from local timber mill situated in Nanning city, China. And it was sieved to uniform size of 0.45 mm.
2.2 Preparation of modified sawdust
The sawdust was treated in an alkaline solution (called mercerization) for 24 h at room temperature, followed by extensive washing with water until the solution reached a neutral pH value around 7, and then dried in a dryer at 60 °C.
Then the mercerized sawdust (5 g), 10 mL epichlorohydrin (EPI), 10% (v/v) ethanol (80 mL) and 0.3 mL perchloric acid were put in a 250 mL three-necked flask equipped with a magnetic stirring bar. The reaction was carried out at 80 °C for 6 h. Upon completion of the reaction, the product was washed with water and acetone in turn until the pH value of 7, and dried at 60 °C.
The treated sawdust was immersed in a mixture of 10% ethanediamine solution (80 mL), and a desired amount of sodium carbonate as a catalyst (1.5 g) was added. Then the reaction system was heated up to 80 °C in nitrogen atmosphere for 4 h. After desired reaction period, nitrogen gas supply was stopped with the graft copolymer cooled to 25 °C. Then the sawdust was filtered out, washed successively with distilled water and acetone.
The sawdust was xanthated by dropwise adding 10% NaOH solution and carbon disulfide into the sealed flask and stirring at 30 °C in a water bath for 3 h until it turned saffron yellow then was left for overnight. It was filtered and washed repeatedly until the suspension become neutral. This material is ready for the following experiments and called modified sawdust for short.
2.3 Characterization of sawdust xanthate modified with ethanediamine
Surface morphology was analyzed by Hitachi S-3400N scanning electron microscopy (SEM). IR analyses were carried out using a fourier transform infrared spectrometer (Nicolet, MAGNAIR-550II). The spectra were recorded from 4000 to 400 cm-1.
2.4 Batch adsorption experiments
The adsorption was determined by mixing the modified sawdust and the Cu(II) or Ni(II) solutions into 150 mL conical flask. The slurry was shaken at a speed of 120 r/min for 2 h. After adsorption, the supematant liquor was periodically filtered through a membrane filter (0.45 μm) and the metal ion concentration was measured by using a TAS-990F atomic absorption spectrophotometer (PERSEE). The sorption capacity of metal ions was calculated by using the mass balance equation for the adsorbent:
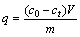 (1)
(1)
where q (mmol/g) is the amount of metal up taken per unit mass of the adsorbent, m (g) is the dry mass of the adsorbent, V (L) is the volume of the test solution, c0 (mmol/L) and ct (mmol/L) represent the initial and final concentration of ions, respectively.
3 Results and discussion
3.1 Characterization
The surface morphology of the nature sawdust, modified sawdust and metal loaded sawdust are shown in Fig. 1. The nature sawdust has a smooth and compact surface, and some knurls. The modified sawdust is a heterogeneous material consisting largely of small spheres, which is irregular and porous.
The IR spectra of the nature sawdust and modified sawdust are shown in Fig. 2. The spectrum of the nature sawdust (curves (a)) exhibits a strong peak at 3456 cm-1 representing the —OH stretching of phenol group of cellulose and lignin. The bands around 2931 and 1163 cm-1 are assigned to —CH stretching and —CO stretching vibration, respectively. The appearance of peak at 1073 cm-1 indicate the presence of —C—O—C— stretching vibration. These absorptions are consistent with those of the typical cellulose backbone [9]. In Fig. 2 (b), the peak intensity at 2901 and 1162 cm-1 increases obviously as a result of reaction with epichlorohydrin. A broad absorption band at 3447 cm-1 is for the —NH stretching vibration. Absorption peaks appear at 2496 cm-1 corresponding to the —SH stretching vibration of the xanthate unit. And the spectra of the —C(=S)—S— groups are displayed at 880 cm-1 [10].
3.2 Effect of adsorbent dosage
The adsorption tests of Cu(II) and Ni(II) on the modified sawdust were carried out at 25 °C by varying the quantity of adsorbent from 0.2 to 4.0 g/L while keeping the volume of metal solutions constant at pH 6. The influence of adsorbent dosage on adsorption rate and adsorption capacity of Cu(II) and Ni(II) is shown in Fig. 3. The removal rate of Cu(II) increases from 18.5% to 84.0%, and that of Ni(II) increases from 14.5% to 84.0%, with increasing the modified sawdust dosage from 0.2 to 4.0 g/L under equilibrium condition. However, the adsorption capacity of Cu(II) decreases from 0.714 mmol/g to 0.165 mmol/g and that of Ni(II) decreases from 0.609 mmol/g to 0.179 mmol/g.
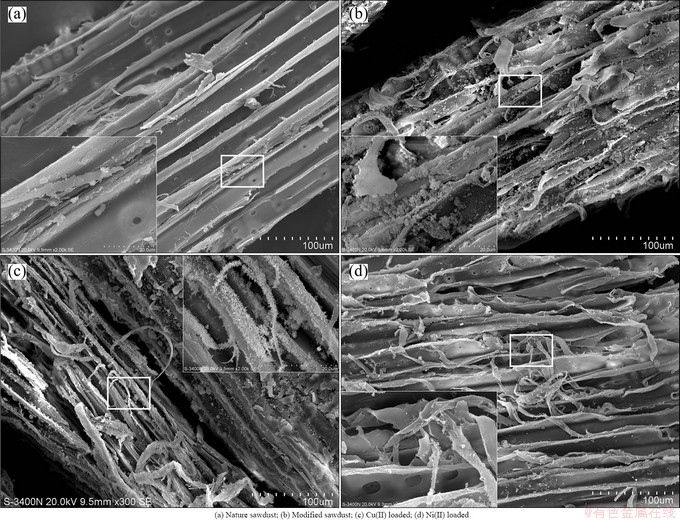
Fig. 1 SEM micrographs of sawdust
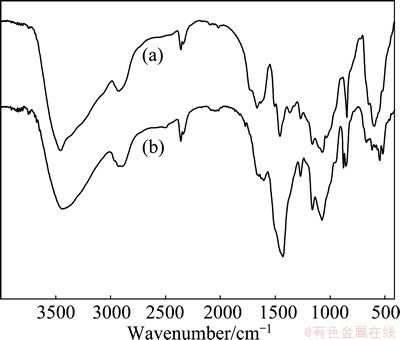
Fig. 2 FTIR spectra of nature (a) and modified (b) sawdust
3.3 Equilibrium adsorption in single and binary systems
The main objective of isotherm is to evaluate the capacity of the modified sawdust to sequester heavy metals from an aqueous solution. It was done by characterizing the equilibrium state of the functionalized adsorbent that had been allowed to react with aqueous solution of the metal of interest. These isotherm, derived at pH=6, are presented in Fig. 4. It was observed that the adsorption capacity decreases with the increase of temperature.
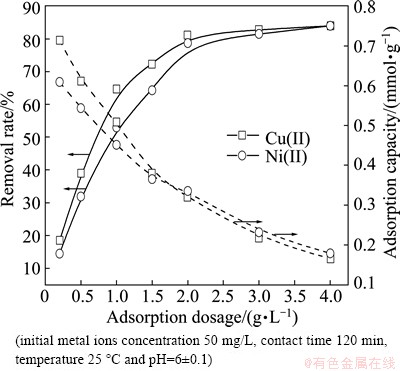
Fig. 3 Effect of adsorbent dosage on adsorption of metal ions
Metal ions adsorption by the modified sawdust can be approximated by a Langmuir adsorption isotherm model [11]:
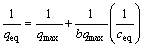 (2)
(2)
where qeq (mmol/g) is the equilibrium metal ions concentration on the adsorbent, qmax (mmol/g) is the Langmuir saturated adsorption capacity of the adsorbent, b (L/mmol) is the Langmuir adsorption constant which is related to the free energy of adsorption, ceq (mmol/L) is the equilibrium metal ions in the solution.
A further analysis of the Langmuir equation can be made on the basis of a dimensionless equilibrium parameter, RL. It is calculated by
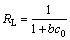 (3)
(3)
where c0 (mmol/L) is the highest initial metal ions concentration. The values of RL calculated are incorporated in Table 1. As the values of RL are between 0 and 1, the adsorption system is favorable. And RL=1 represents linear adsorption while the adsorption process is irreversible if RL=0 [12].
Table 1 Langmuir model constants and correlation coefficients for adsorption of Cu(II) and Ni(II) ions on modified sawdust
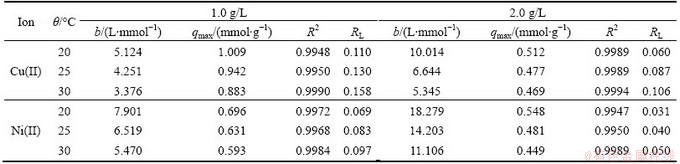

Fig. 4 Single component adsorption isotherms for Cu(II) and Ni(II) under conditions of contact time 120 min, temperature 25 °C, pH=6±0.1 and adsorbent dosage of 1.0 g/L (a) and 2.0 g/L (b)
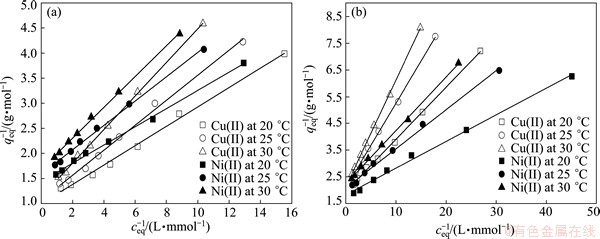
Fig. 5 Langmuir adsorption isotherm plots for adsorption of Cu(II) and Ni(II) ions with adsorbent dosage of 1.0 g/L (a) and 2.0 g/L (b)
The Langmuir adsorption isotherm parameters evaluated from the isotherm plots (Fig. 5) for Cu (II) and Ni(II) are given in Table 1. The adsorption capacity decreases along with the increase of temperature. And the Langmuir saturated adsorption capacity (qmax) decreases along with the increase of adsorbent dosage. As adsorbent dosage increases, the traditional adsorption isotherm (qeq-ceq curve) will decline, and adsorption equilibrium constant also can change [13-15].
In adsorption studies, both energy and entropy considerations should be taken into account to determine that which process will take place spontaneously. Values of thermodynamic parameters are the actual indicators for practical applicability of a process. Langmuir isotherm constant b, its dependence with temperature can be used to estimate the thermodynamic parameters [16]. The changes in free energy (△GΘ), enthalpy (△HΘ) and entropy (△SΘ) were determined by using Eqs. (4) and (5).
△GΘ=-RTlnb (4)
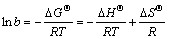 (5)
(5)
The plot of ln b as a function of 1/T (Fig. 6) yields a straight line from which △HΘ and △SΘ are calculated from the slope and intercept, respectively. From Table 2, the enthalpy (△HΘ) and entropy (△SΘ) increase significantly along with an increase of the modified sawdust dosage, which makes the equilibrium constants be fundamentally dependent on the kinetic paths and the reactant concentration condition. PAN [14,17,18] called this phenomenon “adsorbent concentration effect”.
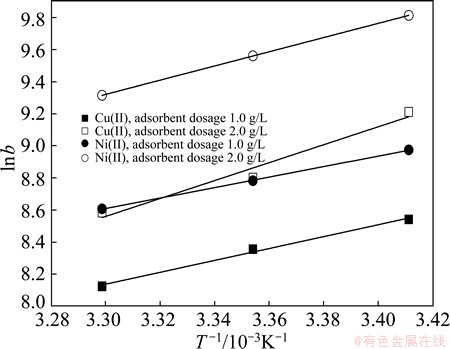
Fig. 6 Plot of ln b vs 1/T for estimation of thermodynamic parameters
The most representative way of depicting the binary equilibrium adsorption data is using a 3D plot in which the metal uptake is plotted as a function of the equilibrium solution concentrations of the two metal ions. In Fig. 7, the mesh represents the adsorption capacity predicted by the competitive modified extended Langmuir model using the parameters derived from single metal isotherms data.
The modified extended Langmuir equation used in this study is give by [19,20]:
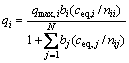 (6)
(6)
where qi is the metal uptake of the component i, N is the number of the components, ceq,i is the equilibrium concentration of the component i, while bi and qmax,i are the Langmuir constants as obtained from the corresponding single metal sorption isotherms, nij is the correction parameter of species i.
3.4 Adsorption kinetics
In order to analyze the metal sorption kinetics in single and binary systems, the pseudo-second order kinetic model shown in Fig. 8 was applied to the data. A simple pseudo-second order equation, which was proposed by HO et al [21,22], was applied to plot the experimental data as t/qt against t, which shows a linear tendency of the data and allows for the determination of the sorption rate constant, namely k2 (g·mmol-1·min-1) in a simple way.
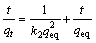 (7)
(7)
where k2 (g·mmol-1·min-1) is the rate constant of the pseudo-second adsorption process, qeq and qt (mmol/g) are the adsorption amount at equilibrium and in time, respectively.
The initial adsorption rate (h) is given by [19]:
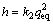 (8)
(8)
Table 2 Thermodynamic parameters calculated from Langmuir isotherm constant (b) for adsorption of Cu (II) and Ni (II)
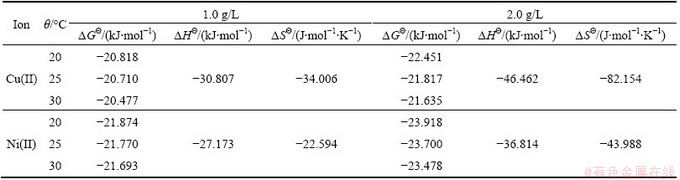
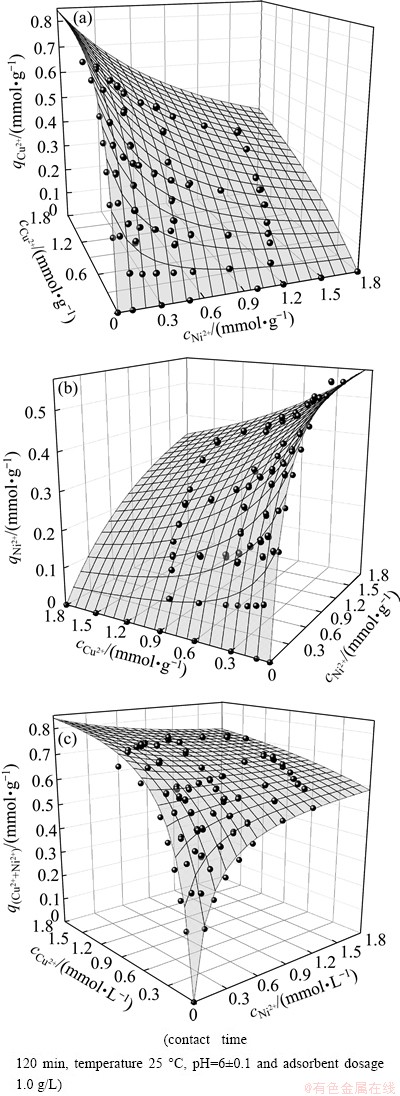
Fig. 7 Total uptake as a function of equilibrium concentration in Cu(II)-Ni(II) binary adsorption system
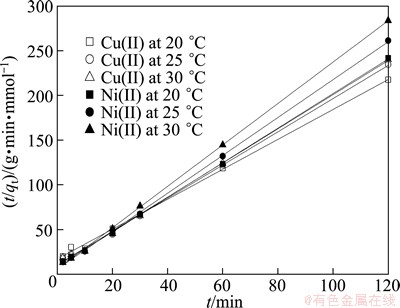
Fig. 8 Pseudo-second order kinetic model plots for adsorption of Cu(II) and Ni(II) under conditions of initial metal ions concentration 50 mg/L, pH=6±0.1 and adsorbent dosage1.0 g/L
The activation energy for Cu(II) and Ni(II) adsorption on the modified sawdust was calculated by using the Arrhenius equation and the equation may be linearized by taking logarithms:
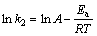 (9)
(9)
where k2 (g·mmol-1·min-1) is the rate constant of the pseudo-second adsorption process, A is the frequency factor, R is the mole gas constant, and Ea is the activation energy for the adsorption process, respectively.
The activation energy for Cu(II) and Ni(II) was obtained from the slope of the plot of ln k2 vs. 1/T using Eq. (9) and was found to be 59.12 and 55.92 kJ/mol.
In binary system, the pseudo-second order kinetic model was fitted better than the single metal sorption data, according to the good correlation coefficient values (R2≈0.999) as shown in Fig. 9. The adsorption rate constant (k2) and the uptake metal capacity at equilibrium (qeq), which are summarized in Table 3 and Table 4.
4 Conclusions
1) The modified sawdust showed a significant capacity to absorb Cu(II) and Ni(II) ions in single- adsorption system as well as in the binary ones.
Table 3 Pseudo-second order constants and correlation coefficients for adsorption of Cu(II) and Ni(II) on modified sawdust
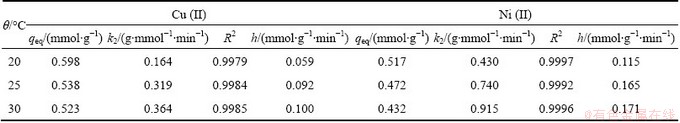
Table 4 Pseudo-second order constants for adsorption of binary system on modified sawdust

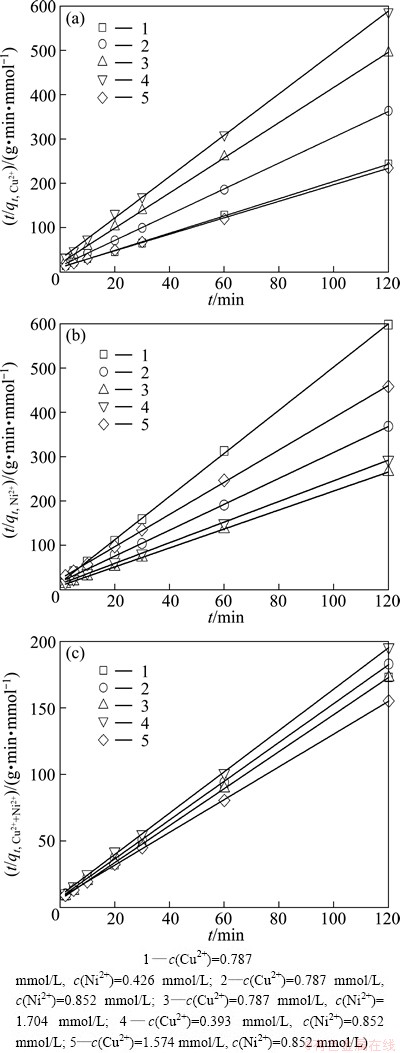
Fig. 9 Pseudo-second order kinetic model plots for adsorption of binary system under conditions of temperature 25 °C, pH 6.0±0.1 and adsorbent dosage 1.0 g/L
2) In single adsorption system, the equilibrium data are described by Langmuir isotherm model. And the equilibrium adsorption capacity and thermodynamic parameters both varied with the change of adsorbent dosage. But the thermodynamic of parameters obtained from Langmuir constant indicated that the adsorption process of Cu(II) and Ni(II) on modified sawdust is spontaneous and exothermic.
3) The equilibrium data of mixtures of Cu(II) and Ni(II) are graphically represented by 3D adsorption surface and modeled by the modified extended Langmuir model.
4) According to the kinetic tests, the pseudo-second order model has fitted better the single metal adsorption data as well as the Cu(II) and Ni(II) binary systems, and the values of activation energy are 59.12 and 55.92 kJ/mol, respectively.
References
[1] SCHWARZENBACH R P, ESCHER B I, FENNER K, HOFSTETTER T B, JOHNSON C A, GUNTEN U V, WEHTLI B. The challenge of micropollutants in aquatic systems [J]. Science, 2006, 313: 1072-1077.
[2] FU F L, WANG Q. Removal of heavy metal ions from wastewaters: A review [J]. J Environ Manage, 2011, 92(3): 407-418.
[3] CUI Z X, REN Q K, AI S, BIAN D. Research progress in heavy metal wastewater treatment and recovery [J]. Environ Sci Technol, 2010, 33(12F): 375-377.
[4] BAILEY S E, OLIN T J, BRICKA R M, ADRIAN D D. A review of potentially low-cost sorbents for heavy metals [J]. Water Res, 1999, 33 (11): 2469-2479.
[5] FENG N C, GUO X Y. Characterization of adsorptive capacity and mechanisms on adsorption of copper, lead and zinc by modified orange peel [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(5): 1224-1231.
[6] WAN NGAH W S, HANAFIAH M A K M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review [J]. Bioresour Technol, 2008, 99(10): 3935-3948.
[7] LIANG S, GUO X Y, FENG N C, TIAN Q H. Effective removal of heavy metals from aqueous solutions by orange peel xanthate [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(s1): s187-s191.
[8] WOJNICKI M, PACLAWSKI K, SOCHA R P, FITZNER K. Adsorption and reduction of platinum(IV) chloride complex ions on activated carbon [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(4): 1147-1156.
[9] ZHANG C, PRICE L M, DALY W H. Synthesis and characterization of a trifunctional aminoamide cellulose derivative [J]. Biomacromolecules, 2006, 7(1): 139-145.
[10] CHANG Q, HAO X K, DUAN L L. Synthesis of crosslinked starch-polyacrylamide-co-sodium xanthate and its performances in wastewater treatment [J]. J Hazard Mater, 2008, 159(2-3): 548-553.
[11] LANGMUIR I. The adsorption of gases on plane surfaces of glass, mica and platinum [J]. J Am Chem Soc, 1918, 40(9): 1361-1403.
[12] HALL K R, EAGLETON L C, ACRIVOS A, VERMEULEN T. Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions [J]. Ind Eng Chem Fundam, 1966, 5(2): 212-223.
[13] CERVERA M L, ARNAL M C. Removal of heavy metals by using adsorption on alumina or chitosan [J]. Anal Bioanal Chem, 2003, 375(6): 820-825.
[14] PAN G, LISS P S, KROM M D. Particle concentration effect and adsorption reversibility [J]. Colloids Surf A, 1999, 151(1-2): 127-133.
[15] COX L, HERMOSIN M C, CELIS R. Sorption of two polar herbicides in soils and soil clay suspensions [J]. Water Res, 1997, 31(6): 1309-1316.
[16] GOK O, OZCAN A, ERDEM B, OZCAN A S. Prediction of the kinetics, equilibrium and thermodynamic parameters of adsorption of copper (II) ions onto 8-hydroxy quinoline immobilized bentonite [J]. Colloids Surf A, 2008, 317(1): 174-185.
[17] PAN G, QIN Y W, LI X L, HU T D, WU Z Y, XIE Y N. EXAFS studies on adsorption desorption reversibility at manganese oxides water interfaces: I. Irreversible adsorption of zinc onto manganite (γ-MnOOH): particle concentration effect and adsorption reversibility [J]. J Colloid Interface Sci, 2004, 271(1): 28-34.
[18] QIN Y W, PAN G, ZHANG M M, LI X L. Adsorption of zinc on manganite (γ-MnOOH): Particle concentration effect and adsorption reversibility [J]. J Environ Sci, 2004, 16 (4): 627-630.
[19] AKSU Z, ACIKEL U, KUTSAL T. Application of multicomponent adsorption isotherms to simultaneous biosorption of Iron(III) and Chromium (VI) on C. vulgaris [J]. J Chem Technol Biotehcnol, 1997, 70(4): 368-378.
[20] PAGNANELLI F, ESPOSUTO A, VEGLIO F. Mutli-metallic modeling for biosorption of binary systems [J]. Water Res, 2002, 36(16): 4095-4105.
[21] HO Y S, MCKAY G. A comparison of chemisorptions kinetic models applied to pollutant removal on various sorbents [J]. Trans IChemE, 1998, 76(4): 332-340.
[22] HO Y S, NG J C Y, MCKAY G. Kinetics of pollutants sorption by biosorbents: Review [J]. Sep Purif Methods, 2000, 29(2): 189-232.
乙二胺改性木屑黄原酸盐对水溶液中Cu(II)和Ni(II)离子吸附平衡及动力学
夏 璐1,2,3,胡伊旭1,张博涵1
1. 广西民族大学 化学化工学院,南宁 530008;
2. 广西民族大学 广西林产化学与工程重点实验室,南宁 530008;
3. 广西民族大学 广西高校化学与生物转化过程新技术重点实验室,南宁 530008
摘 要:使用乙二胺改性的木屑黄原酸盐对水溶液中的Cu(II)、Ni(II)离子进行吸附。在单离子体系中,考虑影响因素(温度、投加量)对Cu(II)、Ni(II)单离子吸附等温线的影响;并计算Cu(II)、Ni(II)离子吸附的热力学参数:吸附吉布斯自由能(△GΘ)、吸附过程的焓变(△HΘ)以及熵变(△SΘ),表明此吸附是一个放热自发的过程。在Cu(II)和Ni(II)双离子体系中,采用修正后的拓展Langmuir模型对体系的吸附情况可以进行很好的预测。在单离子体系和双离子体系中,吸附过程的数据均可通过准二级动力学模型进行描述;计算得到其对Cu(II)和Ni(II)单离子的吸附活化能分别为59.12 和 55.92 kJ/mol。结果表明,金属离子在改性木屑表面的吸附效果会受到另一离子存在的影响。
关键词:铜;镍;吸附;改性木屑
(Edited by Hua YANG)
Foundation item: Project (41061044) supported by the National Natural Science Foundation of China; Projects (2010GXNSFD013016, 2012GXNSFAA053017) supported by the Natural Science Foundation of Guangxi, China
Corresponding author: Lu XIA; Tel: +86-771-3260558; E-mail: xialugx@163.com
DOI: 10.1016/S1003-6326(14)63137-X