
Strengthening bulk metallic glasses with minor alloying additions
KONG Jian(孔 见), XIONG Dang-sheng(熊党生), YUAN Qun-xing(袁群星), YE Zhi-tao(叶治淘)
Department of Materials Science and Engineering, Nanjing University of Science and Technology,
Nanjing 210094, China
Received 10 April 2006; accepted 25 April 2006
Abstract: Cu47Ti34Zr11Ni8, (Cu47Ti34Zr11Ni8)99Si and (Cu47Ti34Zr11Ni8)99Al bulk metallic glass were prepared by copper mold casting method, and the thermal stability, mechanical properties and microstructures of them were studied. With minor alloying of Si and Al additions, the glass transition temperature (Tg), crystallization temperature (Tx1) and temperature interval of supercooled liquid region ?Tx (=Tx1-Tg) and reduced glass transition temperature (Trg) were proved to be changed from 672 K, 734 K, 62 K, 0.575 to 691 K, 752 K, 61 K, 0.592 and to 681 K, 729 K, 48 K, 0.590, respectively. The results indicate that the glass-forming ability (GFA) are improved with minor alloying additions. And the bulk glasses also exhibits high three point-bending flexural strength. Because of the additions of Si and Al, three point-bending flexural strength and flexural modulus of the bulk glass change from 2 350 MPa, 102 GPa to 3 260 MPa, 102 GPa and 2 970 MPa, 108GPa respectively. The obvious strengthening is due to the appearance of the medium-range ordered regions with a size of 2-5 nm under the high-resolution TEM image. The reason that the mixed amorphous and nanocrystalline phases caused by minor alloying of Si and Al additions, is that Si or Al is the third kind of elements, which are different from other constituting elements, and there are a strong bonding and atoms size effects between constituting elements, which cause the glass-forming ability (GFA) and the bulk metallic glasses strength improving.
Key words: bulk metallic glass (BMG); copper-titanium-zirconium-nickel alloy; glass-forming ability (GFA); thermal stability; mechanical property.
1 Introduction
A number of previous results[1-5] indicated that bulk metallic glass(BMGs) could be added in a small amount of other element to expect to cause the second phase appearance and further increase the mechanical properties. The mechanical properties of Cu47Ti34Zr11- Ni8, Cu47Ti33Zr11Ni8Fe, and Cu47Ti33Zr11Ni8Si bulk metallic glasses were investigated by CALIN et al[1], with in situ formed nanoscale precipitates in glassy matrix the Cu47Ti33Zr11Ni8Si exhibits distinct plastic strain in room temperature compression tests. DAS et al[4, 5] reported a novel class of ‘‘work-hardenable’’ metallic glass. The addition of 5%Al (mole fraction) to the Cu50Zr50 glass increases the yield strength significantly and improves the room temperature deformability even though the glass transition temperature of the BMG is quite high. This intrinsic ductility is attributed to the special microstructural features of the heterogeneities at the different length scales, which enable easy and homogeneous nucleation of the shear bands and continuous multiplication during deformation.
In this paper, we report the obviously strengthening phenomena when minor Al or Si is added in the Cu-Ti-Zr-Ni BMGs, and the reason is due to the appearance of the medium-range ordered regions with a size of 2-5 nm in amorphous matrix.
2 Experimental
Quaternary Cu-based alloy ingots with compositions of Cu47Ti34Zr11Ni8, (Cu47Ti34Zr11- Ni8)99Si, (Cu47Ti34Zr11Ni8)99Al were prepared by arc melting the mixture of pure metals (purity ≥99.9%) in an argon atmosphere, and the alloys were remelted several times in order to obtain homogeneity and finally bulk alloy sheets with a length of 50 mm and cross section of 1 mm×5 mm were fabricated in an suction casting facility attached to the arc melter. The glassy structure was identified by X-ray diffraction (XRD) with Cu, Kα radiation (λ=0.154 nm) source calibrated with a silicon standard, optical microscopy (OM), scanning electron microscopy (SEM, Philips Quanta-200) and high resolution transmission electron microscope (HRTEM, Philips Tecnai 20U-Twin). The thermal stability associated with glass transition, crystallization temperature and supercooled liquid region was examined by differential scanning calorimetry (DSC) at a heating rate of 20 ℃/min under ultra-high purity argon with PE DSC-7 detector type. The melting and liquidus temperatures were measured by differential thermal analysis (DTA) at a heating rate of 20 ℃/min under ultrahigh purity argon with Shimadzu DTA-50 detector type. Three points bending test was performed with AGS-10KND machine. The support spans and the cross head speed in the bend test were 20 mm and 0.5 mm/min, respectively. The width and the thickness for the specimen were 5mm and 1mm, respectively.
3 Results and discussion
Fig.1 shows the XRD patterns of the cast Cu47Ti34Zr11Ni8, (Cu47Ti33Zr11Ni8)99Si and (Cu47- Ti34Zr11Ni8)99Al sheets. Only a broad peak exists with no distinct crystalline peaks appears, which indicates the formation of a glassy single phase. The results are also supported by XRD patterns of the central region, OM observation and SEM backscattered election image.
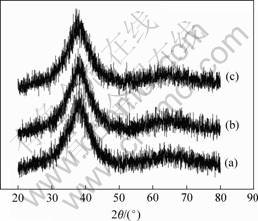
Fig.1 XRD patterns of BMGs: (a) Cu47Ti34Zr11Ni8; (b) (Cu47Ti34Zr11Ni8)99Si; (c) (Cu47Ti34Zr11Ni8)99Al
The DSC traces of three kinds Cu-Ti-Zr-Ni BMGs are shown in Fig.2. The glass transition temperature, Tg, is here defined as the onset of the endothermic event. The primary crystallization temperature, Tx1, is defined as the onset temperature of the first exothermic event. Based on the DSC pattern, it is observed that Tg and Tx1 are 672 K, 734 K for Cu47Ti34Zr11Ni8 alloy, 691 K, 752 K for (Cu47Ti33Zr11Ni8)99Si alloy and 681 K, 729 K for (Cu47Ti34Zr11Ni8)99Al alloy. As a result, the supercooled liquid region defined by the temperature interval between Tg and Tx1 , ?Tx (=Tx1-Tg) changes from 62 K to 61 K after 1%Si addition, and 48 K after 1%Al addition respectively. To prove the alloy crystallizing behavior, the alloys begin to melt at a solidus temperature Ts and completely melting at the liquidus temperature Tl, a high temperature DTA scan was used with a heating rate of 20 K/min. The results are shown in Fig.3. It can be seen that the Ts and Tl are 1 118 K, 1 161 K for Cu47Ti34Zr11Ni8 alloy, respectively, 1 128 K,
1 167 K for (Cu47Ti33Zr11Ni8)99Si alloy and 1 116 K,
1 154 K for (Cu47Ti34Zr11Ni8)99Al alloy. The reduced glass transition temperature Trg (=Tg/Tl) increases from from 0.575 to 0.592 and 0.590, respectively. In Cu-based glass-forming alloys systems[1, 6, 7], the reduced glass transition temperature Trg always plays a dominant role in determining the glass-forming ability (GFA), therefore, these changes may reflect the improving of GFA.
Fig.4 shows the bending flexural stress-strain curves of Cu47Ti34Zr11Ni8, (Cu47Ti33Zr11Ni8)99Si and
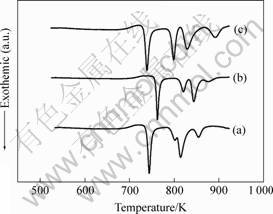
Fig.2 DSC thermograms of BMGs: (a) Cu47Ti34Zr11Ni8; (b) (Cu47Ti34Zr11Ni8)99Si; (c) (Cu47Ti34Zr11Ni8)99Al
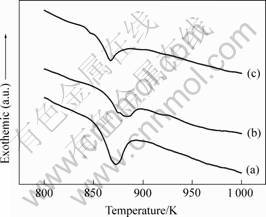
Fig.3 DTA thermograms of BMGs: (a) Cu47Ti34Zr11Ni8; (b) (Cu47Ti34Zr11Ni8)99Si; (c) (Cu47Ti34Zr11Ni8)99Al
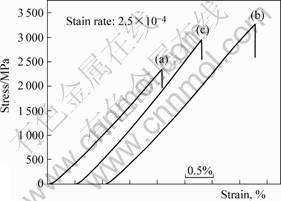
Fig.4 Bending flexural stress-strain curves of BMG sheets: (a) Cu47Ti34Zr11Ni8; (b) (Cu47Ti34Zr11Ni8)99Si; (c) (Cu47Ti34- Zr11Ni8)99Al
(Cu47Ti34Zr11Ni8)99Al amorphous alloy sheets subjected to the three points bend test at room temperature. By applying the load at three points and causing bending, a tensile force acts on the material opposite the midpoint, and the fracture begins at this location. The three point-bending flexural strength σb,f, fracture strain εb,f and flexural modulus Eb,f are evaluated to be 2 350 MPa, 2.1% and 102 GPa for the Cu47Ti34Zr11Ni8 sheets, respectively, 3 260 MPa, 2.7% and 102 GPa for the (Cu47Ti34Zr11Ni8)99Si sheets, respectively, 2 970 MPa, 2.5% and 108 GPa for the (Cu47Ti34Zr11Ni8)99Al sheets, respectively. The bending test is a useful way to measure the plasticity of the materials. No obvious plastic strain could be seen for the BMG sheets, the possible reason is that the ratio of width to thickness (5∶1) is too high. But the properties, which are examined at the same conditions, are also useful to be compared. After the Si or Al addition, the flexural strength is proved to be increased obviously.
With the aim of investigating the reasons of the remarkable improvement of the bending deflection, we examined the microstructure of the BMGs. Fig.5 shows the high-resolution TEM images and selected-area electron diffraction (SAED) patterns of the cast Cu47Ti34Zr11Ni8, (Cu47Ti34Zr11Ni8)99Si and (Cu47- Ti34Zr11Ni8)99Al sheets. The SAED patterns of the BMGs show amorphous halo rings without reflection spots, indicating that the three BMGs have amorphous structure nature in statistic meaning as having been proved by XRD. In Fig.5(a), the high resolution TEM image from the bulk Cu47Ti34Zr11Ni8 specimen shows a contrast typical of the glassy structure, indicating a formation of glassy phase without appreciable crystalline phase. Figs.5(b) and (c) show the HRTEM images of the bulk amorphous (Cu47Ti34Zr11Ni8)99Si and (Cu47Ti34- Zr11Ni8)99Al alloys. The HRTEM images consist of a number of fringe contrast regions with a size of 2 to
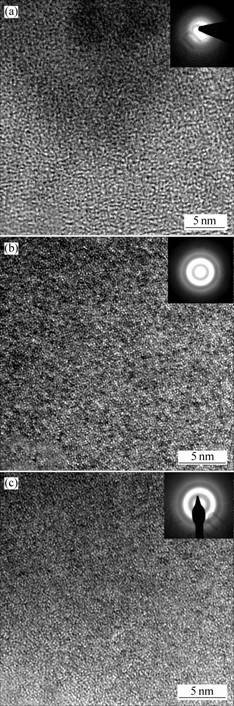
Fig.5 HRTEM images and SAED patterns of cast Cu47Ti34- Zr11Ni8(a), (Cu47Ti34Zr11Ni8)99Si (b) and Cu47Ti34Zr11- Ni8)99Al sheets(c)
5 nm embedded homogeneously in the glassy matrix. These kinds of microstructures have been found by YOKOYAMA et al[8] in Zr-based BMGs. Their studies indicated that the addition of a small amount of Nb which had a positive heat of mixing against Zr (+2 kJ/ mol), caused the generation of homogeneous crystal nucleation in supercooled liquid to Zr-Al-Ni-Cu bulk glassy alloys, and also caused the formation of a finely mixed structure consisting of nanocrystalline particles with a size of 2 to 6 nm embedded in the glassy phase, then the three-point bending flexural strength and flexural modulus are both increased with the 1%Cu substituted by Nb.
But in the present study, the addition element Si or Al has a negative heat of mixing against Ti, Zr, Ni[9]. This means that there is a different mechanism working here. The alloy components of bulk metallic glass can be divided into five groups after examining in more detail the features of the alloy components of the bulk glassy alloy systems[6]. Si or Al plays an important role in group which consists of ETM (early transition metal)-SM (simple metal)-LTM (late transition metal) as exemplified by Zr-Al-(Ni, Cu), Ln-Al-( Ni, Cu) and Cu-Al-Zr. There is a strong bonding between SM and ETM or LTM. Only two types of ETM and LTM are contained in Cu-Ti-Zr-Ni alloy system. There is some difference in atomic size and a large mixing enthalphies between Si or Al and the constituting elements, the Goldschmidt atomic radius[9] of Si is 0.117 nm and Al is 0.143 nm, which are different from 0.128, 0.160, 0.147 and 0.125 nm for Cu, Zr, Ti and Ni, respectively. The mixing enthalphies[9] of Si-Cu, Si-Ti, Si-Zr and Si-Ni are -8, -49, -67 and -23 kJ/mol, respectively, and Al-Cu, Al-Ti, Al-Zr and Al-Ni are -1, -30, -44 and -22 kJ/mol, respectively. The large mixing enthalphies between Si or Al and constituted elements cause a strong bonding in supercooled liquids near the Tg which increase the Tg, and cause the structure of the bulk metallic glass changing which induce the appearance of the medium-range ordered regions with a size of 2-5 nm under HRTEM image and lead to the variation of mechanical properties. All of these changes reflect the atomic bonding nature of Si and Al against the other constituent elements. In one word, the reason of the appearance of nanocrystalline glassy alloy is that Si or Al is the third kind of elements, which are different from other constituting elements, and there are a strong bonding and atoms size effect between constituting elements, which cause the glass-forming ability (GFA) and the bulk metallic glasses strength improving. Here, it may be important to point out that the conflicting behaviors of enhancing GFA and nuclear phenomena mean that the crystalline nuclei have a peculiar structure which is different from the equilibrium crystalline structures obtained after the completion of solidification.
With minor alloying additions, the obvious strengthening of Cu-Ti-Zr-Ni BMGs is due to the appearance of the medium-range ordered regions which act as the second phase and cause the shear bands propagating. In general, BMGs fail due to the formation of shear bands on loading[1-4, 10-15], and this usually results in catastrophic failure, since the initiation of the shear band and its propagation take place almost simultaneously. As such, methods for improving the plasticity and strength of bulk amorphous alloys revolve around the question of how to generate a large number of shear bands and how to impede their sudden propagation. The addition of a second phase[1-5, 8, 10-15] has long been utilized as a means of improving the ductility of brittle materials. The principle behind this method is to introduce stress disturbance near the second phase within the amorphous matrix during the process of loading. This stress disturbance caused by the second phase can alter both the location and the contour, to which the maximum stress (or strain) is imposed. It also interacts with the stress field caused by the propagating shear bands, leading to multiplication, branching and deflection of the shear bands. Therefore, the stress field near the second phase can serve as an effective barrier to the direct propagation of the shear bands, and it causes the strength and ductility of BMGs improved.
4 Conclusions
Cu47Ti34Zr11Ni8, (Cu47Ti34Zr11Ni8)99Si and (Cu47Ti34Zr11Ni8)99Al bulk metallic glass were prepared by copper mold casting method and the effects of minor added elements in BMGs were studied. With minor alloying of Si and Al additions, the glass transition temperature (Tg), crystallization temperature (Tx1) and temperature interval of supercooled liquid region ?Tx and reduced glass transition temperature (Trg) were proved to be changed from 672 K, 734 K, 62 K, 0.575 to 691 K, 752 K, 61 K, 0.592 and then to 681 K, 729 K, 48 K, 0.590, respectively. And the bulk glasses also exhibited high three point-bending flexural strength. Because of the additions of Si and Al, three point-bending flexural strength and flexural modulus of the bulk glass were changed from 2 350 MPa, 102 GPa to 3 260 MPa, 102 GPa and 2 970 MPa, 108 GPa respectively. The obvious strengthening was due to the appearance of the medium-range ordered regions with a size of 2-5 nm under the high-resolution TEM image.
References
[1] CALIN M, ECKERT J, SCHULTZ L. Improved mechanical behavior of Cu-Ti-based bulk metallic glass by in situ formation of nanoscale precipitates [J]. Scripta Materialia, 2003, 48: 653-658.
[2] SAIDA J, DENY A, SETYAWAN H, KATO H, INOUE A. Nanoscale multistep shear band formation by deformation-induced nanocrystallization in Zr-Al-Ni-Pd bulk metallic glass [J]. Applied Physics Letters, 2005, 87: 151907.
[3] WANG W H, BAI H Y. Carbon-addition-induced bulk ZrTiCuNiBe amorphous matrix composite containing ZrC particles [J]. Materials Letters, 2000, 44: 59-63.
[4] DAS J, TANG M B, KIM K B, THEISSMANN R, BAIER F, WANG W H, ECKERT J. ‘‘Work-hardenable’’ ductile bulk metallic glass [J]. Physical Review Letters, 2005, 94: 205501.
[5] KIM K B, DAS J, BAIER F, TANG M B, WANG W H, ECKERT J. Heterogeneity of a Cu47.5Zr47.5Al5 bulk metallic glass [J]. Applied Physics Letters, 2006, 88: 051911.
[6] INOUE A, TAKEUCHI A. Recent progress in bulk glassy alloys [J]. Mater Trans JIM, 2002, 43: 1892-1906.
[7] KONG Jian, XIONG Dang-sheng, YE Zhi-tao. Effect of minor aluminium addition in Cu47Ti34Zr11Ni8 bulk metallic glasses [J]. Trans Nonferrous Met Soc China, 2005, 15(S3): 176-180.
[8] YOKOYAMA Y, YAMANO K, FUKAURA K, SUNADA H, INOUE A. Nanocrystalline Zr-based bulk glassy alloys with high flexural strength [J]. Mater Trans JIM, 1999, 40: 1015-1018
[9] BOER F R, BOOM R, MATTENS W C M, MIEDEMA A R, NIESSEN A K. Cohesion in Metals [M]. Amsterdam: North-Holland, 1988.
[10] HUFNAGEL T C, OTT R T, ALMER J. Structural aspects of elastic deformation of a metallic glass [J]. Physical Review B, 2006, 73: 064204.
[11] CHOI-YIM H, BUSCH R, KOOESTER U, JOHNSON W L. Synthesis and characterization of particulate reinforced Zr57Nb5Al10Cu15.4Ni12.6 bulk metallic glass composites [J]. Acta Materialia, 1999, 47(8): 2455-2462.
[12] KATO H, HIRANO T, MATSUO A, KAWAMURA Y, INOUE A. High stength and good ductility of Zr55Al10Ni5Cu30 bulk glass containing ZrC pacticles [J]. Scripta Materialia, 2000, 43: 503-507.
[13] HAYS C C, KIM C P, JOHNSON W L. Microstructure controlled shear band pattern formation and enhanced plasticity of bulk metallic glasses containing in situ formed ductile phase dendrite dispersions [J]. Physical Review Letters, 2000, 84(13): 2901-2904.
[14] FAN C, OTT R T, HUFNAGEL T C. Metallic glass matrix composite with precipitated ductile reinforcement [J]. Applied Physics Letters, 2002, 81(6): 1020-1022.
[15] KIM H S. Strengthening mechanisms of Zr-based devitrified amorphous alloy nanocomposites [J]. Scripta Materialia, 2003, 48: 43-49.
(Edited by YUAN Sai-qian)
Foundation item: Project(50575106) supported by the National Natural Science Foundation of China; Project (AB41325) supported by the Young Scholar Foundation of Nanjing University of Science and Technology
Corresponding author: KONG Jian; Tel: +86-25-84315325; Fax: +86-25-84315325; E-mail: kongjian68@mail.njust.edu.cn