
New technology for comprehensive utilization of
aluminum-chromium residue from chromium salts production
LI Xiao-bin(李小斌), QI Tian-gui(齐天贵)1, JIANG Xin-min(江新民)1,
ZHOU Qiu-sheng(周秋生)1, LIU Gui-hua(刘桂华)1, PENG Zhi-hong(彭志宏)1,
HAN Deng-lun(韩登仑)2, ZHANG Zhong-yuan(张忠元)2, YANG Kun-shan(杨昆山)2
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Gansu Jinshi Chemical Engineering Corporation, Ltd., Zhangye 734000, China
Received 20 April 2007; accepted 24 August 2007
Abstract: Colloidal aluminum-chromium residue(ACR) was mass-produced in chromate production process, and the large energy consumption and high recovery cost existed in traditional methods of utilizing such ACR. To overcome those problems, a new comprehensive method was proposed to deal with the ACR, and was proven valid in industry. In the new process, the chromate was separated firstly from the colloidal ACR by ripening and washing with additives, by which more than 95% hexavalent chromium was recovered. The chromium-free aluminum residue(CFAR), after properly dispersed, was digested at 120-130 ℃ and more than 90% alumina can be recovered. And then the pregnant aluminate solution obtained from digestion was seeded to precipitate aluminum hydroxide. This new method can successfully recover both alumina and sodium chromate, and thus realize the comprehensive utilization of ACR from chromate industry.
Key words: utilization; aluminum-chromate residue(ACR); aluminum hydroxide; sodium chromate hydrate
1 Introduction
The production of chromate with chromite as raw material is one of the main parts of inorganic chemical industry. But the waste residues produced in chromate industry result in pollution seriously, which restrains the further development of chromate[1-2]. One of the main solid wastes in chromate plant is aluminum-chromate residue(ACR), the amount of which depends on the components of the chromite and the condition of the oxidative sintering process in chromate production. In the typical lime-free chromium extraction process for sodium chromate production, 600-800 kg ACR (Na2Cr2O7·2H2O 22%-28%, Al(OH)3 8%-15%, and H2O 50%-65%) is generated when 1 t sodium chromate is produced. The ACR is composed of aluminum tri-hydrate and amount of sodium chromate with virulent Cr(Ⅵ) that causes severe pollution to the environment. It is necessary and urgent to eliminate chrome pollution in the production of chromate[2-5].
Until now, several processes for chromate recovery from ACRs have been proposed, such as roasting- washing method[1,6-7], precipitation with phosphate co- precipitation[1,6-7], washing with pressure filter[1,7], re-dispersed flocculation process[1,7-8]. Although the roasting-washing method operated at 700-1 000 ℃ can obtain high chromate recovery rate and high chromate concentration washing solution, this method was limited for its costly equipment investment, intensive labor, poor operational circumstance and high sodium chromate recovery cost[6]. The phosphate co-precipitation method can be operated at an improved operation environment, but the unstable efficiency, expensive recovery cost and being apt to leading secondary pollution made it difficult to realize industrial production[1,7]. In the washing with pressure filter method and the re-dispersed flocculation process, due to the poor osmosis in the ACR particles, only 20%-40% of chromate recovery rate can be achieved[7]. Therefore, those methods mentioned above can hardly be utilized in practical way.
In addition, the previous processes for dealing with the ACR focused on the recovery of chromate to reduce the environment pollution, but unfortunately the extraction of aluminum was ignored. To comprehensively utilize both chromate and aluminum in ACR, a new method was proposed in this work.
2 Experimental
2.1 Materials
The ACR used in the experiments was obtained from Gansu Jinshi Chemical Engineering Corporation and originally contained 50%-65% water. The chemical composition of dry ACR is listed in Table 1.
Table 1 Chemical composition of dry ACR (mass fraction, %)
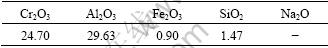
Spent liquor was prepared by dissolving Al(OH)3 (industrial pure) in NaOH (industrial pure). Na2CO3 and Na2SO4 added in the spent liquor were in analytical grade. Table 2 gives the chemical analysis results of the spent liquor that was diluted to preset concentration before experiments.
Table 2 Chemical analysis results of spent liquor (g/L)
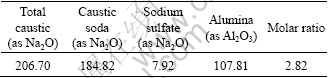
2.2 Equipments
Autoclave with eight volume reaction bombs of 150 mL, with glycerine as heating medium, and temperature controlling accuracy ±1 ℃, was made in CSU (Central South University, China). Settling container with 300 mL glass cylinder, with water as heating insulation medium, and temperature controlling accuracy ±0.5 ℃, was assembled. The seed precipitation tanker had a volume of 1.5 L, with water as heating insulation medium, and the temperature was controlled in the range of 0.5 ℃ automatically.
2.3 Methodology
ACR, water and additives were weighed accurately and put into the reactor bombs, which were then put into autoclave. The re-crystallization reaction was carried out at 70-80℃ for 1-2 h with additives, then the bombs were taken out and cooled by water. The slurry was separated by vacuum filter and the filter cake was washed with water. The separation of aluminum and chromate was realized and the CFAR was thus obtained.
The digestion was carried out in autoclave. The CFAR, reducer agents and spent liquor were charged into the reactor bombs, digested at 120-130 ℃. The slurry obtained was then separated by vacuum filtration and the filter cake was washed, dried and weighted. The concentration of caustic, alumina and chromate in filtrate were analysed by volume analytical method, in order to calculate the aluminum recovery rate.
The sedimentation test was operated in glass cylinder at 96 ℃ in a transparent glass container. The height and volume of the cylinder was 300 mm and 300 mL respectively. Put the digestion slurry and polymer flocculant into glass cylinders and mix them with blender. Then the height of the slurry in different settling time was recorded, then the sedimentation curve was plotted and the settling velocity was calculated.
The seed precipitation was studied in the precipitation tankers. The pregnant aluminate solution, chromate liquor and aluminum hydroxide seed were charged in the tankers with stirrer and automatic temperature regulator.
3 Results and discussion
3.1 Separation of chromate and aluminum
The aluminum exists in the form of amorphous boehmite in the ACR while the chromate is sodium chromate 2-hydrate. Fig.1 shows the XRD pattern of the ACR.
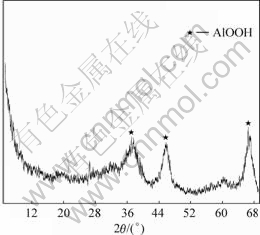
Fig.1 XRD pattern of ACR
The ACR is in a gel structure where the alumina colloid forms a spatial reticulation, in which the sodium chromate fully fills. The colloid particles have a high affinity to the chromate solution, so it is difficult to separate chromate from this colloidal reticular structure [1,9]. To separate the chromate from the ACR, the reticular structure must be destroyed. The new method proposed in this paper is to ripen to break the gel structure in hydro-system with additions, thereby making the chromate escape from the ACR. And after washing less than 0.5% virulent Cr(Ⅵ) remains in the ACR, i.e. more than 95% hexavalent chromium was recovered.
Fig.2 shows the XRD pattern of the CFAR obtained by the new chromate separation process. Compared with Fig.1, the crystallization degree of the CFAR is superior to the untreated ACR, which illustrates that part of the colloidal aluminum hydroxide is converted to higher crystallinity mainly due to the destroyed colloidal structure.
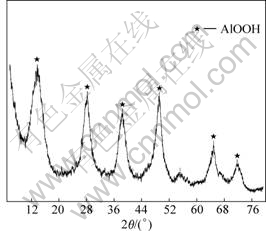
Fig.2 XRD pattern of CFAR
3.2 Digestion of CFAR
The wet CFAR obtained from the chromate separa- tion process contains 50%-65% water. The chemical
analysis shows that the dry CFAR consists of 54.44% Al2O3, 2.19% Cr2O3 and 2.96% SiO2, which is suitable raw material for aluminum hydroxide production by Bayer process. The digesticability in Bayer process of the ground CFAR dried at 90-95 ℃ was tested, and the results are listed in Table 3.
Table 3 Digestion results of CFAR
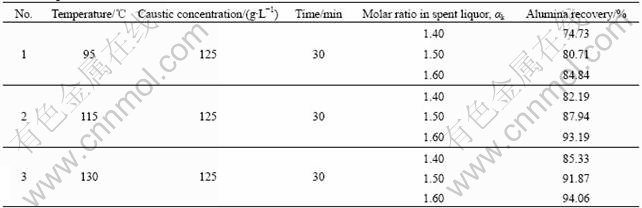
Table 3 show that the alumina recovery rate is enhanced dramatically with increasing digestion temperature and molar ratio of caustic soda to alumina (αk) in spent liquor. With the digestion temperature above 115 ℃ and the αk in spent liquor being 1.50-1.60, more than 90% alumina recovery could be obtained within 30 min. Therefore, it is proved that the CFAR can be digested with relatively lower temperature and caustic concentration in a short retention time.
It is found that the dispersion of CFAR in spent liqour also strongly influences the digestion capability (listed in Table 4), which is related to the microstructure of CFAR. As discussed above, although the crystallinity of the CFAR is improved during the chromate separation process, most of the alumina in residue still remains in the aqueous colloidal structure. Those colloid aluminum is subject to aggregating because of the strong attractive molecular force. Those aggregates cannot be dispersed by low shear stress. In addition, the poor osmosis of those aggregates minimizes the reaction areas when the wet CFAR is poorly dispersed. Thus the alumina recovery will be dominated by the dispersion degree when the residue is not well dispersed. The industry practice indicates that the alumina recovery can reach more than 90% with proper dispersion and ratio of liquor to solid.
Table 4 Influence of dispersion method on alumina recovery

3.3 Sedimentation of digestion slurry
The liquor-solid separation is a very important procedure in the aluminum hydroxide producing process. Because the slurry has a very high liquor/solid ratio, theoretically the pressure filter should be an ideal separation method for the low solid content slurry[10]. But the practice in both laboratory and industry indicated that the pressure filter was not suitable for the slurry produced from CFAR digestion, probably due to the highly dispersed solid particles in the slurry which block the poles of the cloth filter, and thus obstruct liquid to pass through.
Sedimentation, as another important liquor-solid separation method, had been widely adopted in alumina production industry[10]. The electrical double layers on solid particle surface and the flocculant are very important for the sedimentation ability. In this study, a flocculant was selected and some surface potential adjusted additives were added to the slurry to increase the settling velocity. The experiment results of the sedimentation are plotted in Fig.3, showing that the settling velocity in the first 10 min reaches 1.06 m/h.
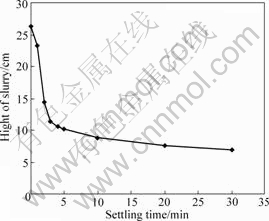
Fig.3 Sedimentation curve of digestion slurry (Digestion condition: temperature 120 ℃, caustic concentration 125 g/L, time 30 min, mole ratio in spent 1.6)
3.4 Influence of chromate on alumina hydroxide production system
The hexavalent chromium in the CFAR will partly dissolve and accumulate during the Bayer recycling process, thus influencing aluminum hydroxide production.
ZOU et al[11] and WANG et al[12] studied the equilibrium of NaOH-Na2CrO4-NaAlO2-H2O system and discovered that the alumina solubility in aluminate solution could be affected by chromate, but the alkali concentration in their studies was much higher than that in popular Bayer digestion process. Thus the influence of the chromate on the digestion process was studied in this work, and the result is listed in Table 5.
Table 5 Influence of sodium chromate on digestion efficiency of alumina

Table 5 shows that the alumina digestion efficiency has little fluctuation even as the concentration of sodium chromate dissolved in the recycle reaches about 8 g/L (as
Cr2O3), which implies that a few sodium chromate in the recycling liquor does not affect the digestion process under the experimental conditions.
Impurities in pregnant liquor have profound influences on the precipitation rate and the product purity. ZOU and ZHANG considered that a spot of chromate in pregnant liquor has little effect on the precipitation rate and the product quality, while the profound influences would emerge with high chromate concentration in pregnant liquor[13]. The influences of different chromate concentration on precipitation were studied and the precipitation curves are plotted in Fig.4.
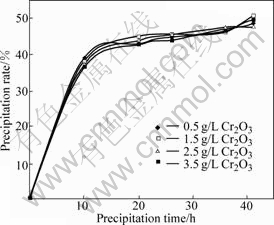
Fig.4 Precipitation curves of pregnant liquors with different chromate concentration (Seed precipitation conditions: Al2O3 concentration 130 g/L, caustic molar ratio 1.6, starting temperature 65 ℃, end temperature 50 ℃, seed ratio 2.0, chromate concentration measured as Cr2O3)
Fig.4 shows that the precipitation rate for all of the pregnant liquors with different chromate concentration reaches 47%-49% in 41 h without obvious difference. This shows that low concentration of chromate has little effect on precipitation, which is consistent with ZOU’s results.
After the precipitation, the aluminum hydroxide were separated and washed. The impurities in the aluminum hydroxide produced from the pregnant with the highest chromate concentration were analyzed by inductive coupled plasma emission spectrometer (ICPES). The results are listed in Table 6.
Table 6 Impurities in alumina hydroxide from precipitation (mass fraction, %)
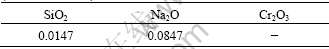
Table 6 shows that the chromium can’t be found in the products by ICPES, which indicates that low concentrated chromate in pregnant has no effect on product purity.
Although it is proven that low concentration of chromate has little effects on digestion and precipitation process, the concentration of chromate would accumulate during the Bayer cycling process. Therefore, how to prevent the chromate from accumulation within Bayer process is still an essential problem to be solved. In this study, reduction agents were added to the digestion system to convert the hexavalent chromium to tervalent chromium. Due to the less solubility of the latter in aluminate solution, the chromate could be removed from the recycling liquor[14-15]. The industrial practice shows that the concentration of chromate in aluminate solution could be kept below 5 g/L (as Na2Cr2O7·2H2O) when some reduction agents were used.
In addition, the chromium was concentrated in digestion residue when the reduce agent was added in digestion process. The digestion residue has a high concentration of chromium and can be returned to sodium chromate production process as raw material for further chromium recovery. With this process, the chromium in CFAR can be utilized completely and the pollution of Cr(Ⅵ) could be eliminated.
4 Recommended flow and industrialization
Based on the studies above, the schematic flow sheet to process ACR was recommended as Fig.5.
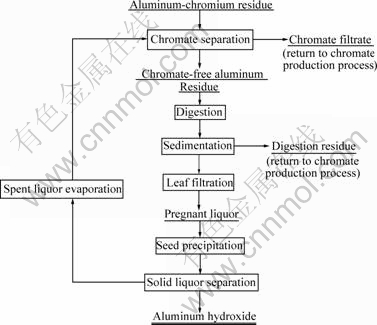
Fig.5 Schematic flow sheet for comprehensive utilization of ACR
This method has been put into industrial practice after fundamental studies in laboratory and semi-commercial test, in which both the alumina and sodium chromate can be recovered without other waste residue produced. It is believed that this method will promote the clearer production for chromate production, and realize comprehensive utilization of ACR residue.
5 Conclusions
1) The chromate can be separated from aluminum containing residue and more than 95% hexavalent chromium can be recovered with a ripening process in hydro-system.
2) CFAR can be digested at 120-130 ℃, and more than 90% alumina recovery can be obtained.
3) The slurry of digestion of CFAR has a good sedimentation performance with proper flocculent and modification of surface potential charge.
4) Chromates with low concentration in recycling liquor have little effect on the digestion and seed precipitation process, and the low chromate concentration can be kept in Bayer recycling spent solutions with adding reduction agents in digestion.
5) This new process for recovering both alumina and sodium chromate, has been found industrial application, which would help to minimize the pollution of chromate and realize the comprehensive utilization of ACR.
References
[1] DING Yi, JI Zhu. Production and application of chromic compound [M]. Beijing: Chemical Industry Press, 2003. (in Chinese)
[2] DARRIE G. Commercial extraction technology and process waste disposal in the manufacture of chromium chemicals from ore [J]. Environmental Geochemistry and Health, 2001, 23: 187-193.
[3] JI Zhu. Reaction mechanism of lime-free sinter process for chromate production [J]. Inorganic Chemicals Industry, 1997(1): 18-21. (in Chinese)
[4] XU Hong-bin, ZHANG Yi, LI Zou-hu, ZHENG Shi-li, WANG Zhi-kuan, QI Tao, LI Hui-quan. Development of a new cleaner production process for producing chromic oxide from chromate ore [J]. Journal of Cleaner Production, 2006(2): 211-219.
[5] KOWALSKI Z, WZOREK Z. Utilization of chromic waste in the sodium chromate (VI) production process [J]. Journal of Loss Prevention in Process Industries, 2002, 15: 169-178.
[6] DING Yi. Comprehensive utilization of chromium-contained by-product in the production of chromium salts [J]. Inorganic Chemicals Industry, 1999, 31(4): 37-39. (in Chinese)
[7] CHENG Liang-rong. Summary of recovery and application for aluminum-chromium residue [J]. Chromate Industry, 1992(2): 55-62. (in Chinese)
[8] DENG Xiang-yi, CHEN Xue-mei. Chromium recovery in aluminum enriched residue [J]. Hubei Chemical Industry, 1995(3): 35-37. (in Chinese)
[9] HIEMENZ P C. Colloidal and surface chemical principles [M]. ZHOU Zu-kang, MA Ji-ming, transl. Beijing: Peking University Press, 1986. (in Chinese)
[10] TANG Mo-tang. Hydrometallurgical equipment [M]. Changsha: Central South University Press, 2004. (in Chinese)
[11] ZOU Xing, ZHANG Yi, LI Zuo-hu, HAN Qi-yong. Phase equilibria of NaOH-NaAlO2-Na2CrO4-H2O saline system [J]. Engineering Chemistry & Metallurgy, 1998, 19(2): 118-121. (in Chinese)
[12] WANG Xing-dong, CUI Jin-lian, GE Xin-lei, ZHENG Shi-li, ZHANG Mei, ZHANG Yi. Thermodynamic study of K2CrO4- KAlO2-KOH-H2O and Na2CrO4-NaAlO2-NaOH-H2O systems [J]. Journal of University of Science and Technology Beijing: Mineral Metallurgy Materials, 2004, 11(6): 500-504.
[13] ZOU Xing, ZHANG Yi. The effect of Na2CrO4 on NaAl(OH)4 solution hydrolysis [J]. Environmental Chemistry, 2000, 19(5): 470-473. (in Chinese)
[14] ABRMOVA V Ya. Physical chemistry principle for utilizing raw containing aluminum with alkaline methods [M]. CHEN Qian-de, TANG Xian-liu, HUANG Ji-fen, LI Xiao-bin, transl. Changsha: Central South University of Technology Press, 1988: 34-65. (in Chinese)
[15] JAMES B. Remediation-by-reduction strategies for chromate- contaminated soils [J]. Environmental Geochemistry Health, 2001, 23: 175-179.
Corresponding author: LI Xiao-bin; Tel: +86-731-8830454; E-mail: X.B.Li@mail.csu.edu.cn
(Edited by YUAN Sai-qian)