
Flotation techniques for separation of diaspore from bauxite using Gemini collector and starch depressant
XIA Liu-yin(夏柳荫), ZHONG Hong(钟 宏), LIU Guang-yi(刘广义)
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 19 February 2009; accepted 13 July 2009
Abstract: The floatability of diaspore and three kinds of silicate minerals, including kaolinite, illite and pyrophyllite, by a cationic Gemini surfactant as collector and starch as depressant was investigated as function of reagents concentration and pulp pH. Further studies of artificially mixed minerals and bauxite ore were also detailedly conducted. At last, the pre-desliming reverse flotation separation process was adopted. It can be concluded that the combinational use of a Gemini cationic collector and the starch depressant is effective. The concentrates with Al2O3 to SiO2 mass ratio of 9.66 and the recovery of Al2O3 of 71.73% are obtained from natural bauxite ore (A/S=5.70) at pH value of around 10.
Key words: diaspore; bauxite; reverse flotation; Gemini surfactant
1 Introduction
There are about 2 500 million tonnes of bauxite reserves in China, but more than 98% of the bauxite is of diasporic characteristics with low mass ratio of Al2O3 to SiO2 (designed as A/S), typically ranging from 4 to 6[1-2]. The gangue minerals in bauxites are mainly kaolinite, pyrophyllite and illite, containing titanium and iron impurities. It is known that high grade bauxite, with A/S greater than 8, can be processed directly by Bayer processing[3-4]. For such low grade Chinese bauxite, a sintering process[5] or a combination of sintering and Bayer process[6] has been used in industry. The sintering process is extremely energy-consumed and environmentally unfriendly, incurring a high alumina production cost. Therefore, it is highly desirable to increase the A/S of the bauxite ores by a low cost physical separation process.
Froth flotation is one of the most widely used processes to concentrate valuable minerals in beneficiation ores. It is particularly useful for separating finely ground ores from their associated gangue minerals. As for diaspore bauxites, flotation is applied to obtain high quality material for Bayer process by increasing the A/S of the diasporic bauxite ores[7-9]. Direct flotation has been shown to be an efficient method for the desilicating of diasporic bauxite[2, 10-11]. However, reverse flotation has more advantages for diasporic bauxite desilicating than direct flotation, such as lower cost, and ease of dewatering and less effect on metallurgy[9, 12-15].
As discussed in our previous reports[16], the dimethyl dodecyl ammonium bromide (12-4-12) collector molecules that are positively charged have been adsorbed onto mineral surfaces through electrostatic force. It was proved that the Zeta potential of the minerals conducted with 12-4-12 becomes much more positive. Whereas, in the region where the mineral surfaces are positively charged, the mineral particles and the cationic surfactants are electrostatic alloy repulsive, and hydrogen bonds exist between the aluminosilicate minerals and 12-4-12. The comparative studies of a Gemini cationic collector and a corresponding traditional one, both used in flotation of the aluminosilicates, were reported[17]. The results showed that due to the special structure of Gemini surfactant, 12-4-12 revealed much stronger collecting ability than dodecyl trimethyl ammonium bromide (DTAB). It was also reported[18] that the origin and nature of starch-diaspore interaction between the soluble starch depressant and diaspore mineral is chemisorption. Starch was chemisorbed onto the diaspore surfaces by forming five membered starch-aluminum ring complexes. The number of broken Al—O bonds on the surface of kaolinite is much less than that of diaspore, so the interaction of starch on minerals is selective between diaspore and kaolinite.
Therefore, in this work, a systematical reverse flotation separation experiments of diaspore from bauxite ores was conducted in the presence of a Gemini cationic collector and the starch depressant.
2 Experimental
2.1 Minerals and reagents
Handpicked pure minerals of diaspore, illite and pyrophyllite were obtained from Mianchi Bauxites, Ohai Mine, Qingtian Mine in China. Kaolinite was obtained from the geological museum of China. They were 90% in purity based on mineralogical analysis, X-ray diffraction, and chemical analysis. The chemical composition of pure minerals is listed in Table 1. Each mineral was porcelain ground to a diameter smaller than 0.074 mm. The specific surface area was measured to be 0.703 m2/g for diaspore, 1.300 m2/g for illite, 1.170 m2/g for pyrophyllite and 0.835 m2/g for kaolinite. Diasporic-bauxite ore used in the bench-scale flotation tests was obtained from Henan Province, China. This sample with Al2O3 head assay of 64.55% and SiO2 head assay of 11.32% was mainly composed of 67.56% diaspore, 10.8% kaolinite, 6.9% illite, 3.1% pyrophyllite and 0.7% chlorite by the X-ray diffraction analysis and mineralogical analysis, and the A/S of the bauxite was 5.70.
Table 1 Chemical composition of pure minerals (mass fraction, %)

Gemini quaternary ammonium salt butane-α, ω-bis (dimethyl dodecyl ammonium bromide) (12-4-12) used as cationic collector was provided by Daochun Chemical Engineering and Technology Corporation of Henan Province, China. The soluble starch was bought from Sinopharm Chemical Reagent Co. Ltd in Shanghai, China. The reagent solutions were prepared freshly each day by dispersing a known mass in cold distilled water and then dissolving it with hot distilled water. Solutions of HCl and NaOH were used to adjust the pH of the system.
2.2 Micro-flotation
Pure mineral particles (3 g) or artificially mixed bauxite samples (3 g) were placed in a plexiglass cell (40 mL), which was then filled with a known concentration of depressant solution. The suspension was agitated for 2 min, and the pH was adjusted to be about 10.5. After adding the desired amount of collector, a 6 min flotation period was conducted. The concentrates, middlings and tailings were weighed separately after filtration and drying, and the recovery was calculated. The A/S was determined by silicon-molybdenum blue colorimetry.
2.3 Bench scale flotation
Bauxite ore sample (500 g crushed to <2 mm during sampling) with a pH modifier and dispersant sodium carbonate was ground to 75% passing 0.074 mm in a d200 mm×400 mm XMB-type steel mill at a pulp density of 50% (mass fraction). The pulp was conditioned at 30% solids with the collector and depressant was added in a 1.5 L XFD-type flotation machines at pulp pH of around 10. The experiments were repeated three times, and the results shown in this work were the averages.
3 Results and discussion
3.1 Flotation behavior of pure minerals
Fig.1 presents the influence of a Gemini collector concentration on the floatation of diaspore, kaolinite, illite and pyrophyllite at pH 6. With 1.0×10-4 mol/L 12-4-12, about 70% of illite and kaolinite can be floated, but the recovery of pyrophyllite is not more than 50%. When the dosage of collector goes up to 3.5×10-4 mol/L, the floated recoveries of the four kinds of minerals are all above 90%. The Gemini surfactant displays superior collecting power for clay minerals with the moderate concentration. Fig.2 shows the flotation behavior of the four kinds of pure minerals when the depressant is added. As can be observed, the four kinds of minerals are all affected by the presence of starch, but the polysaccharide is more effective for diaspore than for kaolinite, illite and pyrophyllite. With the increase of the polymer concentration, the floatability of diaspore indicates a further depression. When the soluble starch dosage reached 180 mg/L, the flotation selectivity is basically formed at pH 10.5 with 12-4-12 as collector.

Fig.1 Effect of Gemini collector concentration on floatability of diaspore and silicate minerals at pH 6
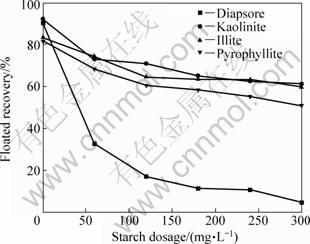
Fig.2 Effect of starch depressant concentration on floatability of diaspore and silicate minerals at pH 10.5 using 2.0×10-4 mol/L Gemini collector
Fig.3 presents the floatability variety of pure minerals as a function of pH in the absence and presence of depressant. In the case of diaspore, the floated recovery keeps at about 30% from pH 2 to 4, and with the increase of pulp pH, it is sharply down until the value of pH reaches around 10, at which the recovery of diaspore is not more than 10%. As for the three silicate minerals, the floated recovery is also slightly decreased with increasing pH, but to a much less extent than the decrease of diaspore. Furthermore, the trends of recovery curves of kaolinite, illite and pyrophyllite treated with starch are in good correlation with those of floatability in the absence of depressant.

Fig.3 Effect of pulp pH on floatability of diaspore and three silicate minerals in absence and presence of starch depressant
The pH dependence of polysaccharide depression is in fact first observed by IWASAKI and LAI[19], who measured the adsorption of a corn starch on the hematite and found that the adsorption was much higher at pH 6.8 than at pH 11.3. After then, many scientists have studied the effect of pulp pH. It is found that co-precipitation is related to the isoelectric point (iep) of the metal- hydroxides, which is linear with the postulation that the polysaccharides interact with surface metal hydroxylated species, and the maximum adsorption of polysaccharides will always occur at the iep of minerals. However, the iep of diaspore is reported at around pH 6, but not at pH 10. This phenomenon that the interaction of polusaccharides and minerals is independent of the iep is also published by many scientists. LIU and LASKOWSKI[20] found that, due to the incomplete Pb coverage of the quarze surface, Pb-coated quartz had negative Zeta potentials in the pH range of 3-13 and probably had an iep at around pH 2, and the maximum adsorption of dextrin was observed not at pH 2 but at pH 10.5. RATH and SUBRAMANIAN[21] also observed that the iep for a galena and a sphalerite was around pH 3; however, the maximum adsorption of dextrin was observed at pH 11.5 and 7.5.
3.2 Separation of artificial mixed minerals
Pure mineral micro-flotation showed that the combined use of Gemini cationic collector and starch depressant demonstrates excellent separation ability of diaspore from kaolinite, illite and pyrophyllite at pH 10. We adopted three kinds of artificially mixed mineral samples to investigate the reverse flotation separation of diaspore from the gangue minerals.
Diaspore and gangue minerals (the mass ratio of illite to pyrophyllite and to kaolinite is about 2?1?2, with illite and kaolinite as the major gangue minerals) were mixed at mass ratios of 1?1, 2?1 and 3?1, corresponding to A/S ratios of 2.70, 4.45 and 6.13, respectively. The result of separation is listed in Table 2. It is indicated that a satisfactory separation between diaspore and the three kinds of silicate minerals is obtained by reverse flotation with 300 mg/L starch and 2.0×10-4 mol/L 12-4-12 collector at pH 10. More than 80% of alumina is recovered and the A/S is 10.74 for sample ?, 12.11 for sample Ⅱ and 12.74 for sample Ⅲ.
Table 2 Reverse flotation separation of three artificially mixed minerals

3.3 Separation of diaspore from bauxite ore
Bench scale flotation tests with bauxite ore were systemically conducted. Firstly, the open-circuit aiming to find the appropriate dosage of reagents were tested. The flotation separation flowchart is shown in Fig.4, and the results of separation are listed in Table 3. From tests 1-3, we can see that the optimum Na2CO3 dosage is 6.6 kg/t, and more or less than this dosage can cause the decrease of A/S of concentrates. The results of tests 4 and 5 tell us that, properly increasing cationic collector dosage at a certain depressant used condition, is beneficial for separation of diaspore from bauxite. Tests 6-9 show that too much collector dosage or too much depressant used in flotation may increase A/S, but Al2O3 recovery is sharply decreased. The bauxite concentrate with A/S of 9.85 and the recovery of Al2O3 of 70.48% are obtained from the low grade bauxite ore (Al to Si mass ratio of 5.70) at pH of around 10, when the dosage of Na2CO3, 12-4-12 and starch are 6 600, 500 and 1 000 g/t, respectively.

Fig.4 Flowsheet of open-circuit reverse flotation of bauxite ore (Dosage of frother: 2 or 3 drops)
Table 3 Reverse flotation separation of bauxite ore with variable reagents

Secondly, the pre-desliming treatment step was inserted before reverse flotation of bauxite to lower the influence of the aluminosilicates slimes. The flowsheet of desliming-flotation processing is plotted in Fig.5, and the results are shown in Table 4. A concentrate with A/S of 10.16, and Al2O3 recovery of 70.83% is obtained.

Fig.5 Flowsheet of pre-desliming reverse flotation of bauxite
Table 4 Results of pre-desliming reverse flotation separation of bauxite

At last, the closed-circuit flotation separation of bauxite was studied. In this closed circuit experiment, the concentrate grade of Al2O3 is 69.07%; the Al to Si mass ratio is 9.66; and Al2O3 recovery can reach 71.73%. It is confirmed that the combined use of Gemini cationic collector and the starch depressant has the superior separation ability for the silicate minerals in bauxite ore and has the selective depression effect on diaspore floatability. The flowsheet of closed-circuit separation of bauxite is shown in Fig.6, and the results of mass transfer are revealed in Fig.7.

Fig.6 Flowsheet of closed-circuit pre-desliming reverse flotation of bauxite

Fig.7 Mass balance chart of flotation separation of bauxite
4 Conclusions
1) Diaspore and aluminosilicate minerals float readily with medium concentration of Gemini cationic collector of 2×10-4-4×10-4 mol/L at pH 6. Starch has a strong depressant effect for diaspore at the alkaline pH region.
2) It is possible to reverse floatation separate diaspore from bauxite ore with 12-4-12 collector and soluble starch at pH 10. The concentrate with Al to Si mass ratio of 9.85 and the recovery of Al2O3 of 70.48% is obtained from low grade bauxite ore (A/S=5.70).
3) By adding pre-desliming step, the slim effect in reverse flotation can be depressed, and the concentrate with Al to Si mass ratio of 10.16 and the recovery of Al2O3 of 70.83% can be reached. In the similar condition, a closed-circuit flotation separation of diaspore from bauxite ore was conducted. The balance Al to Si mass ratio is 9.66, and the recovery of Al2O3 is 71.73%.
References
[1] ZHAO Z D. Bauxite and alumina industry of world [M]. Beijing: Science Press, 1994. (in Chinese)
[2] LIU Guang-yi. A study on flotation and desilication of diaspore bauxites [D]. Changsha: Central South University of Technology, 1999. (in Chinese)
[3] PAPANASTASSIOU D, CSOKE B, SOLYMAR K. Improved preparation of the Greek diasporic bauxite for Bayer-process [C]// Light Metals: Proceedings of TMS Annual Meeting. TMS, 2002: 67-74.
[4] MA C, TIAN X, LIU R, YANG X. Selection of Bayer digestion technology and equipment for Chinese bauxite [C]//Light Metals: Proceedings of TMS Annual Meeting. TMS, 1996: 187-191.
[5] JIANG T, Li G, HUANG Z, FAN X, QIU G. Thermal behaviors of kaolinite-diasporic bauxite and desilication from it by roasting-alkali leaching processing [C]//Light Metals: Proceedings of TMS Annual Meeting. TMS, 2002: 89-94.
[6] CHEN Wan-kun, PENG Guan-cai. The digestion technology of diasporic bauxite [M]. Beijing: Metallurgical Industry Press, 1997. (in Chinese)
[7] HU Yue-hua. Progress in flotation de-silica [J]. Trans Nonferrous Met Soc China, 2003, 13(3): 656-662.
[8] WANG Y H, HU Y H, HE P B, GU G H. Reverse flotation for removal of silicates from diasporic-bauxite [J]. Minerals Engineering, 2004, 17(1): 63-68.
[9] XU Z H, PLITT V, LIU Q. Recent advances in reverse flotation of diasporic ores––A Chinese experience [J]. Minerals Engineering, 2004, 17(9): 1007-1015.
[10] FENG Q M, LIU G Y, LU Y P. The 90’s research and outlook of bauxite on impurity removing by mineral processing [J]. Light Metals, 1998(4): 9-13. (in Chinese)
[11] LU Y P, ZHANG G F, FENG Q M, OU L M. A novel collector RL for flotation of bauxite [J]. J Cent South Univ Technol, 2002, 9(1): 21-24. (in Chinese)
[12] CAO Xue-feng, HU Yue-hua, JIANG Yu-ren, LI Hai-pu. Flotation mechanism of aluminium silicate minerals with N-dodecyl-1, 3-diaminopropane [J]. The Chinese Journal of Nonferrous Metals, 2001, 11(4): 693-696. (in Chinese)
[13] LUO Zhao-jun, HU Yue-hua, WANG Yu-hua, QIU Guan-zhou. Mechanism of dispersion and aggregation in reverse flotation for bauxite [J]. The Chinese Journal of Nonferrous Metals, 2001, 11(4): 680-683. (in Chinese)
[14] HU Yue-hua, LI Hai-pu, JIANG Yu-ren. Effect of hydroxamic acid starch on reverse flotation desilicate from diasporic bauxite [J]. Trans Nonferrous Met Soc China, 2002, 12 (5): 974-978.
[15] HU Y, LIU X, XU Z. Role of crystal structure in flotation separation of diaspore from kaolinite, pyrophyllite and illite [J]. Minerals Engineering, 2003, 16(3): 219-227.
[16] XIA L Y, ZHONG H, LIU G Y, HUANG Z Q, CHANG Q W. Flotation separation of the aluminosilicates from diaspore by a Gemini cationic collector [J]. International Journal of Mineral Processing, 2009, 92(1/2): 74-83.
[17] XIA Liu-yin, ZHONG Hong, LIU Guang-yi, LI Xin-gang. Comparative studies on the flotation of illite, pyrophyllite and kaolinite with Gemini and conventional cationic surfactants [J]. Trans Nonferrous Met Soc China, 2009, 19(2): 446-453.
[18] XIA L Y, ZHONG H, LIU G Y, WANG S. Utilization of soluble starch as a depressant for the reverse flotation of diaspore from kaolinite [J]. Minerals Engineering, 2009, 22(6): 560-565.
[19] IWASAKI I, LAI R W. Starches and starch products as depressants in soap flotation of activated silica from iron ores [J]. Trans AIME, 1965, 232: 364-371.
[20] LIU Q, LASKOWKI J S. The role of metal hydroxides at mineral surfaces in dextrin adsorption I. Studies on modified quartz samples [J]. International Journal of Mineral Processing, 1989, 26(3/4): 297-316.
[21] RATH R K, SUBRAMANIAN S. Adsorption, electrokinetic and differential flotation studies on sphalerite and galens using dextrin [J]. International Journal of Mineral Processing, 1999, 57(4): 265-283.
Foundation item: Project(50874118) supported by the National Natural Science Foundation of China; Project(2007B52) supported by the Foundation for the Author of National Excellent Doctoral Dissertation of China; Project(2005CB623701) supported by the National Basic Research Program of China
Corresponding author: ZHONG Hong; Tel: +86-731-88830603; E-mail: zhongh@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(09)60168-0
(Edited by YANG Bing)