
Effect of electropolymer sizing of carbon fiber on mechanical properties of phenolic resin composites
LI Jin(李 劲), FAN Qun(范 群), CHEN Zhen-hua(陈振华),
HUANG Kai-bing(黄凯兵), CHENG Ying-liang(程英亮)
College of Materials Science and Engineering, Hunan University, Changsha 410082, China
Received 10 April 2006; accepted 25 April 2006
Abstract: Carbon fiber/phenolic resin composites were reinforced by the carbon fiber sized with the polymer films of phenol, m-phenylenediamine or acrylic acid, which was electropolymerized by cyclic voltammetry or chronopotentiometry. The contact angles of the sized carbon fibers with deionized water and diiodomethane were measured by the wicking method based on the modified Washburn equation, to show the effects of the different electropolymer film on the surface free energy of the carbon fiber after sizing by the electropolymerization. Compared with the unsized carbon fiber, which has 85.6°of contact angle of water,52.2° of contact angle of diiodomethane, and 33.1 mJ/m2 of surface free energy with 29.3 mJ/m2 of dispersive components (γL) and 3.8 mJ/m2 of polar components (γsp), respectively. It is found that the electropolymer sized carbon fiber tends to reduce the surface energy due to the decrease of dispersive γL with the increase of the polymer film on the surface of the carbon fiber that plays an important role in improving the mechanical properties of carbon/phenolic resin composites. Compared with the phenolic resin composites reinforced by the unsized carbon fiber, the impact, flexural and interlaminar shear strength of the phenolic resin composites were improved by 44 %, 68% and 87% when reinforced with the carbon fiber sized by the electropolymer of m-phenylenediamine, 66%, 100%, and 112% by the electropolymer of phenol, and 20%, 80 %, 100% by the electropolymer of acrylic acid. The results indicate the skills of electropolymerization may provide a feasible method for the sizing of carbon fiber in a composite system, so as to improve the interfacial performance between the reinforce materials and the matrix and to increase the mechanical properties of the composites.
Keywords: carbon fibers; electropolymer sizing; contact angle; mechanical property; phenolic composites
1 Introduction
Carbon fibers present extremely high strength and modulus, good stiffness, and creep resistance etc., have been widely employed as the reinforcing material in the high performance resin composites which have been extensively used in many industrial fields[1]. It is significant to develop the composite reinforced by carbon fiber with good mechanical properties, which are governed by both the composed components and the interface between them. A strong and durable interface adhesion can efficiently pass the stress from the matrix to the fiber, which may be required to the outstanding mechanical properties of the composites. Many researches show that interfacial adhesion is attributed to the interfacial chemical bonds formed from an interaction between the polar groups such as hydroxyl or carboxyl on the surface of the reinforcing fiber and the active groups present in the matrix resin[2-4]. The improvement of strength of the fiber-matrix bond may be due to the possibility of forming attractive bonds including polar interactions, hydrogen and covalent bonds between the fibers and the matrix, but these interfacial bonds cannot be formed unless the fiber surface can contact intimately with the matrix resin, so it is necessary for high performance resin composites developing that the fibers can be suitably wetted by the liquid polymeric matrix during the formation of the composites which is decided by the nature of the fiber and liquid polymeric surface, especially the hydrophobic-hydrophilic properties or the London dispersive-polar components of surface free energy.
In order to achieve good adhesion between the reinforcement and the matrix materials, carbon fibers are usually modified by the surface treatment to enhance the physical and chemical properties of their surface such as wetting properties, bond forming possibility. The surface modification is mainly based on various oxidizing and surface coating processes by various means like oxidation in acid solutions, anodic oxidation, plasma treatments, mild fluorination, and metallic coating, then fibers are generally sized or coated with thin polymer film after surface treatment[5-7].
Electrochemical polymerization is a simple and attractive approach for forming a polymer film on the electrode surface[8-11], the thickness of the film can be controlled and the functionality of the formed coating film can be selected through processing parameters (the current density, monomer concentration, temperature, etc.) [12-14]. In this paper, the surface treatments of the carbon fiber sizing with the electropolymer films of phenol, m-phenylenediamine or acrylic acid were studied in terms of the surface free energy of fibers and the mechanical characteristic of the phenolic resin composites reinforced by those sized carbon fiber.
2 Experimental
2.1 Electropolymerization procedure
The polymer was deposited electrochemically onto the carbon fiber by cyclic voltammetry or chronopotentiometry performed in a solution with the required amount of monomer using an instrument of CHI-660B electrochemical workstation. A three-elect-
rode cell was used and consisted of carbon fiber as a working electrode, a platinum wire auxiliary electrode, and Ag/AgCl reference electrode. The mixture was stirred continuously during the polymerization. The sized carbon fiber was washed by distilled water and dried in a vacuum.
2.2 Contact angle measurement
Contact angle was used as a parameter to characterize the wetting performance and surface free energy of the electropolymer sized carbon fibers. The surface free energy of carbon fibers was measured by wicking method based on the modified Washburn equation [4, 15]. A definite mass of carbon fibers was packed into a polytetrafluoroethylene tube and then mounted on the measuring arm of the balance. The polytetrafluoroethylene tube packed carbon fibers was immersed in the wetting liquid, and the relation of the wetting time and weight of the liquid was obtained. The test wetting liquids used for contact angle measurements were deionized water, diiodomethane and n-hexane.
The contact angle of carbon fibers using two wetting liquid of distilled water and diiodomethane was calculated by the modified Washburn equation:
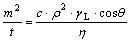 (1)
(1)
where m is the mass of the penetrating liquid; t is the flow time; ρ is the density of measuring liquid; η is the viscosity; γL is the surface free energy of liquid; θ is the contact angle and c is the packing factor. The packing factor is an empirical constant, which depends on the size and degree of the packing. To measure the packing factor, n-hexane was selected as the wetting liquid. The contact angle of the carbon fibers was assumed to be 0?, so the packing factor c could be calculated.
The surface free energy of carbon fibers is make up of London dispersive and polar components, such as
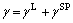 (2)
(2)
The London dispersive and polar components of surface free energy were calculated by
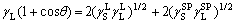 (3)
(3)
where the subscripts L and S represent the liquid and solid states; the superscripts ‘L’ and ‘SP’ represent the London dispersive and polar components of surface free energy.
In this paper, the London dispersive component, polar component and the surface free energy were measured by Eqns.(2) and (3), which the wetting liquid used deionized water, diiodomethane.
2.3 Mechanical properties
The samples of carbon fiber/phenolic resin composites were made according to the method in Ref.[16]. A three-point bending test was performed to measure the mechanical properties of the composites on DW-1 Model mechanical tester. The mechanical strength tests were carried out according to the process of ASTM D-2344, GB1042-70, and GB1043-70. At least, five specimens were tested for each set of samples and the values were reported.
The fracture surfaces of the samples were examined by scanning electron microscopy (SEM).
3 Results and discussion
To improve the surface properties of carbon fibers, electrochemical polymerization of electro-active monomers given by phenol, m-phenylenediamine and acrylic acid were performed in this experiment, which may size the carbon fiber with the polymer film remaining different agent such as —OH, —NH2, and —COOH using cyclic voltammetry or chronopotentio-
metry skills. The interfacial properties of the phenolic composites could be improved by those rudimental agents that act as the reactive coupling groups between the sized carbon fibers and the matrix resulting an increase of the mechanical properties. The SEM images of the electropolymer sized carbon fiber are shown in Fig.1.

Fig.1 SEM images of carbon fiber sized by electropolymer from different monomer: (a) Phenol; (b) m-phenylenediamine; (c) Acrylic acid
Furthermore, the surface free energy of the carbon fiber has been observed to change after being sized by the electropolymer due to the hydrophobic properties of the polymer film and polar agents on the film. The varieties of the contact angle (θ) with the electropolymerization time are shown in Fig.2, the changes of the dispersive components (γL) and polar components (γsp) of surface free energy (γ) are shown in Fig.3. Compared with the unsized carbon fiber, of which contact angle of water is 85.6°, contact angle of diiodomethane is 52.2° and surface free energy is 33.1 mJ/m2 with γL of 29.3 mJ/m2 and γsp of 3.8 mJ/m2, the electropolymer sized carbon fiber tends to reduce the surface energy due to the decrease of dispersive γL with the increase of the polymer film on the carbon fiber during the electropolymerization. By the means of electropolymer sizing the carbon fibers can modify their surface nature such as surface free energy for a suitable wettability in the composite system. The optimum mechanical properties of the composite were obtained after electopolymerizing for about 30 min by cyclic voltammetry, or for about 2 h by chronopotentiometry (data not shown).
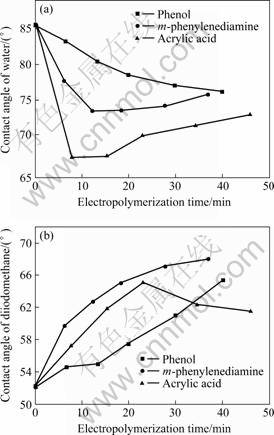
Fig.2 Contact angles varied with electropolymerization time of different monomer: (a) Water; (b) Diiodomethane
As can be seen from Fig.3, during the electropolymer sizing, the treatment results primarily in an increase of the polar component of surface energy which is probably due to the increase of polar functional groups of the polymeric films on the surface of the carbon fiber. γsp of the carbon fibers sized by poly (m-phenylenediamine) or poly (acrylic acid) begins to decrease after reaction for about 30 min while to poly
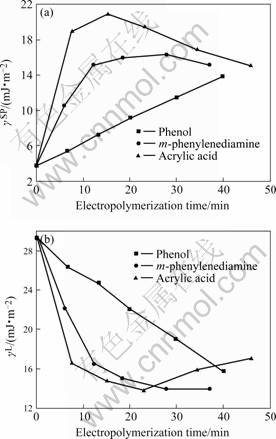
Fig.3 Variation of dispersive components (γL) and polar components (γsp) of surface free energy (γ) with electropolymerization time of different monomer: (a) Polar components; (b) Dispersive Components
(phenol) it keeps increase within the observing time which show that the eletropolymerization of phenol may be reaction rather slow. This indicates the nature of the carbon fibers surfaces may depend on the percentage of polar groups of the polymeric film on them, which can be controlled by the electropolymerization to produce the most favorable wetting properties of the sized carbon fibers for high performance composites developing.
It can be seen in Fig.4, the mechanical strength of the composites increased with the contents of the carbon fiber regardless the surface treatments. At a fix carbon fiber content of 4%, the impact, flexural and interlaminar shear strength of the phenolic resin composites reinforced with the carbon fiber sized by the electropolymer of m-phenylenediamine were improved about 44 %, 68% and 87%, those reinforced with the carbon fiber sized by the electropolymer of phenol were improved about 66%, 100%, and 112%, and by the electropolymer of acrylic acid that were about 20%,80%, and 100%, which was compared with the unsized carbon fiber reinforced composites. The composites
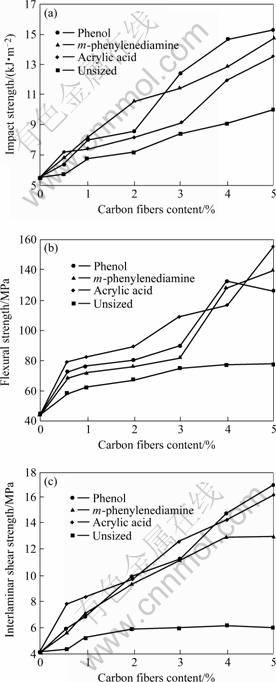
Fig.4 Variation of mechanical properties of composites with content of carbon fibers sized by different electropolymer: (a) Impact strength; (b) Flexural strength; (c) Interlaminar shear strength
reinforced by the carbon fiber sized with the electropolymer of phenol has a higher mechanical strength than that of the others, which indicates that the coupling agent of —OH remained on the electropolymer may improve the interfacial strength between the carbon fiber and the phenolic resin.
Fig.5 shows the SEM images of the fractured surface of the composites. On the fractured surface of the
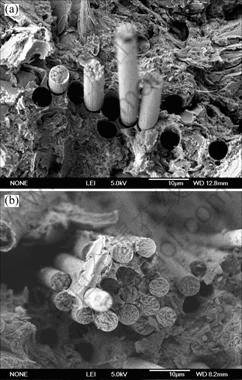
Fig.5 SEM photographs of impact fracture of phenolic composites reinforced by carbon fiber: (a) Unsized; (b): Sized with electropolymer of phenol
composite reinforced by the unsized carbon fibers, there are some holes formed by pulling carbon fibers out of the matrix resin during the mechanical test, and the pulling out carbon fibers are randomly dispersed in the matrix resin, as shown in Fig. 5(a). It means that the adhesion between the carbon fiber and the phenolic resin is very poor. But the holes and the pulling out fibers are very few on the fractured surface of the composite reinforced by the sized carbon fibers and the fibers were bonded together with the matrix as shown in Fig.5(b). Thus, it reveals that the interfacial adhesion between carbon fiber and phenolic resin is improved by treating carbon fibers with the electropolymer sizing.
4 Conclusions
The electropolymer of phenol, m-phenylenediamine, and acrylic acid were sizing on carbon fiber surface conveniently and were characterized in terms of the surface free energy of the carbon fibers and the mechanical properties of the composites. In this way an interface given active agents between the reinforcing materials and the matrix was developed that led to resulting the high mechanical properties of the composites. It seems to be a simple way to control the degree of fiber-matrix adhesion and govern the final mechanical behavior of composites. The results also indicate that the electropolymerization may provide a feasible method for carbon fiber sizing in a composite system.
References
[1] Donnet J B, Bansal R C. Carbon Fibers[M]. 2nd ed. New York: Dekker, 1990.
[2] PARK S J, JANG Y S. Interfacial characteristics and fracture toughness of electrolytically Ni-plated carbon-fiber-reinforced phenolic resin matrix composites[J]. Journal of Colloid and Interface Science, 2001, 237: 91-97.
[3] CHOI M H, JEON B H, CHUNG I J, The effect of coupling agent on electrical and mechanical properties of carbon fiber/phenolic resin composites[J]. Polymer, 2000, 41: 3243-3252.
[4] PARK S J, KIM M H LEE J R CHOI S. Effect of fiber-polymer interactions on fracture toughness behavior of carbon fiber-reinforced epoxy matrix composites[J]. Journal of Colloid and Interface Science, 2000, 228: 287-291.
[5] SARAC A S, BARDAVIT Y. Electrografting of copolymer of poly[N-vinylcarbazole-co-styrene] and poly[N-vinylcarbazole-co- acrylonitrile] onto carbon fiber: cyclovoltammetric(CV), spectroscopic (UN-Vis, FT-IR-ATR), and morphological study (SEM)[J]. Progress in Organic Coating, 2004, 49: 85-94.
[6] SEVERINI F, FORMARO L, PEGORARO M, POSCA L. Chemical modification of carbon fiber surfaces[J]. Carbon, 2002, 40: 735-741.
[7] WU G W. Oxygen plasma treatment of high performance fibers for composites[J]. Materials Chemistry and Physics, 2004, 85: 81-87.
[8] LU W, SMELA E, ADAMS P, ZUCCARELLO G, MATTES B R. Development of solid-in-hollow electrochemical linear actuators using highly conductive polyaniline[J]. Chem Mater, 2004, 16: 1615-1621.
[9] FUKUHARA T, AKIYAMA Y, YONEDA N, TADA T, HARA S. Effective synthesis of difluorocyclohexadienones by electrochemical oxidation of phenols[J]. Tetrahedron Letters, 2002, 43: 6583-6585.
[10] MATSUSHITA M, KURAMITZ H, TANAKA S. Electrochemical oxidation for low concentration of aniline in neutral pH medium: Application to the removal of aniline based on the electrochemical polymerization on a carbon fiber[J]. Environ Sci Technol, 2005, 39: 3805-3810.
[11] CAO H, HUANG Y, ZHIQIAN, SUN J. Uniform modification of carbon fibers surface in 3-D fabrics using intermittent electrochemical treatment[J]. Composites Science and Technology, 2005, 65: 1655-1662.
[12] SEZAI A S, BARDAVIT Y. Electrografting of copolymer of poly[N-vinylcarbazole-co-styrene] and poly[N-vinylcarbazole-co- acrylonitrile] onto carbon fiber: cyclovoltammetric(CV), spectroscopic (UN-Vis, FT-IR-ATR), and morphological study (SEM)[J]. Progress in Organic Coating, 2004, 49: 85-94.
[13] HUA S, BRISENO A L, SHI X, MAH D A, ZHOU F. Polyelectrolyte-coated nanosphere lithographic patterning of surfaces: Fabrication and characterization of electropolymerized thin polaniline honeycomb films[J]. J Phys Chem B, 2002, 106: 6465-6472.
[14] KUMRU E M, SPRINGER J, SARAC A S, BISMARK A. Electrografting of thiophene, carbazole, pyrrole and their copolymers onto carbon fibers: electrokinetic mearements, surface composition and morphology[J]. Synthetic Metals, 2001, 123: 391-402.
[15] LEE Y S, LEE B K. Surface properties of oxyfluorinated PAN-based carbon fibers[J]. Carbon, 2002, 40: 2461-2468.
[16] LI Jin, LI Wei, CHEN Zhen-hua. The reinforced effect of continuous separated carbon fiber tape on mechanical properties of phenolic resin composites[J]. Acta Materiae Compositae Sinica, 2006, 23(1): 51-55.
(Edited by YANG Hua)
Corresponding author: LI Jin; Tel: +86-731-8821564; E-mail: ljnhd@hnu.cn