Solution behavior of ZnS and ZnO in eutectic Na2CO3-NaCl molten salt used for Sb smelting
来源期刊:中南大学学报(英文版)2017年第6期
论文作者:叶龙刚 胡宇杰 夏志美 唐朝波 陈永明 唐谟堂
文章页码:1269 - 1274
Key words:solubility; molten salt; sulfur-fixing smelting; sedimentation
Abstract: The solution behavior, including solubility, reactivity and sedimentation, of ZnO and ZnS in a Na2CO3-NaCl molten salt used for Sb smelting was investigated in the temperature range of 700-1000 oC. The saturated amount of dissolved ZnO in the molten salt remained constant at 0.02% and was unaffected by temperature; additionally, ZnO did not react with the molten salt. In contrast, the saturated amount of dissolved ZnS in the eutectic molten salt increased with increasing temperature, and the content of ZnS was 0.53% at 1000 oC. In addition, ZnS reacted with Na2CO3 above 900 oC to give ZnO. The sedimentation rates of these three species in the molten salt followed the order of Sb>ZnS>ZnO. It was thus concluded that ZnO is an appropriate sulfur-fixing agent for low-temperature Sb smelting in a Na2CO3-NaCl molten medium, and that the optimal smelting temperature is below 900 oC.
Cite this article as: YE Long-gang, HU Yu-jie, XIA Zhi-mei, TANG Chao-bo, CHEN Yong-ming, TANG Mo-tang. Solution behavior of ZnS and ZnO in eutectic Na2CO3-NaCl molten salt used for Sb smelting [J]. Journal of Central South University, 2017, 24(6): 1269-1274. DOI: 10.1007/s11771-017-3531-8.
J. Cent. South Univ. (2017) 24: 1269-1274
DOI: 10.1007/s11771-017-3531-8

YE Long-gang(叶龙刚)1, 2, HU Yu-jie(胡宇杰)1, 2, XIA Zhi-mei(夏志美)1, 2,
TANG Chao-bo(唐朝波)2, CHEN Yong-ming(陈永明)2, TANG Mo-tang(唐谟堂)2
1. School of Metallurgical Engineering, Hunan University of Technology, Zhuzhou 412007, China;
2. School of Metallurgy and Environment, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Abstract: The solution behavior, including solubility, reactivity and sedimentation, of ZnO and ZnS in a Na2CO3-NaCl molten salt used for Sb smelting was investigated in the temperature range of 700-1000°C. The saturated amount of dissolved ZnO in the molten salt remained constant at 0.02% and was unaffected by temperature; additionally, ZnO did not react with the molten salt. In contrast, the saturated amount of dissolved ZnS in the eutectic molten salt increased with increasing temperature, and the content of ZnS was 0.53% at 1000°C. In addition, ZnS reacted with Na2CO3 above 900°C to give ZnO. The sedimentation rates of these three species in the molten salt followed the order of Sb>ZnS>ZnO. It was thus concluded that ZnO is an appropriate sulfur-fixing agent for low-temperature Sb smelting in a Na2CO3-NaCl molten medium, and that the optimal smelting temperature is below 900°C.
Key words: solubility; molten salt; sulfur-fixing smelting; sedimentation
1 Introduction
Molten salts have excellent thermal and electrical properties, including high-temperature stability; low vapor pressure and viscosity; and high conductivity, ionic migration rate and thermal capacity; and are widely used in materials preparation [1-3], solar power plants [4-6], the nuclear industry, metallurgy process and other engineering fields [7, 8]. As the simplest binary system, eutectic Na2CO3–NaCl molten salts are commonly used as solvents in quenching baths for steel [9, 10], the preparation of ceramic materials [11, 12] and municipal waste disposal [13].
Recently, low-temperature molten salt metallurgy has been proposed by numerous researchers as a solution to the problems of high energy consumption and low-concentration SO2 emissions in traditional processes [14, 15]. Low-temperature molten salt metallurgy uses low-cost chloride, hydroxide and carbonate salts of alkali metals (e.g., Na2CO3 and NaOH) to replace the commonly used ternary FeO–SiO2–CaO slag, the sulfide ore is smelted at low temperatures and the molten salt acts simply as an inert reaction medium [16, 17]. Our group has long focused on the low-temperature smelting of stibnite (Sb2S3) using a molten salt sulfur-fixing process. Sb2S3 is reduced to Sb metal by carbothermic and sulfur-fixing reactions, and collects at the bottom of the melt through natural sedimentation in an alkalescent Na2CO3–NaCl molten salt in the presence of the reductant and sulfur-fixing agent ZnO. As shown in the schematic diagram in Fig. 1, sulfur was fixed by ZnO in the form of ZnS and also settled at the bottom of the melt, but on top of the molten metal. This process has considerable advantages over the conventional smelting process, namely, reduction in the smelting temperature, elimination of SO2 discharge and comprehensive utilization of the fluoride- and chloride-containing ZnO ash produced in lead and zinc smelting plants.
The product of the sulfur-fixing reaction of ZnO is ZnS. However, data on the solution behavior of both ZnO and ZnS, including their solubility and reactions with molten salts, are scarce. A high solubility of ZnO and ZnS in the molten salt will increase the viscosity of the melt because both ZnO and ZnS have high melting points, which reduces the fluidity of the melt. Additionally, recycling of the molten salt becomes difficult if it reacts with ZnO and ZnS. In the present study, the solution behavior of both ZnO and ZnS in a Na2CO3–NaCl melt was investigated over a temperature range of 700–1000°C, and the sedimentation rates of ZnO, ZnS and Sb in a Na2CO3–NaCl eutectic molten salt were compared. The results can be used to determine whether it is appropriate to use ZnO as a sulfur-fixing agent for low-temperature Sb smelting in a Na2CO3– NaCl molten salt.
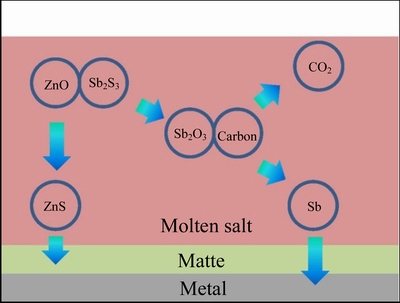
Fig. 1 Schematic diagram of low-temperature smelting of Sb2S3 using ZnO as a sulfur-fixing reagent in a Na2CO3–NaCl molten salt
2 Experimental
The Na2CO3 and NaCl used in this experiment were of analytical grade (99%) and were dried in an oven at 270°C for 24 h. The ZnO and ZnS were of high purity (99.99%). As the phase diagram in FactSage shows, the eutectic temperature for a composition of n(Na2CO3)/ [n(Na2CO3)+n(NaCl)]=0.423, where n represents the number of moles, is 635°C. Na2CO3 and NaCl were charged into a corundum crucible (diameter of 3 cm, and height of 5 cm) in the appropriate ratio and heated until they had completely melted. Then, ZnO or ZnS (5 g or 1 g of the salt or powder sample, respectively) was submerged in the melt, and this moment was defined as the experiment start time. All solubility experiments were carried out under dehydrated high-purity argon gas, and the samples were held at the given temperature for 3 h without stirring. After the predetermined experimental time, the sample was quenched by flushing with argon gas. Insoluble ZnO or ZnS separates from the molten salt as a result of the density difference, and settles under the molten salt. The salt sample containing dissolved ZnO or ZnS was then separated for chemical analysis, and inductively coupled plasma atomic emission spectroscopy was used to analyze the content of ZnO and ZnS in the solid salt. All experiments were conducted three times. A schematic diagram of the process is shown in Fig. 2.
After heat treatment, X-ray diffraction (XRD) studies were performed using Rigaku D/max 2550VB+18 kW powder diffractometer with a Cu Kα X-ray source at 40 kV and 300 mA. To investigate the diffusion homogenization of ZnO (ZnS) in molten salt, the saturation salt was quenched to room temperature, and the block sample was cut along the diameter to obtain a flat cross-section. The distribution of ZnO (ZnS) along the diameter was monitored by a JEOL JSM- 6360LV scanning electron microscope (SEM) coupled with an X-ray electron dispersive spectroscope (EDS).
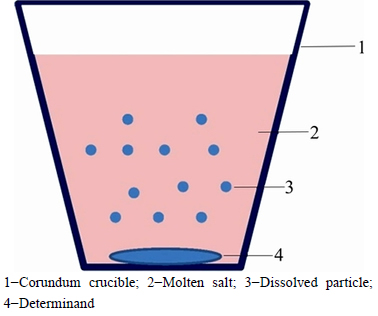
Fig. 2 Schematic diagram of solubility measurements:
3 Results and discussion
3.1 Solubility of ZnO in Na2CO3-NaCl eutectic molten salt
The ZnO concentration in the Na2CO3–NaCl eutectic molten salt is presented in Fig. 3 as a function of temperature. The figure shows that temperature has a weak influence on the solubility of ZnO. The dissolved amount of ZnO at equilibrium was 0.02% and this value did not change significantly. Owing to the acceleration of ionic migration and molecular diffusion at high temperatures, the dissolution equilibration was achieved quickly above 700°C, and further increases in temperature did not result in increased solubility of ZnO.
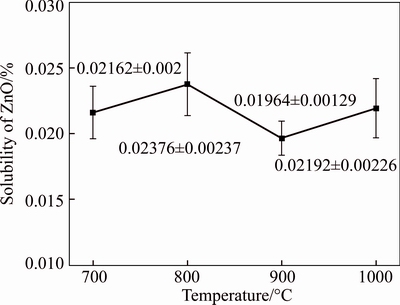
Fig. 3 Effect of temperature on solubility of ZnO in Na2CO3-NaCl eutectic salt
To examine whether the eutectic salts and ZnO undergo a phase change after treatment at high temperature, quenched specimens of the supernatant salt and the interfacial mixture from the ZnO layer were studied by X-ray diffraction (XRD). Figure 4 shows the XRD patterns of the two samples after 3 h of heat treatment from 700 °C to 1000 °C. Comparison of the patterns with the standard cards confirmed that all of the peaks in Fig. 4(a) arise from Na2CO3 (JCPDS card No. 37-0451) and NaCl (JCPDS card No. 05-0628). Furthermore, all of the peaks in Fig. 4(b), besides those belonging to Na2CO3 and NaCl, were identified as ZnO (JCPDS card No. 65-3411). The Na2CO3–NaCl molten salt permeates into the infusible ZnO solid, and their characteristic peaks were thus observed on the XRD patterns of the interfacial mixture. The results illustrate the excellent clarification of the molten salt and ZnO. The lack of ZnO peaks in the salt mixture may be due to the insufficient content of ZnO. Therefore, ZnO does not react with the molten salt and can exist stably in the smelting system.
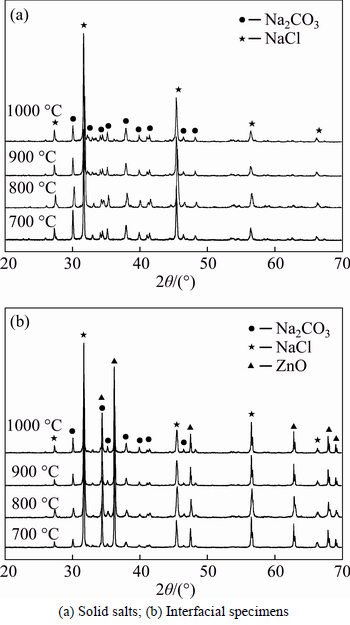
Fig. 4 XRD patterns of samples after dissolution of ZnO in molten salt:
The morphology of the salt sample obtained after heat treatment at 900°C for 3 h was also studied. Figure 5 shows the SEM-BSE image and EDX spectrum of the sample. The image shows that the ZnO particles were randomly embedded in the salt throughout the solid granular powder (Fig. 5(a)), with no regular morphology. The ZnO particles, the bright points in the image, were tightly embedded in the surface of the granular salt and had diameters of less than 10 μm.
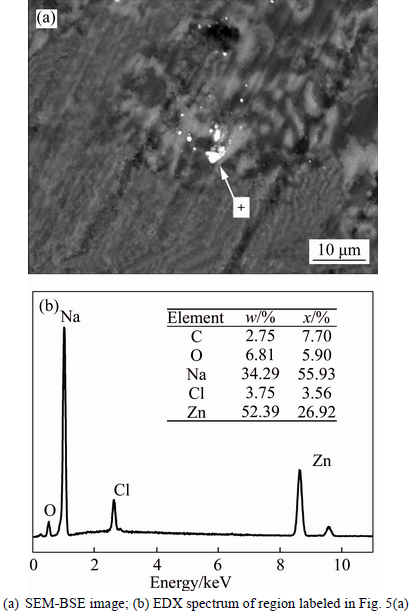
Fig. 5 SEM-BSE image and energy spectrum of ZnO dissolved in solid salt after treatment at 900°C for 3 h:
3.2 Solubility of ZnS in Na2CO3–NaCl eutectic molten salt
The product of the sulfur-fixing reaction of ZnO is ZnS, and a high solubility of ZnS in the smelting melt will increase the viscosity of the molten salt and the difficulty of separating the slag from the molten salt. Thus, this issue requires attention. The equilibrium solubility of ZnS in the eutectic molten salt after 3 h at different temperatures is shown in Fig. 6.
Figure 6 clearly shows that the solubility of ZnS increases with increasing temperature, which is markedly different to the behavior of ZnO. As the temperature increases from 700 °C to 1000 °C, the solubility of ZnS increases from 0.05713% to 0.5343%. The dissolved ZnS was distributed in the melt, and the insoluble part collected at the sulfide layer with other sulfides from the raw ore through natural sedimentation during the smelting process. However, the saturated solubility of ZnS remained low in the temperature range of 700–900 °C, which is the most common temperature range for low-temperature smelting using the Na2CO3-NaCl system.
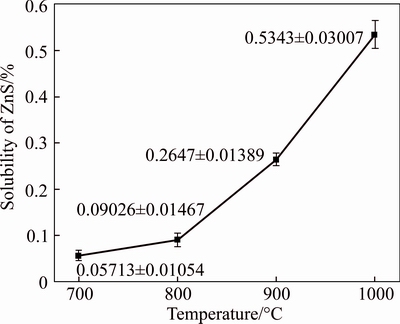
Fig. 6 Effect of temperature on solubility of ZnS in Na2CO3-NaCl eutectic salt
Figure 7 shows the XRD patterns of the supernatant salt and interfacial mixture from the ZnS layer of quenched specimens that were heat treated at 700–1000 °C for 3 h. Figure 7(a) indicates that, up to a temperature of 800 °C, the primary condensate of the solid salt is a mixture of Na2CO3 and NaCl, with no Zn phase. However, both ZnO (JCPDS card No. 65-3411) and ZnS (JCPDS card No. 05-0492) were present in the molten salt sample after treatment above 900°C. This confirms that ZnS can react with Na2CO3 to generate ZnO, and the equation is as follows:
ZnS+Na2CO3=Na2S+ZnO+CO2(g) (1)
ΔGΘ=181.8038-0.1654T+2.4063×10-5T 2
Furthermore, for the interfacial specimens, shown in Fig. 7(b), only ZnS, Na2CO3 and NaCl were observed after treatment below 900°C, but a large amount of ZnO was present at 1000°C. This can be explained by the fact that the tendency for reaction between ZnS and Na2CO3 increases as the temperature increases. It also illustrates the increased solubility of ZnS at increased temperatures, which means that more ZnS is available for reaction with Na2CO3 as the temperature increases. The results therefore show that ZnS is stable in the molten Na2CO3-NaCl system below 900 °C, and, at the same time, ZnS has lower solubility at lower temperatures.
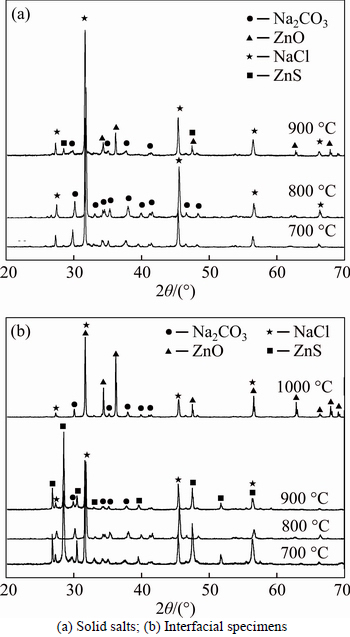
Fig. 7 XRD patterns of molten salt samples after dissolution of ZnS:
The morphology of the salt samples containing dissolved ZnS was also studied after the solubility measurements. Figure 8 shows the SEM-BSE image and EDX spectrum of the cooled specimen after 3 h of heat treatment at 900°C. From the EDX spectrum, the bright regions in the SEM image were determined to be ZnS, and the ZnS particles were randomly distributed in the molten salt. There were three particles with irregular shapes that were randomly embedded across the solid granular powder and had obvious interfaces. The dissolved ZnS was thus mixed in the molten salt in the form of solid particles.
3.3 Settling velocity calculation
The solution behavior of Sb in the Na2CO3-NaCl molten salt was described in our previous work [18]. The ZnO, ZnS and Sb particles are deposited by the action of gravity in the molten salt, and the settling velocity of the particles is affected by the density and radius of the particles and the viscosity of the melt. This relationship can be expressed by the Stokes equation as follows:
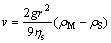 (2)
(2)
where v is the average settling velocity of the particle in m·s-1; r is the radius of the particle in m; η is the viscosity of the molten salt in Pa·s; ρM and ρSare the density of the settling particle and the molten salt, respectively, in kg·m-3; and g is the acceleration of gravity.
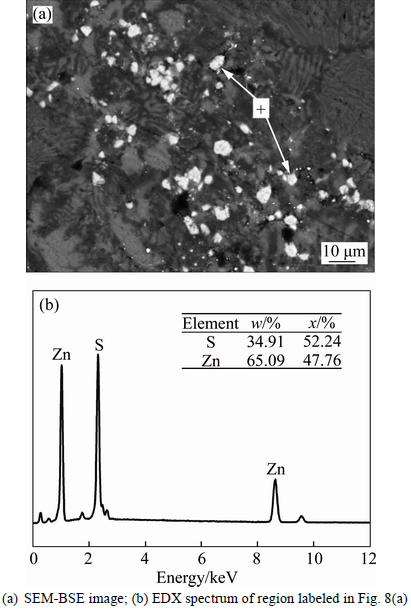
Fig. 8 SEM-BSE image and energy spectrum of ZnO dissolved in solid salt after treatment at 900°C for 3 h:
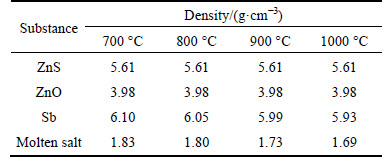
The SEM morphology clearly shows that all of the ZnO, ZnS and Sb particles were less than 10 μm in diameter. Therefore, the average diameter of these three types of particles was approximated to 10 μm in the calculation of the settling velocity. The densities of the three compounds, shown in Table 1, were obtained from the publishing handbook [19], and the viscosity of the molten salt was measured in a previous study [20].
Table 1 Density of relative substances in reaction system at different temperatures
The calculated settling velocities are shown in Fig. 9, and show that those of ZnS and Sb were approached, and were faster than that of ZnO. This is beneficial for smelting because it means that the products (Sb and ZnS) will quickly sink to the bottom of the melt.
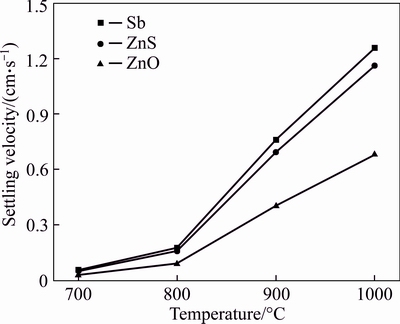
Fig. 9 Settling velocities of ZnO, ZnS and Sb in Na2CO3–NaCl eutectic salt
In this way, the separation of the products and molten salt is efficient, and the products do not increase the viscosity of the smelting melt. The low settling velocity of ZnO is also beneficial for the reaction because this slow sedimentation allows sufficient contact with the reactant for sulfur fixing. Therefore, a deep pool depth is required for complete reaction. In reality, the practical settling velocities will be slower than the calculated values owing to the stirring effect of the bubbles.
4 Conclusions
The solubility of ZnO and ZnS in the Na2CO3–NaCl molten salt and the interaction between the two compounds and the molten salt were measured over a temperature range of 700-1000°C. The results show that temperature has different effects on the dissolution of ZnO and ZnS. ZnO was scarcely dissolved in the Na2CO3–NaCl molten salt, with an equilibrium concentration of around 0.02%. Temperature had a minimal effect on the solubility of ZnO, and the compound did not react with the molten salt, even at 1000°C. In contrast, the equilibrium amount of ZnS dissolved in the eutectic molten salt increased from 0.057% to 0.53% as the temperature was increased from 700 °C to 1000 °C. In addition, ZnS reacted with Na2CO3 at temperatures above 900 °C to generate ZnO. By using the Stokes equation as an approximation, the sedimentation rate of three main species in the smelting molten salt was determined to be in the order of Sb>ZnS>ZnO. In conclusion, ZnO is a suitable sulfur-fixing agent for low-temperature Sb smelting in a Na2CO3-NaCl molten medium in a temperature range of 700-900°C.
References
[1] CHEN G Z, FRAY D F, FARTHING T W. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride [J]. Nature, 2000, 407(6802): 361-364.
[2] CHEN Z Y, ZHU W, ZHU H L, ZHANG J L, LI Q F. Electrochemical performances of LiFePO4/C composites prepared by molten salt method [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(5): 809-813.
[3] GUO C P, LI C G, DU Z M. Thermodynamic optimization of the NaCl-PrCl3 system and the LiCl-NaCl-PrCl3 system [J]. Thermochimica Acta, 2012, 540(7): 85-90.
[4] ZHOU D, ZHAO C Y, TIAN Y. Review on thermal energy storage with phase change (PCMS) in building applications [J]. Applied Energy, 2012, 92(4): 593-605.
[5] ZHAO C Y, WU Z G. Thermal property characterization of a low melting-temperature ternary nitrate salt mixture for thermal energy storage system [J]. Solar Energy Materials and Solar Cells, 2011, 96(12): 3341-3346.
[6] FUJIWARA S, INABA M, TASAKA A. New molten salt systems for high temperature molten salt batteries: Ternary and quaternary molten salt systems based on LiF-LiCl; LiF-LiBr, and LiCl-LiBr [J]. Journal of Power Sources, 2011, 196(8): 4012-4018.
[7] BAO M, WANG Z W, GAO B L, SHI Z N, HU X W, YU J Y. Electrical conductivity of NaF-AlF3-CaF2-Al2O3-ZrO2 molten salts [J]. Transactions of Nonferrous Metals of China, 2013, 23(12): 3788-3792.
[8] IWASAWA K, MAEDA M. Phase diagram study for the alkali metal-oxychloride system [J]. Metallurgy and Materials Transaction B-Process Metallurgy and Materials Processing Science, 2000, 31B(8): 795-799.
[9] JUN Y, KATSUNARI O, KOICHI A. Thermodynamic assessment of the KCl-K2CO3-NaCl-Na2CO3 system [J]. Calphad-Computer Coupling of Phase Diagrams and Thermochemistry, 2007, 31(2): 155-163.
[10] YAOKAWA J, MIURA D, ANZAI K, YAMADA Y, YOSHII H. Strength of salt core composed of alkali carbonate and alkali chloride mixture made by casting technique [J]. Materials Transaction, 2007, 48(5): 1034-1041.
[11] QIAN J G, ZHAO T. Electrodeposition of Ir on platinum in NaCl-KCl molten salt [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(11): 2855-2862.
[12] LV W Y, ZENG C L. Preparation of cohesive graphite films by electroreduction of 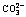 in molten Na2CO3-NaCl [J]. Surface and Coatings Technology, 2012, 206(219-220): 4287-4292.
in molten Na2CO3-NaCl [J]. Surface and Coatings Technology, 2012, 206(219-220): 4287-4292.
[13] IWASAWA K, YAMAGUCHI S, MAEDA M. Phase relation and thermodynamic properties of NaCl-Na2CO3 system as a basic system for secondary fly ash in incineration processes of municipal wastes [J]. Materials Transaction, 2001, 42(12): 2480-2486.
[14] YANG J G, HE D W, TANG C B, CHEN Y M, SUN Y H. Thermodynamics calculation and experimental study on separation of bismuth from a bismuth glance concentrate through a low-temperature molten salt smelting process [J]. Metallurgy and Materials Transaction B-Process Metallurgy and Materials Processing Science, 2011, 42(4): 730-737.
[15] LIU Jing-xin, GUO Xue-yi, LIU Yang. Fractal leaching kinetics of alkaline smelting product with metal enrichment from waste printed circuit boards [J]. The Chinese Journal of Nonferrous Metals, 2015, 25(2): 545-552. (in Chinese)
[16] YE L G, TANG C B, TANG M T, YANG J G, CHEN Y M, YANG S H, HE J. Separation antimony from stibnite concentrate through a low temperature smelting [J]. Journal of Central South University (Science and Technology), 2012, 43(9): 3338-3343. (in Chinese)
[17] YE L G, TANG C B, CHEN Y M, YANG S H, YANG J G, ZHANG W H. One-step extraction of antimony from low-grade stibnite in sodium carbonate-sodium chloride binary molten salt [J]. Journal of Cleaner Production, 2015, 93: 134-139.
[18] CHEN Y M, YE L G, TANG C B, YANG S H, TANG M T, ZHANG W H. Solubility of Sb in binary Na2CO3-NaCl molten salt [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(9): 3146-3151.
[19] LIDE D R, HAYNES W M. CRC Handbook of chemistry and physics (90th Edition) [M]. Floride: CRC Press Inc, 2010: 444-498.
[20] YE L G, TANG C B, CHEN Y M, YANG S H, TANG M T. Thermal physical properties and stability of the eutectic composition in a Na2CO3-NaCl binary system [J]. Thermochimica Acta, 2014, 596(10): 14-20.
(Edited by YANG Bing)
Cite this article as: YE Long-gang, HU Yu-jie, XIA Zhi-mei, TANG Chao-bo, CHEN Yong-ming, TANG Mo-tang. Solution behavior of ZnS and ZnO in eutectic Na2CO3-NaCl molten salt used for Sb smelting [J]. Journal of Central South University, 2017, 24(6): 1269-1274. DOI: 10.1007/s11771-017-3531-8.
Foundation item: Projects(51104128, 51234009) supported by the National Natural Science Foundation of China
Received date: 2015-09-21; Accepted date: 2016-03-22
Corresponding author: YE Long-gang, PhD; Tel: +86-731-22183453; E-mail: yelonggang@sina.cn

