DOI: 10.11817/j.issn.1672-7207.2016.11.014
Dy3+对NaLaF4:Yb3+/Er3+/Dy3+双功能纳米晶的上转换发光及顺磁性调制作用
胡仕刚1,吴笑峰1,席在芳1,唐志军1,刘云新2
(1. 湖南科技大学 信息与电气工程学院,湖南 湘潭,411201;
2. 湖南科技大学 物理与电子科学学院,湖南 湘潭,411201)
摘要:利用热溶剂法制备NaLaF4:Yb3+/Er3+/Dy3+光磁双功能纳米晶。结合能级跃迁图,阐述Dy3+的6FJ和6HJ系列能级与Er3+之间的能量传递及由此引起的特殊光调制现象。研究结果表明:样品在波长为980 nm的红外光子激发下可以发射中心波长为522 nm和547 nm的绿光;随着激发光功率增大,绿光发射强度也相应增强;调节Dy3+的掺杂摩尔百分比,可以同时调制样品的上转换发光和顺磁特性;随着Dy3+的摩尔百分比从0增加到5%,样品的522 nm发光相对于547 nm发光峰逐渐增强;若进一步增加Dy3+的摩尔百分比到10%,其相对强度反而减弱。随着Dy3+的摩尔百分比从0增大到10%,样品的顺磁性单调提升,但伴随着总体发光强度衰减。
关键词:光磁;双功能;稀土离子;掺杂
中图分类号:TB34 文献标志码:A 文章编号:1672-7207(2016)11-3715-06
Tuning upconversion luminescence and paramagnetic property of NaLaF4:Yb3+/Er3+/Dy3+ bifunctional nanocrystals by Dy3+
HU Shigang1, WU Xiaofeng1, XI Zaifang1, TANG Zhijun1, LIU Yunxin2
(1. School of Information and Electrical Engineering, Hunan University of Science and Technology,
Xiangtan 411201, China;
2. Department of Physics and Electronic Science, Hunan University of Science and Technology, Xiangtan 411201, China)
Abstract: NaLaF4:Yb3+/Er3+/Dy3+ bifunctional magnetic-optical nanocrystals were synthesized by solvothermal method. Combined with the energy level transition diagram, the energy transfer between the 6FJ and 6HJ series level of Dy3+ and Er3+ was discussed. The results show that the energy transfer can cause a special magnetic-optical modulation phenomenon. Green light is emitted which is centered at 522 nm and 547 nm under the excitation of 980 nm infrared light. With the increase of the laser power, the intensity of the green emission increases. The upconversion luminescence and paramagnetic characteristics of the samples can be modulated by adjusting the doping concentration of Dy3+.As the concentration of Dy3+ increases from 0 to 5%, emission peaks centered at 522 nm gradually increase with respect to the 547 nm emission peaks. If the concentration of Dy3+ is further increased to 10%, the relative intensity decreases. With the increase of the concentration of Dy3+ from 0 to 10%, the paramagnetic properties of the sample are enhanced, but the overall emission intensity is attenuated.
Key words: magnetic-optical; bifunctional; rare earth ions; doping
上转换材料在低能量红外光子的激发下可以发射从红光到紫外波长的高能量光子,在传感器、生物高清成像及红外探测等领域得到广泛应用[1-6]。上转换发光材料通常由基体材料和掺杂的发光离子组成[7-9],如单一的NaLaF4基体材料并不发光,但掺杂稀土Er3+之后能发射红光和绿光。上转换材料的发光效率高度与基体材料的声子能量、晶体场特性及晶体缺陷有关,选择合适的基体材料对于实现高效上转换发光至关重要。到目前为止,NaLnF4 (Ln即镧系)被公认为具有最高上转换发光效率的基体材料[10-12],其中,人们对NaYF4的研究最多。本文作者对NaLaF4体系的上转换发光特性进行研究。La3+与Y3+属于元素周期表的同一族,物理化学性质相近。但由于La3+比Y3+的半径更大,电负性更高,因此,对应的NaLaF4基体材料与NaYF4相比,具有更低的晶格点对称性和热传导性。晶格点对称性越低,在该类格点上的发光中心离子可以获得更高的上转换发光效率;而低的热传导性有利于光热聚集。上转换纳米探针用于肿瘤细胞的探测和热效应治疗领域具有广阔的应用前景。常用的掺杂离子为Yb3+/Er3+和Yb3+/Tm3+对,可以实现红、绿、蓝三基色上转换发光[13-19]。Yb3+/Er3+/Tm3+最外层都是由非饱和电子填充,具有顺磁或铁磁性。本文拟引入稀土Dy3+同时调制Yb3+/Er3+对掺杂的NaLaF4上转换纳米颗粒的发光与磁性。
1 样品制备与表征
1.1 原材料
原材料为:油酸,十八烯和三氟醋酸钠,购于阿法埃莎并直接使用;环己烷、氯化铒、氯化镱、氯化镝、氯化镧等,购于国药集团并直接使用;去离子水,自制。
1.2 样品制备
1) 将0.4 mL浓度为2 mol/L的氯化稀土溶液与7 mL油酸和7 mL十八烯混合,在氮气流保护下,于150 ℃的温度中加热0.5 h去除水分,然后升温到305 ℃,得样品A,备用。
2) 将1.6 mmol三氟醋酸钠溶于2 mL油酸和2 mL十八烯,在160 ℃和氮气流保护下加热搅拌40min至三氟醋酸钠完全溶于油酸和十八烯混合溶剂,得样品B。
3) 将B注入A,并在305 ℃反应0.5 h。反应完毕,冷却到室温,加入无水乙醇析出纳米晶产物,将产物用乙醇和环己烷各清洗2次,最后分散在环己烷中备用。
1.3 表征
透射电镜成像用JEM 3010进行表征,工作电压为200 kV;荧光光谱测试仪器为日立F-4700,激发光源为980 nm可调功率半导体激光器;利用布鲁克 D8 ADVANCE X线粉末衍射仪测试XRD谱,角度重现性为±0.000 1°;磁性测量采用Quantum Design公司的PPMS测试系统。
2 结果和讨论
2.1 晶体结构
NaLaF4:25%Yb3+/2%Er3+/5%Dy3+ TEM像见图1。从图1可以看到:合成的NaLaF4:25%Yb3+/2%Er3+/ 5%Dy3+上转换纳米晶具有较均一的粒度,具有立方体形貌,平均直径为10 nm。X线衍射谱见图2,这种上转换纳米晶为六角相晶体结构。利用谢乐公式
D=Kλ/(βcosθ) (1)
计算的晶体粒度为9.35 nm,与TEM的观测结果基本一致。式(1)中:K为Scherrer常数;D为晶粒垂直于晶面方向的平均厚度;B为实测样品衍射峰半高宽度;θ为衍射角;λ为X线波长,为0.154 056 nm。
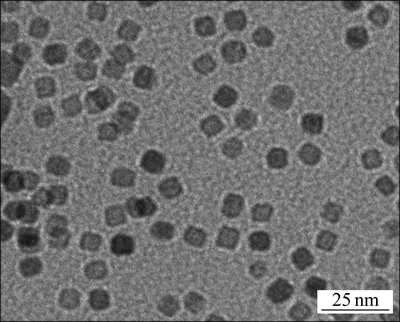
图1 NaLaF4:25%Yb3+/2%Er3+/5%Dy3+光磁双功能纳米晶的TEM像
Fig. 1 TEM image of NaLaF4:25%Yb3+/2%Er3+/5%Dy3+ bifunctional magnetic-optical nanocrystals
2.2 上转换发光特性
NaLaF4:25%Yb3+/2%Er3+/5%Dy3+光磁双功能纳米晶在980 nm红外光激发下的发射谱见图3。在波长为980 nm的红外光激发下,NaLaF4:25%Yb3+/2%Er3+/ 5%Dy3+上转换纳米晶的发射强绿光,即使在0.2 W/mm2的低功率光源激发下,也会发现肉眼可见的闪亮绿光。绿光由2条光谱带组成,中心波长分别位于547 nm和522 nm,前者对应Er3+ 4f壳层电子的4S3/2-4I15/2跃迁,后者对应Er3+ 4f壳层电子的2H11/2-4I15/2跃迁。虽然发光离子为Er3+,但Yb3+在实现上转换发光的过程中起重要作用。简化能级图及可能存在的激发与发射过程见图4。在一般情况下,Yb3+处于2F7/2基态的电子首先吸收980 nm红外激发光子,向上跃迁到2F5/2能级,在跃迁回2F7/2基态的过程中会将释放的能量传递给位于4I15/2和4I11/2能级上的Er3+电子[4-6]。Er3+的4I15/2基态上的电子吸收Yb3+的能量后可以继续向上跃迁到4I11/2能级,4I11/2态电子吸收1个波长为 980 nm的光子能量后可以向上跃迁到4F7/2能级。4F7/2能级上的电子又可以向下弛豫到2H11/2和4S3/2能级,而2H11/2和4S3/2能级上的电子在跃迁回基态4I15/2的过程中分别发射522 nm和547 nm的绿光。虽然Er3+的4I15/2基态和4I11/2激发态电子都可以直接吸收激发光源的980 nm光子,但是Er3+的吸收截面只有Yb3+吸收截面的几分之一,吸收效率显著小于Yb3+的吸收效率;另一方面,Yb3+的2F5/2态电子与Er3+的4F7/2和4I11/2态电子之间可以实现共振能量传递,因此,Yb3+在上转换发光领域被广泛用于Er3+,Tm3+和Ho3+等发光离子的敏化剂,从而提高发光离子的上转换发光效率。Dy3+在Er3+的发光过程中同样起敏化作用。Dy3+的6H7/2至6F3/2能级之间分布有非常丰富的梯形能级,其能隙刚好对应于Er3+的4F7/2与2H11/2能级的能隙,因而,可以为Er3+的4F7/2→2H11/2弛豫提供能量储存和反馈,从而提升其转换效率。另外,Dy3+也可以与Yb3+之间通过2F5/2(Yb)+6H15/2(Dy)→2F7/2(Yb)+6H5/2(Dy)交换能量,从而将Yb3+的无辐射能量储存并传递给Er3+。值得注意的是:Dy3+也可以直接吸收980 nm激发光子,但因为其吸收截面远比Yb3+的小,所以,与起直接敏化作用的Yb3+相比,Dy3+起间接敏化作用。
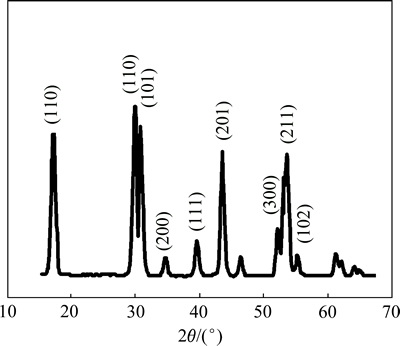
图2 NaLaF4:25%Yb3+/2%Er3+/5%Dy3+光磁双功能纳米晶的XRD谱
Fig. 2 XRD pattern of NaLaF4:25%Yb3+/2%Er3+/5%Dy3+ bifunctional magnetic-optical nanocrystal
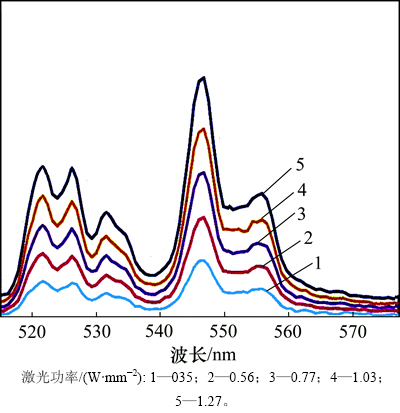
图3 NaLaF4:25%Yb3+/2%Er3+/5%Dy3+光磁双功能纳米晶在980 nm红外光激发下的发射谱
Fig. 3 Upconversion luminescence spectra of NaLaF4:25%Yb3+/2%Er3+/5%Dy3+ bifunctional magnetic-optical nanocrystals under excitation of 980 nm infrared light
从图3可知:随着激发功率增强,上转换绿光的强度也随之增强,但光谱的形状未出现明显变化。根据Auzel定律,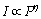 (其中,I为发光强度,P为激发光功率,
(其中,I为发光强度,P为激发光功率,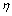 为发射1个可见光光子所需的红外光子数)。图5所示为激发功率与发光强度的关系线性拟合,
为发射1个可见光光子所需的红外光子数)。图5所示为激发功率与发光强度的关系线性拟合,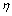 分别为1.78(对于547 nm绿光发射)和1.82(对于522 nm绿光发射)。这说明无论发射1个522 nm绿光光子,还是发射1个547 nm绿光光子,都需要吸收2个980 nm红外光子。由光子的能量正比于波长的倒数,可以推知2个980 nm红外光子的能量大于1个522 nm或547 nm绿光光子的能量,这与根据Auzel理论计算的2光子能量完全一致。需注意的是:尽管都是双子上转换发光,但522 nm绿光的
分别为1.78(对于547 nm绿光发射)和1.82(对于522 nm绿光发射)。这说明无论发射1个522 nm绿光光子,还是发射1个547 nm绿光光子,都需要吸收2个980 nm红外光子。由光子的能量正比于波长的倒数,可以推知2个980 nm红外光子的能量大于1个522 nm或547 nm绿光光子的能量,这与根据Auzel理论计算的2光子能量完全一致。需注意的是:尽管都是双子上转换发光,但522 nm绿光的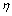 要比547 nm绿光带的
要比547 nm绿光带的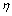 大,这说明522 nm绿光对应的Er3+2H11/2能级上的电子布居概率要比547 nm绿光对应的4S3/2能级的电子布居概率略低。
大,这说明522 nm绿光对应的Er3+2H11/2能级上的电子布居概率要比547 nm绿光对应的4S3/2能级的电子布居概率略低。
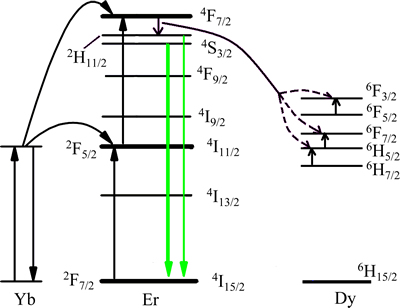
图4 简化能级图及可能激发与发射过程
Fig. 4 Schematic energy-level diagram and possible excitation and emission processes
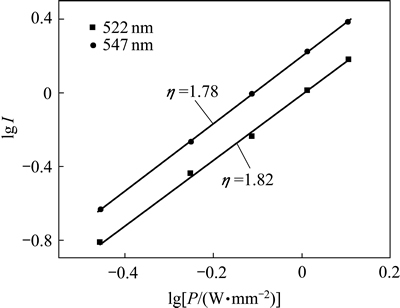
图5 NaLaF4:25%Yb3+/2%Er3+/5%Dy3+光磁双功能纳米晶在980 nm红外光激发下的发射谱与激发功率之间的拟合
Fig. 5 Plots of emission intensity versus excitation power in NaLaF4:25%Yb3+/2%Er3+/5%Dy3+ bifunctional magnetic-optical nanocrystals under excitation of 980 nm infrared light
Dy3+属于重稀土离子,其电子能级相当多。Dy3+在紫外光的激发下,本身可以发射绿光和黄光。Dy3+对980 nm的红外光子也有一定吸收,但吸收截面远比Er3+和Yb3+的小。图6所示为NaLaF4:25%Yb3+/2%Er3+/ Dy3+上转换纳米晶的发光强度随掺杂离子Dy3+的摩尔百分比所产生的变化,所有光谱以547 nm发射峰为基础进行了归一化处理。从图6可见:随着Dy3+摩尔百分比从0增大到10%,522 nm发射带相对于 547 nm发射带先逐渐增强,然后减弱。这说明Dy3+与Er3+之间存在显著的能量交换。从图3可知:522 nm发射带对应2H11/2-4I15/2电子跃迁,而2H11/2能级上的电子布居数取决于4F7/2能级上的电子向下弛豫的概率。在没有Dy3+掺杂的情况下,Er3+的4F7/2能级上电子向下跃迁到2H11/2能级的概率比4S3/2能级的概率略低,由此导致522 nm发射峰的强度比547 nm发射峰的强度低。从图3可知:Dy3+存在密集的6FJ和6HJ系列梯形能级分布,而这2个系列能级之间的间隔刚好与4F7/2-2H11/2弛豫的能量相当,可以有效耗散4F7/2- 2H11/2弛豫产生的能量,这有利于加速4F7/2-2H11/2弛豫过程,由此导致2H11/2能级上的电子数显著提升及2H11/2-4I15/2跃迁效率提高。图6表明:522 nm绿光发射强度并不是随着Dy3+摩尔百分比的增大而单调增大,当Dy3+摩尔百分比为5%时,其发射强度达到最大值;但在Dy3+摩尔百分比从5%继续提升到10%的过程中,522 nm发射光的强度减弱。结合文献[20]和[21]的报道,这主要是猝灭效应所致。当Dy3+摩尔百分比达到1 0%时,Dy3+之间的热振动会显著提升,从而大量耗散Er3+和Yb3+所储存的光子能量。
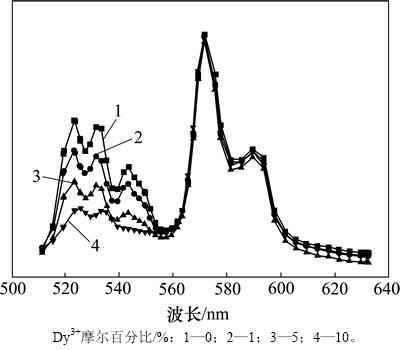
图6 NaLaF4:25%Yb3+/2%Er3+/x%Dy3+ (x=0,1,5,10)光磁双功能纳米晶在980 nm红外光激发下的发射谱
Fig. 6 Upconversion luminescence spectra of NaLaF4:25%Yb3+/2%Er3+/x%Dy3+ (x=0,1,5,10) bifunctional magnetic-optical nanocrystals under excitation of 980 nm infrared light
2.3 室温顺磁性
图7所示为NaLaF4:25%Yb3+/2%Er3+纳米晶的室温顺磁特性。这种顺磁特性主要是稀土磁性离子Yb3+和Er3+存在所致。基体材料NaLaF4并无顺磁特性。在NaLaF4:25%Yb3+/2%Er3+纳米晶中掺杂Dy3+可以显著调制其顺磁特性。随着Dy3+摩尔百分比从0增大到10%,在12×105 A/m外加磁场强度作用下,其磁化强度最高可达到1.33 Am2/kg,见图8。这主要是由于Dy3+具有优异顺磁特性。若继续提高Dy3+的掺杂摩尔百分比,其磁化强度还会进一步提高,但其总体发光强度会衰减,且对522 nm发射光的相对调制效果也会有所下降。
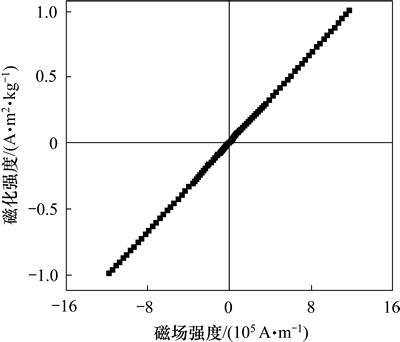
图7 NaLaF4:25%Yb3+/2%Er3+纳米晶的顺磁特性
Fig. 7 Paramagnetic properties of NaLaF4:25%Yb3+/2%Er3+ nanocrystals
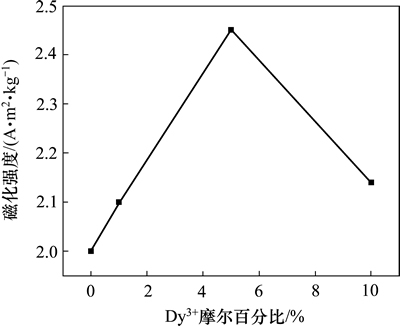
图8 NaLaF4:25%Yb3+/2%Er3+/x%Dy3+ (x=0,1,5,10)纳米晶的顺磁特性与掺杂离子Dy3+的摩尔百分比关系曲线
Fig. 8 Relationship between paramagnetic properties of NaLaF4:25%Yb3+/2%Er3+/x%Dy3+(x=0,1,5,10) nanocrystals and concentration of doped ions Dy3+
3 结论
1) 利用热溶剂法制备了光磁双功能纳米晶NaLnF4:25%Yb3+/2%Er3+/x%Dy3+ (x=0,1,5,10)。该光磁双功能纳米晶具有立方体形貌,属六角相晶体,平均尺寸为10 nm。
2) 样品在980 nm红外光子的激发下可以发射中心波长为522 nm和547 nm的绿光。随着激发光功率增大,绿光发射强度也相应增大。
3) 调节Dy3+的掺杂摩尔百分比,可以同时调制样品的上转换发光和顺磁特性。随着Dy3+摩尔百分比从0增加到5%,样品的522 nm发光相对于547 nm发光峰逐渐增强;若进一步增大Dy3+的摩尔百分比到10%,其相对强度反而减弱。结合能级跃迁图,可阐述Dy3+的6FJ和6HJ系列能级与Er3+之间的能量传递,即由此引起的特殊光调制现象。
4) 随着Dy3+摩尔百分比从0增大到10%,样品的顺磁性单调提升,但伴随着总体发光强度衰减。
参考文献:
[1] LIU Yunxin, WANG Dingsheng, LI Lingling, et al. Energy upconversion in lanthanide-doped core/porous-shell nanoparticles[J]. Inorganic Chemistry, 2014, 53(7): 3257-3259.
[2] LI Li, CAO Xueqin, ZHANG You, et al. Synthesis and upconversion luminescence of Lu2O3:Yb3+,Tm3+ nanocrystals[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(2): 373-379.
[3] CHEN Lei, WEI Xianhua, FU Xu. Effect of Er substituting sites on upconversion luminescence of Er3+-doped BaTiO3 films[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(5): 1156-1160.
[4] XU Wei, GAO Xiaoyang, ZHENG Longjiang, et al. An optical temperature sensor based on the upconversion luminescence from Tm3+/Yb3+ co-doped oxyfluoride glass ceramic[J]. Sensors and Actuators B: Chemical, 2012, 173(12): 250-253.
[5] ZHENG Kezhi, LIU Zhenyu, L Changjian, et al. Temperature sensor based on the UV upconversion luminescence of Gd3+ in Yb3+–Tm3+–Gd3+ co-doped NaLuF4 microcrystals[J]. Journal of Materials Chemistry C, 2013, 1(35): 5502-5507.
Changjian, et al. Temperature sensor based on the UV upconversion luminescence of Gd3+ in Yb3+–Tm3+–Gd3+ co-doped NaLuF4 microcrystals[J]. Journal of Materials Chemistry C, 2013, 1(35): 5502-5507.
[6] GU Xiaorong, HUANG Kun, PAN Haifeng, et al. Efficient mid-infrared single-photon frequency upconversion detection with ultra-low background counts[J]. Laser Physics Letters, 2013, 10(5): 527-535.
[7] B NZLI J C G. Lanthanide luminescence for biomedical analyses and imaging[J]. Chemical Reviews, 2010, 110(5): 2729-2755.
NZLI J C G. Lanthanide luminescence for biomedical analyses and imaging[J]. Chemical Reviews, 2010, 110(5): 2729-2755.
[8] CHEN Guangying, QIU Hailong, PRASAD P N, et al. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics[J]. Chemical Reviews, 2014, 114(10): 5161-5214.
[9] YIN P T, SHAH S, CHHOWALLA M, et al. Design, synthesis, and characterization of graphene-nanoparticle hybrid materials for bioapplications[J]. Chemical Reviews, 2015, 115(7): 2483–2531.
[10] ZHAO G, TONG L, CAO P, et al. Functional PEG–PAMAM- tetraphosphonate capped NaLnF4 nanoparticles and their colloidal stability in phosphate buffer[J]. Langmuir, 2014, 30(23): 6980-6989.
[11] HU Rongxuan, YE Song, WANG Huiyun, et al. Upconversion luminescence properties of phase and size controlled NaLnF4: Yb3+,Er3+(Ln=Y,Gd) Nanoparticles[J]. Journal of Nanoscience and Nanotechnology, 2015, 15(1): 368-372.
[12] SARAKOVSKIS A, KRIEKE G, DOKE G, et al. Comprehensive study on different crystal field environments in highly efficient NaLaF4:Er3+ upconversion phosphor[J]. Optical Materials, 2015, 39: 90-96.
[13] NIU Na, YANG Piaoping, HE Fei, et al. Tunable multicolor and bright white emission of one-dimensional NaLuF4:Yb3+,Ln3+ (Ln=Er,Tm,Ho,Er/Tm,Tm/Ho) microstructures[J]. Journal of Materials Chemistry, 2012, 22(21): 10889-10899.
[14] GAO Yu, ZHAO Qian, XU Zhenhe, et al. Hydrothermally derived NaLuF4:Yb3+,Ln3+(Ln3+=Er3+,Tm3+,Ho3+) microstructures with controllable synthesis, morphology evolution and multicolor luminescence properties[J]. New Journal of Chemistry, 2014, 38(6): 2629-2638.
[15] XU Zhenhe, ZHAO Qian, REN Baoyi, et al. Facile synthesis and luminescence properties of Y2O3:Ln3+(Ln3+=Eu3+,Tb3+,Dy3+,Sm3+, Er3+,Ho3+,Tm3+,Yb3+/Er3+,Yb3+/Tm3+,Yb3+/Ho3+) microspheres[J]. Journal of Nanoscience and Nanotechnology, 2014, 14(8): 5781-5789.
[16] HU Shigang, LIU Yunxin, WU Xiaofeng, et al. Remarkable red-shift of upconversion luminescence and anti-ferromagnetic coupling in NaLuF4:Yb3+/Tm3+/Gd3+/Sm3+ bifunctional microcrystals[J]. Journal of Rare Earths, 2016, 34(2): 166-173.
[17] CHEN Zenghui, WU Xiaofneg, HU Shigang, et al. Upconversion NaLuF4 fluorescent nanoprobes for jellyfish cell imaging and irritation assessment of organic dyes[J]. Journal of Materials Chemistry C, 2015, 3(23): 6067-6076.
[18] HU Pan, WU Xiaofeng, HU Shigang, et al. Enhanced upconversion luminescence through core/shell structures and its application for detecting organic dyes in opaque fishes[J]. Photochemical & Photobiological Sciences, 2016, 15(2): 260-265.
[19] CHEN Zenghui, WU Xiaofneg, HU Shigang, et al. Multicolor upconversion NaLuF4 fluorescent nanoprobe for plant cell imaging and detection of sodium fluorescein[J]. Journal of Materials Chemistry C, 2015, 3(1): 153-161.
[20] KUMAR K, RAI S B, RAI D K. Upconversion and concentration quenching in Er3+-doped TeO2-Na2O binary glasses[J]. Journal of Non-crystalline Solids, 2007, 353(13): 1383-1387.
[21] DAI Shixun, YU Chunlei, ZHOU Gang, et al. Concentration quenching in erbium-doped tellurite glasses[J]. Journal of Luminescence, 2006, 117(1): 39-45.
(编辑 陈灿华)
收稿日期:2015-11-22;修回日期:2016-01-26
基金项目(Foundation item):国家自然科学基金资助项目(61376076, 61274026, 21301058, 61377024);湖南省教育厅资助项目(14B060);湖南省科技计划项目(2014FJ2017, 2013FJ2011) (Projects(61376076, 61274026, 21301058, 61377024) supported by the National Natural Science Foundation of China; Project(14B060) supported by the Scientific Research Fund of Education Department of Hunan Province; Projects(2014FJ2017, 2013FJ2011) supported by the Science and Technology Plan Foundation of Hunan Province)
通信作者:刘云新,博士,副教授,从事光电信息材料与器件研究;E-mail: lyunxin@163.com