Preparation, characterization and catalytic performance of Cu nanowire catalyst for CO2 hydrogenation
来源期刊:中南大学学报(英文版)2018年第4期
论文作者:翟玉春 王明华 张晓艳 陈忠翼 P. XIAO P. WEBLEY
文章页码:691 - 700
Key words:CO2 hydrogenation; methanol; Cu nanowire; migration; sintering; flow blockage
Abstract: Pure Cu nanowires as catalyst were prepared by electrochemical deposition and were used in CO2 hydrogenation to methanol. The active sites of the Cu based catalyst were discussed. The performance and structural development of the catalyst were observed during CO2 hydrogenation. A mechanism for the deactivation of the catalyst was discussed. The key factors that affect the deactivation of the catalyst were found. Cu nanowire sample was characterized by SEM, EDS, XRD, and BET. The results show that Cu nanowires have very high sintering resistance and catalytic stability. This helps to develop high performance catalysts. The changes in the grain size, SEM morphology and catalytic properties of the sample during CO2 hydrogenation show that the migration of the Cu atoms on the surface of the Cu nanowires can occur. Continuous migration of Cu atoms and sintering of Cu grains can lead to flow blockage in gas channels. The gas channel flow blockage or the sintering of Cu grains can lead to deactivation of the catalyst. However, the shape of catalytic performance curve indicates that the main reason for the deactivation of the catalyst is the gas channel flow blockage.
Cite this article as: ZHANG Xiao-yan, WANG Ming-hua, CHEN Zhong-yi, P. XIAO, P. WEBLEY, ZHAI Yu-chun. Preparation, characterization and catalytic performance of Cu nanowire catalyst for CO2 hydrogenation [J]. Journal of Central South University, 2018, 25(4): 691–700. DOI: https://doi.org/10.1007/s11771-018-3773-0.

J. Cent. South Univ. (2018) 25: 691-700
DOI: https://doi.org/10.1007/s11771-018-3773-0

ZHANG Xiao-yan(张晓艳)1, 3, WANG Ming-hua(王明华)1, CHEN Zhong-yi(陈忠翼)1,P. XIAO2, P. WEBLEY2, ZHAI Yu-chun(翟玉春)1
1. School of Metallurgy, Northeastern University, Shenyang 110819, China;
2. Department of Chemical and Biomolecular Engineering, The University of Melbourne,Victoria 3010, Australia;
3. Library of Tongren College, Tongren 554300, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract: Pure Cu nanowires as catalyst were prepared by electrochemical deposition and were used in CO2 hydrogenation to methanol. The active sites of the Cu based catalyst were discussed. The performance and structural development of the catalyst were observed during CO2 hydrogenation. A mechanism for the deactivation of the catalyst was discussed. The key factors that affect the deactivation of the catalyst were found. Cu nanowire sample was characterized by SEM, EDS, XRD, and BET. The results show that Cu nanowires have very high sintering resistance and catalytic stability. This helps to develop high performance catalysts. The changes in the grain size, SEM morphology and catalytic properties of the sample during CO2 hydrogenation show that the migration of the Cu atoms on the surface of the Cu nanowires can occur. Continuous migration of Cu atoms and sintering of Cu grains can lead to flow blockage in gas channels. The gas channel flow blockage or the sintering of Cu grains can lead to deactivation of the catalyst. However, the shape of catalytic performance curve indicates that the main reason for the deactivation of the catalyst is the gas channel flow blockage.
Key words: CO2 hydrogenation; methanol; Cu nanowire; migration; sintering; flow blockage
Cite this article as: ZHANG Xiao-yan, WANG Ming-hua, CHEN Zhong-yi, P. XIAO, P. WEBLEY, ZHAI Yu-chun. Preparation, characterization and catalytic performance of Cu nanowire catalyst for CO2 hydrogenation [J]. Journal of Central South University, 2018, 25(4): 691–700. DOI: https://doi.org/10.1007/s11771-018-3773-0.
1 Introduction
At present, there is still debate on active site of Cu based catalysts for CO2 hydrogenation to methanol. One opinion is that Cu is main active site of the catalysts [1–5]. The activity of the catalysts is easily affected by the Cu specific surface area [4–10] or Cu grain size [5, 10, 11], and the catalytic activity of the catalysts with smaller grain size or larger specific surface area is usually higher. ZnO as additive may act important role in dispersing active component, inhibition of sintering, limiting poisoning of active site, and neutralizing the acidity of Al2O3 to prevent the formation of by-products, such as dimethyl ether [8, 12, 13]. ZnO is a good industrial fine desulfurization agent [14, 15]. The second opinion is that Cu–Zn site [16–19] may participate in CO2 hydrogenation to methanol, and there is a strong interaction between Cu and ZnO [9, 20–25]. Cu must be alloyed with Zn to make methanol synthesis better and faster, and the interaction between single Cu and CO2 is very poor [19].The previous research results showed that the activity of skeletal Cu based catalysts for methanol synthesis is higher than that of commercial Cu/ZnO/Al2O3 catalyst [26, 27]. The activity of pure Cu particles is very low for methanol synthesis, as reported in Ref. [18]. This is because the metal atoms have higher mobility [28], and they are easy to sinter in the absence of inert support. Copper micro-crystals can fuse even at a temperature less than 200 °C, and the activity decreases rapidly [29, 30]. The sintering characteristic of pure Cu particles has severely limited the study on pure Cu catalyst.
High concentration CO2 can cause rapid deactivation of Cu-based catalysts [23]. The structure of common Cu based catalysts is more complex, and it is difficult to obtain the information about structural development or sintering mechanism during deactivation. One opinion is that Cu sintering is the main reason of catalyst deactivation [23, 31]. Researchers tried to improve the sintering resistance of Cu based catalysts by adding additive [25, 31–34] or changing the morphology [25, 32–34]. The second opinion is that the H2O produced during CO2 hydrogenation can promote the growth of metal oxide crystals [23, 35] or Cu grains [36], and can accelerate the sintering and deactivation of catalysts [23, 35, 36]. LI et al [2] mentioned that water is strong oxidant for metals such as copper at high temperature. When the reduction rate by H2 or CO is not high enough, most of active site is oxidized to lose its activity. Researchers tried to improve the water resistance of Cu based catalysts by adding ZrO2, SiO2 or other additives [2, 36]. The information about the structural development or the sintering mechanism during deactivation is still currently scarce [37]. The study on the deactivation behavior has important practical significance for improving the performance of catalyst.
The methanol synthesis from CO2 hydrogenation has structurally sensitive character [10, 11]. The morphology affects the performance of catalysts [25, 32–34, 38]. Nanowire has significant surface effect, quantum size effect and macroscopic quantum tunneling effect and exhibits unique structural, electronic and thermal properties, and it becomes an international frontier research hotspot recently. A few metal oxide catalysts for CO2 hydrogenation were prepared to form a fibrous type or were loaded on one-dimensional carrier [25, 32–34]. AN et al [32, 33] reported that a better dispersion of Cu/Zn crystallites and the fibrous structure of catalyst were the main factors for the higher activity and stability. LEI et al [25] found that the activities of catalysts depended strongly on the morphology of ZnO, and CuO/ZnO catalyst prepared with filament-like ZnO exhibited the best activity.
Pure Cu nanowire catalyst was prepared by electrochemical deposition and was used the first time in CO2 hydrogenation to methanol. The catalyst was characterized by various means. The performance of Cu nanowire catalyst was investigated. The mechanism of deactivation and active site of catalyst, which has attracted much attention, was discussed.
2 Experimental
2.1 Preparation of Cu nanowire catalyst
1060 aluminum plate was annealed at 400 °C for 4 h. Anodic aluminum oxide (AAO) template was prepared by one-step anodic oxidation method in 0.3 mol/L oxalic acid solution at 40 V for 8 h under water cooling.
Pure Cu nanowires were prepared by electrochemical deposition at room temperature using AAO template with aluminum substrate as cathode. Pure Cu sheet was used as anode. The distance between the two electrodes was about 3 cm. The electrolyte was comprised [39] of 60 g/L CuSO4·5H2O, 180 g/L H2SO4, 300 mg/L PEG,80 mg/L NaCl and 20 mg/L SPS. At initial stage of electro-deposition, current density was slowly increased to about 1.5 A/dm2 and then kept constant for 20 min. The electrolyte was stirred by means of moving cathode with a frequency of about 60 min–1. The AAO template with Cu nanowires was immersed into 0.75 mol/L NaOH solution with 3 g/L glucose until aluminum plate was completely exposed. After the aluminum plate was removed, remaining solution was stirred under ultrasonic at room temperature. After the AAO template was completely dissolved, the suspended solid particles in liquid were immediately filtered and washed with distilled water, and then red brown Cu nanowire sample was obtained after being washed with 95% ethanol. In order to prevent the Cu nanowires from oxidation in air, the prepared sample was sealed.
2.2 Characterization of catalyst
The morphology and surface composition of sample were observed with SEM (Model SSX-550, Shimadzu, Japan; Model S-3400N, Hitachi, Japan; Model Ultra Plus, Zeiss, Germany) with EDS. Crystal structure of sample was analyzed by XRD (Model PW3040/60, PANALYTICAL B.V, the Netherlands). The specific surface area and pore distribution of sample was tested using BET (Model Kubo X1000, Beijing builder electronic technology CO., LTD).
2.3 Catalytic performance test
The performance of Cu nanowire catalyst was tested by a hybrid instrument-combining reactor system, temperature-controlling system, flux- controlling system, and gas analysis system. The reactor was a stainless steel tubular fixed bed. The gas composition at the inlet and outlet of the reactor was detected by a gas chromatograph (Model GC-2060, Lunan Analytical Instrument CO., LTD). Because Cu nanowire sample mainly exists in metal state, therefore it was not pre-reduced before use and was directly used. Feed gas was composed of V(H2)/V(CO2)/V(N2)≈3/1/1. Pressure was about 3 MPa. CO2 conversion, selectivity of products was calculated by the following formulas:
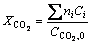
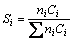
where ni is the number of carbon atoms in product i; Ci is the mole fraction of product i; CCO2,0 is the mole fraction of CO2 in feed gas.
3 Results and discussion
3.1 SEM morphologies of Cu nanowire sample
NaOH solution was used for dissolving AAO template, and a small amount of glucose was added in the NaOH solution to remove dissolved oxygen and to slow down Cu oxidation in alkaline solution. The standard electrode potential of glucose acid salt/glucose is –0.470 V, and the standard electrode potential of Cu2O/Cu is –0.385 V [40]. Figure 1(a) shows that there are hemispherical particles and scattered Cu nanowires in the sample after AAO template was dissolved. Hemispherical particles with an average diameter of about 30 μm have regular shape. Figure 1(b) shows that the average diameter of scattered Cu nanowires is about 80 nm.
Figure 2 shows the SEM morphology of single hemispherical particle in sample at high magnification. Figure 2(a) shows that the Cu perpendicular to the plane of the hemispherical nanowires inside the hemispherical particle are particle and form an array, and the average diameter of the Cu nanowires is also about 80 nm. Figure 2(b) shows that there are pores on the spherical surface of the hemispherical particle, and the tops of some Cu nanowires are faintly visible (see Inset).
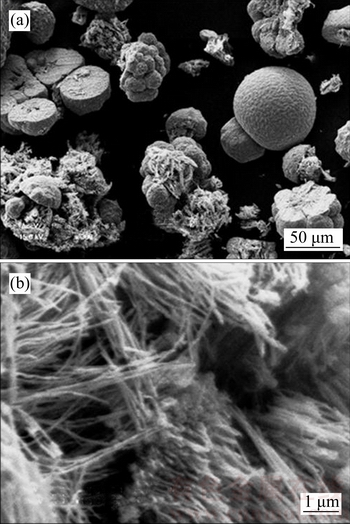
Figure 1 SEM morphologies of Cu nanowire sample (a) and scattered Cu nanowires (b) in sample
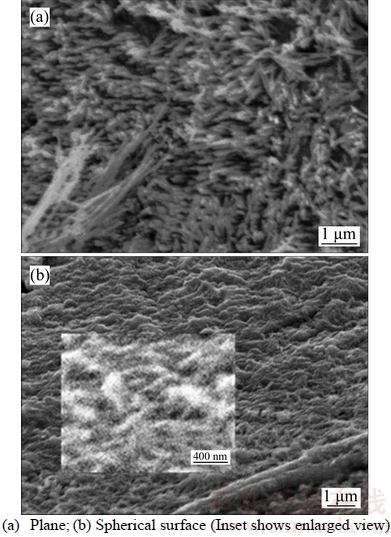
Figure 2 SEM morphologies of single hemispherical particle in sample at high magnification:
3.2 BET specific surface area and pore volume distribution of sample
The N2 adsorption desorption isotherm and pore volume distribution of Cu nanowire sample are shown in Figures 3 and 4, respectively. The results show that the specific surface area of the sample is about 14.8 m2/g. The theoretical specific surface area of Cu nanowires with an average diameter of 80 nm is about 6 m2/g. The actual specific surface area of Cu nanowires is higher than the theoretical one, which is due to the fact that the surface of Cu nanowires is not smooth. Based on these findings and SEM morphologies of hemispherical particle it is speculated that the hemispherical particle is a Cu nanowire array.
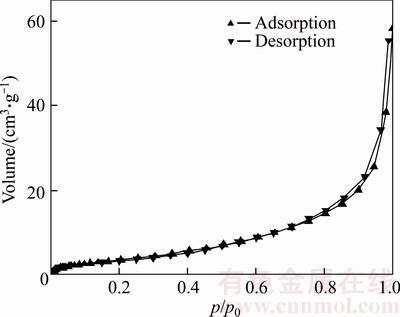
Figure 3 N2 adsorption–desorption isotherm of Cu nanowire sample

Figure 4 Pore volume distribution of Cu nanowire sample
Under the condition of the movement of moving cathode, the shear force generated by stirring is small, and the Cu nanowires deposited outside the template can still keep their own growth. When the Cu nanowires grow to a certain length, some adjacent Cu nanowires gather to form clusters and large numbers of clusters gather to form arrays.
3.3 EDS and XRD of Cu nanowire sample
There is no XRD peak of Cu oxide in Figure 5, but there is a weak EDS peak of oxygen (see Inset of Figure 5), which is because Cu nanowire surface has large number of dangling bonds, and the dangling bonds adsorbed the oxygen in environment form a protective layer of oxide film [41] on the Cu nanowire surface. These results indicate that Cu nanowires mainly exist in metal state (PDF card number 04–0836). The average grain size of Cu grains in the sample is about 26 nm by Jade 6 software.
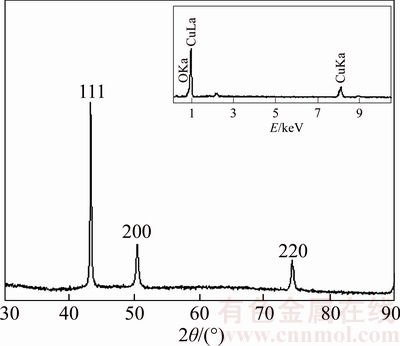
Figure 5 XRD pattern of Cu nanowire sample (Inset shows EDS spectrum)
3.4 Initial activity of Cu nanowire catalyst
The main products of CO2 hydrogenation are CO, methanol and H2O over the Cu nanowire catalyst.
The suitable temperature for methanol synthesis on Cu based catalysts is generally 250 °C [8, 9, 27, 31]. Section I of Figure 6 shows that at 250 °C, CO2 conversion rate is about 7.1%, CO selectivity is about 64.4%, methanol selectivity is about 35.5%, the space time yield (STY) of methanol is about 21.6 mg/(gcat·h) and methanol specific yield is about 1.5 mg/(m2Cu·h) on Cu nanowire catalyst of 1.0 g. The higher CO selectivity and lower methanol selectivity on the catalyst are in agreement with the performance characteristics of Cu based catalysts [1].
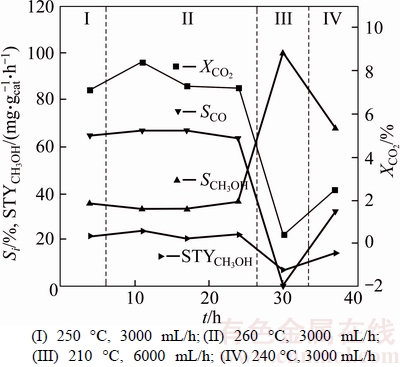
Figure 6 CO2 conversion and product selectivity over Cu nanowire catalyst of 1.0 g:
3.5 Performance of catalyst after change in reaction temperature
The performance of Cu based catalysts is easier to be affected by temperature [1, 9, 31, 42]. The activity of the catalysts is higher at higher temperature and the selectivity for methanol is better at lower temperature generally. Section II of Figure 6 shows that, when the reaction temperature increased to 260 °C, the activity of the Cu nanowire catalyst obviously increased, CO2 conversion rate and the STY of methanol reached 8.4% and 23.9 mg/(gcat·h) respectively, and the selectivity for methanol slightly decreased to about 33.1%. But soon CO2 conversion rate decreased significantly and later gently. It is speculated that some of the catalyst may be deactivated. Reaction temperature was decreased for continuing to react. When the temperature was reduced to 210 °C, the feed gas flow rate was increased from 3000 mL/h to 6000 mL/h in order to prevent lower boiling point product (such as methanol and H2O) from staying. Section III of Figure 6 shows that at 210 °C, the activity of the catalyst was lower, CO2 conversion rate was only about 0.4%, methanol selectivity was higher close to the 100% (CO was not detected), and the STY of methanol was about 6.9 mg/(gcat·h). Section IV of Figure 6 shows that when reaction temperature increased to 240 °C again, the conversion of CO2 and the STY of methanol significantly increased to 2.5% and 14.5 mg/(gcat·h) respectively, and methanol selectivity slightly decreased to 67.5%. The change in performance of Cu nanowire catalyst with temperature is in agreement with the performance characteristic of Cu based catalysts. Combined with above phenomena, it is suggested that Cu may be the active site of catalyst.
3.6 Characterization of catalyst in intermediate stage of reaction
After 37 h reaction, the catalyst was taken out from reactor. Figure 7 shows that there are tiny Cu nano-particles on the surface of some scattered Cu nanowires. Figure 8 shows that the average grain size of sample is about 22 nm (by Jade 6 software), smaller than the original size of 26 nm. It is because FWHM of the diffraction peak of XRD was broadened because of the tiny Cu nanoparticles on the surface of some scattered Cu nanowires (see Figure 7), and therefore the average grain size of the sample became smaller by XRD analysis. Based on above phenomena it is speculated that more small Cu nanoparticles can be generated on the surface of scattered Cu nanowires during CO2 hydrogenation.

Figure 7 Scattered Cu nanowires after 37 h reaction
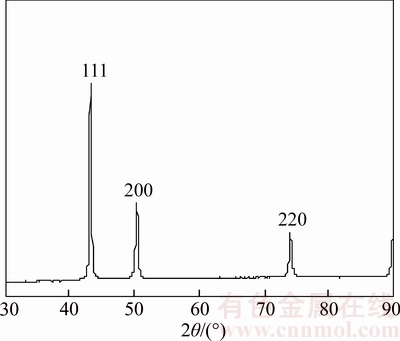
Figure 8 XRD pattern of Cu nanowire catalyst after 37 h reaction
3.7 Separation and analysis of inactive parts of catalyst in intermediate stage of reaction
The catalyst was taken out and was dispersed in alkaline solution again, and it was found that some particles of the sample were easy to precipitate. The precipitate weighs about 0.3 g, and the conversion of CO2 is only about 0.03% (trace amount of CO was detected, and no methanol was detected), indicating that this part of the sample is deactivated. This result is consistent with the obvious reduction of CO2 conversion rate in Section II of Figure 6, which further confirms the above speculation that some of the catalysts are inactive. Figure 9 shows that Cu grains on surface of the deactivated catalyst have been sintered, and the average grain size is about 67 nm by XRD (its XRD pattern is not showed in this paper). Sintering may cause gas channel blockage. Gas channel blockage may impede gas flow and CO2 hydrogenation. This leaded to deactivation of some of the catalyst. It is speculated that the deactivation may be attributed to the contact between some of the catalyst and higher temperature reactor wall.
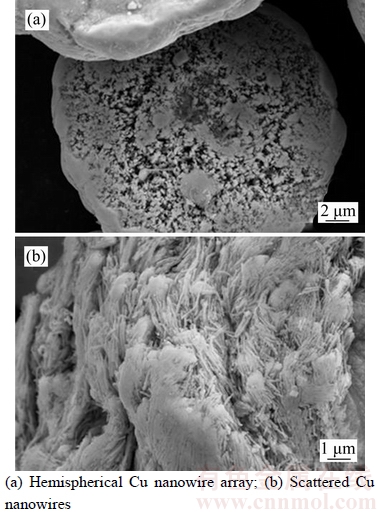
Figure 9 Deactivated part of catalyst after 37 h reaction:
3.8 Separation and performance of active part of catalyst in intermediate stage of reaction
The suspended solid particles in above liquid were processed, and red brown solid powders, which comprise hemispherical Cu nanowire arrays and scattered Cu nanowires, were obtained. 0.5 g of the powders was taken out for performance test. Section I of Figure 10 shows that over the catalyst of 0.5 g, CO2 conversion was about 3.4%, methanol selectivity was about 35.8%, and the STY of methanol was about 20.9 mg/(gcat·h) at 250 °C. Compared with the results in Section I of Figure 6, the changes in methanol STY and methanol selectivity were very little, indicating that the catalyst of 0.5 g was not deactivated. In addition, the decrease in CO2 conversion and methanol STY was very slow with reaction time from about 40 h to about 90 h, and the increase in methanol selectivity was also very slow, indicating that the performance of the catalyst is stable at 250 °C.

Figure 10 CO2 conversion and product selectivity over Cu nanowire catalyst of 0.5 g at 250 °C:
3.9 Performance of catalyst after change in space velocity
Section I of Figure 10 shows that at around 90 h, the conversion of CO2 was about 2.8%, methanol selectivity was about 40.7% and the STY of methanol was about 19.6 mg/(gcat·h). The research of REN et al [1] showed that the STY of methanol over Cu based catalyst increases with increasing gas hourly space velocity (GHSV). The Section II of Figure 10 shows that when gas flow rate doubled, the conversion of CO2 reduced to about 1.5%, methanol selectivity was almost unchanged and still about 40.6%, and the STY of methanol increased to about 20.9 mg/(gcat·h). It is because increasing gas velocity while reducing contact time made CO2 conversion rate decrease, but the amount of gas flowing through catalyst bed layer increased in unit time, which made the STY of methanol of the catalyst increase.
3.10 Stability of Cu nanowire catalyst
Section II of Figure 10 shows that at around 120 h, the conversion of CO2 was about 1.4%, methanol selectivity was about 39.9%, and the STY of methanol was about 19.2 mg/(gcat·h) and decreased by only 11.1% than 21.6 mg/(gcat·h) over the fresh catalyst (see Section I of Figure 6). After a long-time running for up to 120 h, the performance of the Cu nanowire catalyst without oxide support still remained relatively stable in feed gas containing high concentration of CO2, indicating that the Cu nanowires have very high catalytic stability. This helps to develop high performance catalysts.
3.11 Characterization and analysis of deactivated catalyst
Section II of Figure 10 shows that after 120 h reaction, the CO2 conversion, methanol selectivity and methanol STY all quickly decreased, while CO selectivity quickly increased, speculating that the catalyst probably deactivated. Figure 11 shows that after 130 h reaction, the average grain size of sample is about 37 nm (by Jade 6 software), significantly greater than the original size of 26 nm, indicating that sintering for the catalyst may have happened. Literature reported that Cu sintering is the main reason of deactivation of Cu based catalyst [23, 31]. However, according to the shape of performance curve in Section II of Figure 10, it is speculated that the main cause of sudden deactivation of the Cu nanowire catalyst is probably gas channel flow blockage.
Figure 12 shows that the diameter of some scattered Cu nanowires in the deactivated catalyst became fine; however they still retained their nanowire shape that the average diameter is about 70 nm, and a large number of copper agglomerates appeared around these thinner Cu nanowires. Combining these phenomena with the SEM morphology of sample in Figure 7, it is speculated that the Cu atoms on surface of scattered Cu nanowires continuously migrate, which may be completed via species such as CuO [2], trans-COOH [43], CuCO or/and Cu2HCOO [44], finally resulting in the formation of thinner Cu nanowires, while Cu nanoparticles formed during migration of Cu atoms are agglomerated and sintered continuously, which may be accelerated by H2O [36, 45] produced during CO2 hydrogenation via Ostwald ripening, finally resulting in the generation of copper agglomerate. The phenomenon of migration of Cu atoms in common Cu based catalysts [46, 47] is very difficult to be found, and so far there is still lack of relevant reports of migration of Cu atoms. It is speculated that the migration of Cu atoms and sintering of Cu nanoparticles may be avoided by increasing space velocity to remove generated liquid.
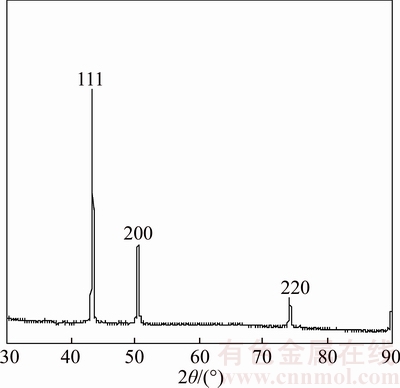
Figure 11 XRD pattern of Cu nanowire catalyst after 130 h reaction
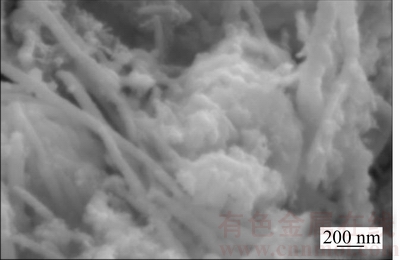
Figure 12 Scattered Cu nanowires after 130 h reaction
In Section I of Figure 10, the phenomena (such as the slow decrease in both CO2 conversion rate and methanol STY and the slow increase in methanol selectivity) may also be attributed to continuous migration of Cu atoms and sintering of Cu grains. Cu grains with smaller size formed during migration of Cu atoms can make catalytic activity, methanol selectivity and methanol STY increase, but Cu grains with larger size formed during sintering can make the catalytic activity, methanol selectivity and methanol STY decrease, and it may be that the cooperation of sintering and migration lead to the above phenomena. It is concluded that the performance of Cu nanowire catalyst can be improved by decreasing Cu grain size.
Figure 13 shows the SEM morphology of hemispherical Cu nanowire array in deactivated catalyst, and the inset shows that some Cu nanowires inside the array have the same average diameter as its original size. The morphology of the Cu nanowires has not obvious changed, which may be attributed to the partial gas channel blockage at the initial stage of CO2 hydrogenation reaction or at the preparation stage of sample. Gas channel blockage may impede gas flow and CO2 hydrogenation, and therefore the morphology of these Cu nanowires has not obvious changed. Figure 14 shows that Cu nanoparticles in inert atmosphere are quickly sintered at 250 °C for only 4 h. These findings indicate that the Cu nanowires have excellent sintering resistance.
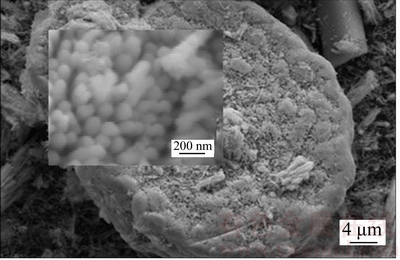
Figure 13 Hemispherical Cu nanowire array after 130 h reaction (Inset shows enlarged view)

Figure 14 SEM morphologies of Cu nanoparticles (a) and their agglomerates after heat treatment at 250 °C for 4 h under N2 (b)
4 Conclusions
1) Cu nanowires have very high sintering resistance and catalytic stability, and they may have potential application value in study on active site or in industrial application.
2) The changes in the grain size, SEM morphology and catalytic performance of sample indicate that migration of Cu atoms on the surface of Cu nanowires can occur during CO2 hydrogenation.
3) Continuous migration of Cu atoms and sintering of Cu grains on surface of Cu nanowires can lead to flow blockage in gas channels. Gas channel flow blockage or sintering of Cu grains can lead to deactivation of the catalyst. However, the shape of catalytic performance curve indicates that the main reason for the deactivation of the catalyst is probably gas channel flow blockage. Further research is needed to improve the performance of Cu nanowire catalyst.
References
[1] REN Hong, XU Cheng-hua, ZHAO Hao-yang, WANG Ya-xue, LIU Jie, LIU Jian-ying. Methanol synthesis from CO2 hydrogenation over Cu/γ-Al2O3 catalysts modified by ZnO, ZrO2 and MgO [J]. Journal of Industrial and Engineering Chemistry, 2015, 28: 261–267.
[2] LI Cong-ming, YUAN Xing-dong, FUJIMOTO K. Development of highly stable catalyst for methanol synthesis from carbon dioxide [J]. Applied Catalysis A: General, 2014, 469: 306–311.
[3] GAO Peng, ZHONG Liang-shu, ZHANG Li-na, WANG Hui, ZHAO Ning, WEI Wei, SUN Yu-han. Yttrium oxide modified Cu/ZnO/Al2O3 catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol [J]. Catalysis Science & Technology, 2015, 5(9): 4365–4377.
[4] LI Zhi-xiong, NA wei, WANG Hua, GAO Wen-gui. Direct syntheses of Cu-Zn-Zr/SBA-15 mesoporous catalysts for CO2 hydrogenation to methanol [J]. Chemical Journal of Chinese Universities, 2014, 35(12): 2616–2623. (in Chinese)
[5] NATESAKHAWAT S, LEKSE J W, BALTRUS J P, OHODNICKI P R Jr, HOWARD B H, DENG Xing-yi, MATRANGA C. Active sites and structure–activity relationships of copper-based catalysts for carbon dioxide hydrogenation to methanol [J]. ACS Catalysis, 2012, 2(8): 1667–1676.
[6] BALTES C, VUKOJEVI S, SCH
S, SCH TH F. Correlations between synthesis, precursor, and catalyst structure and activity of a large set of CuO/ZnO/Al2O3 catalysts for methanol synthesis [J]. Journal of Catalysis, 2008, 258(2): 334–344.
TH F. Correlations between synthesis, precursor, and catalyst structure and activity of a large set of CuO/ZnO/Al2O3 catalysts for methanol synthesis [J]. Journal of Catalysis, 2008, 258(2): 334–344.
[7] SAITO M, MURATA K. Development of high performance Cu/ZnO-based catalysts for methanol synthesis and the water-gas shift reaction [J]. Catalysis Surveys from Asia, 2004, 8(4): 285–294.
[8] TOYIR J, MILOUA R, ELKADRI N E, NAWDALI M, TOUFIK H, MILOUA F, SAITO M. Sustainable process for the production of methanol from CO2 and H2 using Cu/ZnO-based multicomponent catalyst [J]. Physics Procedia, 2009, 2(3): 1075–1079.
[9] AHOUARI H, SOUALAH A, VALANT A L, PINARD L, MAGNOUX P, POUILLOUX Y. Methanol synthesis from CO2 hydrogenation over copper based catalysts [J]. Reaction Kinetics Mechanisms and Catalysis, 2013, 110(1): 131–145.
[10] GAO Peng, LI Feng, ZHAO Ning, WANG Hui, WEI Wei, SUN Yu-han. Preparation of Cu/Zn/Al/(Zr)/(Y) catalysts from hydrotalcite-like precursors and their catalytic performance for the hydrogenation of CO2 to methanol [J]. Acta Physico-Chimica Sinica, 2014, 30(6): 1155–1162. (in Chinese)
[11] ARENA F, BARBERA K, ITALIANO G, BONURA G, SPADARO L, FRUSTERI F. Synthesis, characterization and activity pattern of Cu–ZnO/ZrO2 catalysts in the hydrogenation of carbon dioxide to methanol [J]. Journal of Catalysis, 2007, 249: 185–194.
[12] TWIGG M V, SPENCER M S. Deactivation of supported copper metal catalysts for hydrogenation reactions [J]. Applied Catalysis A: General, 2001, 212: 161–174.
[13] KANG S H, BAE J W, PRASAD P S S, OH J H, JUN K W, SONG S L, MIN K S. Influence of Ga addition on the methanol synthesis activity of Cu/ZnO catalyst in the presence and absence of alumina [J]. Journal of Industrial and Engineering Chemistry, 2009, 15(5): 665–669.
[14] WANG Shou-jian. Comprehensive utilization technology of natural gas [M]. Beijing: Chemical Industry Press, 2003: 105–106. (in Chinese)
[15] HUANG Zhong-tao. Industrial catalyst handbook [M]. Beijing: Chemical Industry Press, 2004: 660–663. (in Chinese)
[16] FUJITANI T, NAKAMURA I, UCHIJIMA T, NAKAMURA J. The kinetics and mechanism of methanol synthesis by hydrogenation of CO2 over a Zn-deposited Cu( 111 ) surface [J]. Surface Science, 1997, 383: 285–298.
[17] CHOI Y, FUTAGAMI K, FUJITANI T, NAKAMURA J. The role of ZnO in Cu/ZnO methanol synthesis catalysts— morphology effect or active site model? [J]. Applied Catalysis A: General, 2001, 208(1): 163–167.
[18] BEHRENS M, STUDT F, KASATKIN I, K HL S, H
HL S, H VECKER M, ABILD-PEDERSEN F, ZANDER S, GIRGSDIES F, KURR P, KNIEP B L, TOVAR M, FISCHER R W,
VECKER M, ABILD-PEDERSEN F, ZANDER S, GIRGSDIES F, KURR P, KNIEP B L, TOVAR M, FISCHER R W,  J K, SCHL
J K, SCHL GL R. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts [J]. Science, 2012, 336(6083): 893–897.
GL R. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts [J]. Science, 2012, 336(6083): 893–897.
[19] QIAN Bo-zhang. Hope of CO2 for efficient methanol synthesis [N]. China Chemical Industry News, 2014-08-05(002). (in Chinese)
[20] LI Zhong, FAN Hui, ZHANG Hua-yan, LIU Yan. Influence of microwave irradiation on precursor microstructure and catalytic performance of Cu/ZnO/Al2O3 for slurry methanol synthesis [J]. Chinese Journal of Catalysis, 2010, 31(4): 471–478. (in Chinese)
[21] LI Zhong, LIU Yan, HE Zhong, FAN Hui, ZHENG Hua-yan. Effects of Cu/Zn on the structure and activity of CuO/ZnO/Al2O3 catalysts prepared under microwave irradiation in aging process [J]. Acta Chimica Sinica, 2011, 69(5): 570–576. (in Chinese)
[22] LIN Sheng-da, TANG Hao-dong, LU Zhao-bo, LIU Cai-lai, CEN Ya-qing, LIU Hua-zhang. Influence of precipitation methods on precursors and properties of Cu-based catalyst for methanol synthesis [J]. Chinese Journal of Catalysis, 2010, 31(10): 1257–1262. (in Chinese)
[23] WANG Dong-sheng, TAN Yi-sheng, HAN Yi-zhuo, TSUBAKI N. Effect of CO2 on stability of Cu-based catalyst for dimethyl ether synthesis in slurry phase [J]. Chinese Journal of Catalysis, 2008, 29(1): 63–68. (in Chinese)
[24] ARENA F, ITALIANO G, BARBERA K, BONURA G, SPADARO L, FRUSTERI F. Basic evidences for methanol synthesis catalyst design [J]. Catalysis Today, 2009, 143: 80–85.
[25] LEI Hong, NIE Ren-feng, WU Guo-qiang, HOU Zhao-yin. Hydrogenation of CO2 to CH3OH over Cu/ZnO catalysts with different ZnO morphology [J]. Fuel, 2015, 154: 161–166.
[26] ERTL G, KN ZINGER H, SCH
ZINGER H, SCH TH F, WEITKAMP J. Handbook of heterogeneous catalysis [M]. 2nd ed. Weinheim: Wiley-VCH, 2008: 97–98.
TH F, WEITKAMP J. Handbook of heterogeneous catalysis [M]. 2nd ed. Weinheim: Wiley-VCH, 2008: 97–98.
[27] TOYIR J, SAITO M, YAMAUCHI I, LUO Sheng-cheng, WU Jin-gang, TAKAHARA I, TAKEUCHI M. Development of high performance Raney Cu-based catalysts for methanol synthesis from CO2 and H2 [J]. Catalysis Today, 1998, 45(1): 245–250.
[28] CHU Wei. Catalyst engineering [M]. Chengdu: Sichuan University Press, 2006: 107. (in Chinese)
[29] ZHANG Yun-liang, LI Yu-long. Manufacture and aapplication of industrial catalysts [M]. Beijing: Chemical Industry Press, 2008: 20. (in Chinese)
[30] YAN Xin, SHU Jun-jie, KONG Yu-hua. Novel integrated methanol process and energy saving [M]. Beijing: Chemical Industry Press, 2009: 294. (in Chinese)
[31] NATESAKHAWAT S, OHODNICKI P R Jr, HOWARD B H, LEKSE J W, BALTRUS J P, MATRANGA C. Adsorption and deactivation characteristics of Cu/ZnO-based catalysts for methanol synthesis from carbon dioxide [J]. Topics in Catalysis, 2013, 56(18): 1752–1763.
[32] AN Xin, REN Fei, LI Jin-lu, WANG Jin-fu. A highly active Cu/ZnO/Al2O3 nanofiber catalyst for methanol synthesis through CO2 and CO hydrogenation [J]. Chinese Journal of Catalysis, 2005, 26(9): 729–730.
[33] AN Xin, LI Jin-lu, ZUO Yi-zan, ZHANG Qiang, WANG De-zheng, WANG Jin-fu. A Cu/Zn/Al/Zr fibrous catalyst that is an improved CO2 hydrogenation to methanol catalyst [J]. Catalysis Letters, 2007, 118(3): 264–269.
[34] WANG Guan-nan, CHEN Li-min, GUO Yuan-yuan, FU Ming-li, WU Jun-liang, HUANG Bi-chun, YE Dai-qi. Effect of chromium doping on the catalytic behavior of Cu/ZrO2/CNTs-NH2 for the synthesis of methanol from carbon dioxide hydrogenation [J]. Acta Physico-Chimica Sinica, 2014, 30(5): 923–931. (in Chinese)
[35] RAZALI N A M, LEE K T, BHATIA S, MOHAMED A R. Heterogeneous catalysts for production of chemicals using carbon dioxide as raw material: A review [J]. Renewable and Sustainable Energy Reviews, 2012, (16): 4951–4964.
[36] SAMEI E, TAGHIZADEH M, BAHMANI M. Enhancement of stability and activity of Cu/ZnO/Al2O3 catalysts by colloidal silica and metal oxides additives for methanol synthesis from a CO2-rich feed [J]. Fuel Processing Technology, 2012, 96: 128–133.
[37] FICHTL M B, SCHLERETH D, JACOBSEN N, KASATKIN I, SCHUMANN J, BEHRENS M A, SCHL GL R, HINRICHSEN O. Kinetics of deactivation on Cu/ZnO/Al2O3 methanol synthesis catalysts [J]. Applied Catalysis A: General, 2015, 502: 262–270.
GL R, HINRICHSEN O. Kinetics of deactivation on Cu/ZnO/Al2O3 methanol synthesis catalysts [J]. Applied Catalysis A: General, 2015, 502: 262–270.
[38] QU Jin, ZHOU Xi-wen, XU Feng, GONG Xue-qing, TSANG S C E. Shape effect of Pd-promoted Ga2O3 nanocatalysts for methanol synthesis by CO2 hydrogenation [J]. The Journal of Physical Chemistry C, 2014, 118: 24452–24466.
[39] CHOWDHURY T, CASEY D P, ROHAN J F. Additive influence on Cu nanotube electrodeposition in anodised aluminium oxide templates [J]. Electrochemistry Communications, 2009, 11: 1203–1206.
[40] SONG Mao-ping, HE Zhan-hang. Basic chemistry experiment and technology [M]. Beijing: Chemical Industry Press, 2008: 559. (in Chinese)
[41] KHALIL A, HASHAIKEH R, JOUIAD M. Synthesis and morphology analysis of electrospun copper nanowires [J]. Journal of Materials Science, 2014, 49(8): 3052–3065.
[42] BANSODE A, TIDONA B, von ROHR P R, URAKAWA A. Impact of K and Ba promoters on CO2 hydrogenation over Cu/Al2O3 catalysts at high pressure [J]. Catalysis Science & Technology, 2013, 3: 767–778.
[43] ZHAO Ya-fan, YANG Yong, MIMS C, PEDEN C H F, LI Jun, MEI Dong-hai. Insight into methanol synthesis from CO2 hydrogenation on Cu(111): Complex reaction network and the effects of H2O [J]. Journal of Catalysis, 2011, 281(2): 199–211.
[44] RASMUSSEN D B, JANSSENS T V W, TEMEL B, BLIGAARD T, HINNEMANN B, HELVEG S, SEHESTED J. The energies of formation and mobilities of Cu surface species on Cu and ZnO in methanol and water gas shift atmospheres studied by DFT [J]. Journal of Catalysis, 2012, 293: 205–214.
[45] KONDO S, ISHIKAWA T, ABE I. Adsorption science [M]. 2nd ed. LI Guo-xi, translation. Beijing: Chemical Industry, Press, 2006: 11. (in Chinese)
[46] YU Qing-chun, DENG Yong, WANG Fei, FENG Yue-bin, YANG Bin, XU Bao-qiang, LIU Da-chun. Comparison of desulfurization kinetics of copper oxide sorbent [J]. Journal of Central South University, 2015, 22(08): 2902–2908.
[47] ZHANG Xiao-yan, WANG Ming-hua, WEBLEY P A, XIAO P, WANG Feng-luan, TAO Yu-zhong, ZHAI Yu-chun. Preparation and performance of catalyst for CO2 hydrogenation [J]. Journal of Materials and Metallurgy, 2016, 15(4): 272–276. (in Chinese)
(Edited by YANG Hua)
中文导读
CO2加氢Cu纳米线催化剂的制备、表征和催化性能
摘要:采用电化学沉积方法制备纯Cu纳米线催化剂,并首次将其用于CO2加氢合成甲醇反应,探讨Cu基催化剂的活性位,这有助于催化剂活性位的研究。观察CO2加氢过程中催化剂的性能和结构变化,探讨催化剂的失活机理,找出影响催化剂失活的关键因素,这有利于提高催化剂的应用性能。通过SEM、EDS、XRD和BET等检测手段对Cu纳米线样品进行表征。结果发现,Cu纳米线具有非常高的抗烧结性能和催化稳定性,这有助于研制高性能催化剂。CO2加氢过程中样品的晶粒尺寸、SEM形貌和催化性能的变化情况表明,Cu纳米线表面的Cu原子可能发生迁移。Cu原子不断的迁移和Cu晶粒不断的烧结可能导致气体通道堵塞。气体通道堵塞或Cu晶粒烧结均可能导致催化剂失活。然而,催化性能曲线的形状表明,该催化剂失活的主要原因是气体通道堵塞。
关键词:CO2加氢; 甲醇; Cu纳米线; 迁移; 烧结; 流动阻塞
Foundation item: Project(51074205) supported by the National Natural Science Foundation of China
Received date: 2016-08-31; Accepted date: 2018-01-20
Corresponding author: ZHAI Yu-chun, PhD, Professor; Tel/Fax: +86-24-83687731; E-mail: zhaiyc@smm.ned.edu.cn; ORCID: 0000- 0002-1161-2390; WANG Ming-hua, PhD; E-mail: wangmh@smm.neu.edu.cn; ORCID: 0000-0001-6789-588x

