J. Cent. South Univ. Technol. (2010) 17: 285-288
DOI: 10.1007/s11771-010-0043-1 
Influences of collector DLZ on chalcopyrite and pyrite flotation
GU Guo-hua(顾帼华)1, SUN Xiao-jun(孙小俊)1, 2, LI Jian-hua(李建华)2, HU Yue-hua(胡岳华)1
1. School of Resource Processing and Bio-engineering, Central South University Changsha 410083, China;
2. Daye Nonferrous Metals Company, Huangshi 435005, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2010
Abstract: The interaction mechanism of collector DLZ in the flotation process of chalcopyrite and pyrite was investigated through flotation experiments, zeta potential measurements and infrared spectrum analysis. Flotation test results indicate that DLZ is the selective collector of chalcopyrite. Especially, the recovery of chalcopyrite is higher than 90% in neutral and weak alkaline systems, while the recovery of pyrite is less than 10%. When using CaO as pH regulator, at pH=7-11, the floatability of pyrite is depressed and the recovery is less than 5%. Zeta potential analysis shows that the zeta potential of chalcopyrite decreases more obviously than that of pyrite after interaction with DLZ, confirming that collector DLZ shows selectivity to chalcopyrite and pyrite. And FTIR results reveal that the flotation selectivity of collector DLZ is due to chemical absorption onto chalcopyrite surface and only physical absorption onto pyrite surface.
Key words: chalcopyrite; pyrite; collector; flotation; zeta potential
1 Introduction
Pyrite is the most widespread and abundant of naturally occurring metal sulfides. It commonly presents in copper sulfides. Pyrite lowers the quality of copper concentrates and increases the amount of sulfur compounds produced in copper extraction processes. Therefore, selective separation of Cu/S by flotation is desirable. In the flotation of complex sulfide ores, high pH value is generally used to separate valuable sulfide minerals from pyrite with xanthate collectors [1-5]. Some researchers revealed the surface reaction and reaction mechanism by many methods such as X-ray photoelectron spectrum (XPS), zeta potential, infrared spectrum analysis and chemical analysis about the flotation behavior of pyrite and chalcopyrite [6-9]. It has been shown that mineral oxidation degree, pulp pH value and potential have a great effect on the flotation behavior of pyrite and chalcopyrite. In conventional flotation of Cu/S separation, it is general to collect Cu and depress S [10-11]. This method often brings about difficulties of activating highly depressed S, high collector expenditure, depression of noble metal such as Au and Ag, and environmental pollution [12-14].
Therefore, it is very important to develop an efficient collector with selectivity for chalcopyrite under neutral and weak alkaline conditions. Based on this conception, some efficient collectors with selectivity for chalcopyrite have been developed by many domestic and foreign scholars in recent years [15-16]. Dialkyl thionocarbamates are powerful collectors for the flotation of copper sulfide minerals and more selective than xanthates against pyrite at mild alkaline pH values [17]. New flotation thionocarbamates collectors such as ethoxycarbonyl thionocarbamates were developed by Cytec Industries Inc. It could well separate Cu from S by floatation at pH value of 8.5 [18-19]. Collector DLZ developed by our laboratory is a new efficient selective collector for chalcopyrite under neutral and weak alkaline conditions. The interaction mechanism of DLZ in flotation of chalcopyrite and pyrite was investigated through flotation experiments, zeta potential measurements and FTIR.
2 Experimental
2.1 Materials
Pyrite and chalcopyrite samples used were from Yunfu Mine of Guangdong Province and Tonglushan Copper Mine of Hubei Province, China, respectively. These pure mineral lumps were crushed, handpicked and ground, and the fraction with size of 32-74 μm was obtained by screening as flotation samples of single mineral test. Chemical analysis of the two mineral samples indicated that their purities were 92.89% and 90.45%, respectively.
In flotation test, DLZ was chosen as the collector, pine oil (frother No.2) was employed as the frother, and hydrochloric acid and sodium hydroxide were used as pH value adjusting reagents. Calcium oxide and cupric sulphate anhydrous (CuSO4) were also used in tests. All reagents, except pine oil, were analytical grade products. Flotation water was distilled water.
2.2 Flotation test
Before flotation test, the mineral sample of 2.0 g was put into a beaker (100 mL) and treated to clean the surface for 5 min using supersonic cleaner. Flotation of the minerals was carried out in flotation machine with a cell of total pulp volume of 40 mL. After adjusting pH value to an appropriate value, the mineral pulp was conditioned with CuSO4 (or depressant calcium oxide) and frother in turn, and the conditioning time was 2, 3 and 1 min correspondingly. The flotation time was 3 min.
2.3 Zeta potential measurement
The zeta potentials were measured with Coulter Delsa 440SX apparatus. 50 mg pyrite (or chalcopyrite) mineral sample with corresponding reagent solutions (DLZ dosage 2.6 μmol/L) was used. The mixture suspension was agitated for 10 min under magnetic stirring. The pH values were adjusted with hydrochloric acid and sodium hydroxide (or calcium oxide) and noted in all the measurements. Repeated measurements were done three times, and the results were obtained by average method.
2.4 Infrared spectrum measurement
0.5 g sample was immersed in 25 mL corresponding reagent solutions for 30 min in mortar hand-ground, and then settled for 30 min, filtrated, and flushed 2-3 times using the corresponding pH buffer solution. The solid obtained was vacuum-dried. Infrared spectra were recorded using NEXUS-470 spectrometer.
3 Results and discussion
3.1 Collecting capability of DLZ
The recoveries of chalcopyrite and pyrite as function of pulp pH value in different conditions with DLZ as collector are shown in Fig.1. Dosages of DLZ, CuSO4, and frother were 2.6 μmol/L, 4 mol/L and 22 mg/L, respectively. The pH values were adjusted with hydrochloric acid and sodium hydroxide, or calcium oxide, respectively.
From Fig.1, it can be seen that when pH value is adjusted by HCl or NaOH, chalcopyrite can be well floated in the range of pH 2.7-12.0, and the maximum recovery is 95.7%. But the floatability of pyrite is weak, the maximum recovery of pyrite is 24.1%, and the recovery of pyrite is less than 10% at pH>6.9. When using CaO as pH adjustor, there is no obvious change for the recovery of chalcopyrite at pH=7-11, while the floatability of pyrite decreases and the recovery of pyrite is less than 5%.
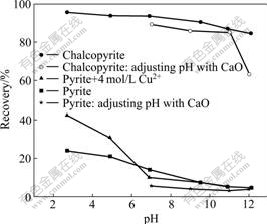
Fig.1 Recoveries of chalcopyrite and pyrite as function of pulp pH value in different conditions with DLZ as collector
The results in Fig.1 also indicate that, in the presence of 4 mol/L Cu2+, the recovery of pyrite increases under acid condition and reaches 42.0% at pH=2.7, but the recovery of pyrite has no change at pH=6.9-12.0 compared with that in the absence of Cu2+. These phenomena are explained that, under acid condition, the adsorption of DLZ on pyrite surface is promoted in the presence of Cu2+; but under alkaline condition, Cu(OH)2 is formed from Cu2+ which can deposit and adhere to pyrite surface and depress the flotation of pyrite.
The results of the dosage tests of DLZ at pH=6.9 are shown in Fig.2. It can be seen that the recovery of chalcopyrite increases from 94.4% to 96.4% with increasing amount of DLZ in a range from 2.6 to 15.6 μmol/L, and the recovery of pyrite increases from 13.8% to 20.4%. The results show that the influence of DLZ dosage on the recovery of chalcopyrite and pyrite is little and DLZ is an efficient selective collector for chalcopyrite.
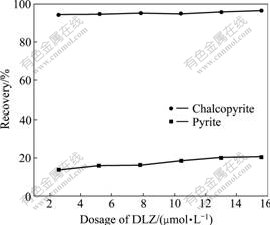
Fig.2 Effect of DLZ dosage on floatability of minerals
The above flotation test results also show that without depressant, the selective flotation separation of Cu/S can be well realized with collector DLZ under neuter and weak alkaline conditions and the low concentration of reagent.
3.2 Mechanism of mineral-DLZ interaction
3.2.1 Zeta potential analysis
Zeta potentials of pyrite and chalcopyrite particles and minerals interaction with DLZ with respect to pH value are presented in Fig.3. It can be seen that the two minerals exhibit acidic isoelectric points of about pH value of 3, and beyond this pH value the electronegative character of the minerals increases with the increase in pH value. After interaction with DLZ for 30 min, the isoelectric point of pyrite shifts to a low pH value, while the isoelectric point of chalcopyrite almost has no change. And zeta potential values of pyrite and chalcopyrite particles decrease in the whole testing range of pH, suggesting that DLZ is a kind of anionic collector. When zeta potential values of mineral are positive, due to the electrostatic force between DLZ and mineral, zeta potential values of mineral transfer negatively. When zeta potentials values of mineral are negative, after interaction with DLZ, zeta potential values of mineral transfer negatively also, suggesting that there is other force between DLZ and mineral. It can also be seen that zeta potential value of chalcopyrite decreases more obviously than that of pyrite. These indicate that there is more DLZ adhesion on chalcopyrite than on pyrite, and confirm that DLZ shows selectivity to chalcopyrite and pyrite.
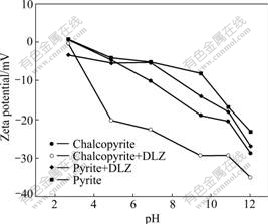
Fig.3 Relationship between zeta potential of minerals and pulp pH value
3.2.2 Infrared spectrum analysis
The infrared spectrum of DLZ is presented in Fig.4. As observed from Fig.4, the peaks identified on DLZ sample show that the —NH stretching vibration peak is located at 3 423.4 cm-1, and 2 962.2 cm-1 comes from N—H asymmetrical stretching vibration. The C=C framework vibrations are corresponding to 1 590.0, 1 497.5 and 1 458.1 cm-1. And peaks in the range of 2 000-1 600 cm-1 come from the characteristic peak of mono-substituted benzenes. Peaks at 1 319.2 and 1 250.8 cm-1 belong to C—N stretching vibration. Peaks at 1 218.5 and 1 174.0 cm-1 belong to inner bending vibration that connects with benzene. Peaks at 1 070.2, 889.1, 753.3, 711.6 and 677.7 cm-1 belong to out bending vibration, which are related to mono-substituted benzenes. Peaks at 1 379.8, 1 143.5 cm-1 come from —(N)—C=S stretching vibration.
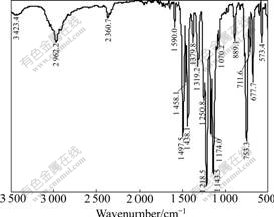
Fig.4 Infrared spectrum of DLZ
The infrared spectra of chalcopyrite and pyrite particles and minerals interaction with reagents are presented in Figs.5 and 6, respectively.
After interacting with DLZ (in Fig.5), the characteristic peaks for pure chalcopyrite become weak and the characteristic peaks (1 594.7, 1 515.8 and 687.2 cm-1) of reagent appear. It can also be seen that the C—N stretching vibration (1 319.2 cm-1) of DLZ shifts to 1 337.7 cm-1, which is 18.5 cm-1 displacement, proving that the chemical absorption occurs when DLZ is absorbed onto chalcopyrite surface. Fig.6 shows the infrared spectra of pyrite treated or no-treated with reagents. It can be seen that the infrared spectrum of pyrite has no obvious change after being treated with DLZ or Cu2+ and DLZ. Therefore, the adsorption of DLZ onto pyrite surface is physical adsorption.
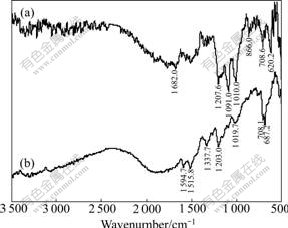
Fig.5 Infrared spectra of chalcopyrite and chalcopyrite interaction with reagent: (a) Chalcopyrite; (b) Chalcopyrite+ DLZ

Fig.6 Infrared spectra of pyrite and pyrite interaction with reagents: (a) Pyrite; (b) Pyrite+Cu2++DLZ; (c) Pyrite+DLZ
4 Conclusions
(1) Flotation experiments show that the collecting ability of DLZ to chalcopyrite is much stronger than that of pyrite at pH=2.7-12.0, the maximum recovery of chalcopyrite is 95.7%. The recovery of pyrite is only about 24%.
(2) Zeta potential experiments show that, after interaction with DLZ, zeta potential values of pyrite and chalcopyrite become more negative. The results indicate that the DLZ belongs to anionic collector adhesive to minerals surface.
(3) Infrared spectrum demonstrates that the adsorption form of DLZ onto chalcopyrite surface is chemical absorption, while it is physical adsorption onto the pyrite surface. This is the reason that causes the flotation selectivity of DLZ between chalcopyrite and pyrite.
References
[1] SUN Wei, LIU Run-qing, CAO Xue-feng. Flotation separation of marmatite from pyrrhotite using DMPS as depressant [J]. Transactions of Nonferrous Metals Society of China, 2006, 16: 671- 675.
[2] OU Le-ming, FENG Qi-ming, SHEN Gang. Reacting mechanism of new collector CSU-A with chalcopyrite [J]. Journal of Central South University of Technology: Science and Technology, 2003, 34(6): 603-605. (in Chinese)
[3] SHEN W Z, FORNASIERO D, RALSTON J. Effect of collectors conditioning pH and gases in the separation of sphalerite from pyrite [J]. Minerals Engineering, 1998, 11: 145-158.
[4] LIU Guang-yi, ZHONG Hong, DAI Tai-gen. The separation of Cu/Fe sulfide minerals at slightly alkaline conditions by using ethoxycarbonyl thionocarbamates as collectors: Theory and practice [J]. Minerals Engineering, 2006, 19: 1380-1384
[5] LOPEZ VALDIVIESO A, CELEDON CERVANTES T, SONG S, ROBLEDO CABRERA A, LASKOWSKI J S. Dextrin as a non-toxic depressant for pyrite in flotation with xanthates as collector [J]. Minerals Engineering, 2004, 17: 1001-1006.
[6] CHANDRA A P, GERSON A R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite [J]. Advances in Colloid and Interface Science, 2009, 145: 97-110.
[7] LEE K, ARCHIBALD D, MCLEAN J, REUTER M A. Flotation of mixed copper oxide and sulphide minerals with xanthate and hydroxamate collectors [J]. Minerals Engineering, 2009, 22: 395-401.
[8] LIU Zhi-lin, XU Fang. The research on the inhibitor of pyrite under the condition with low-pH value [J]. Mining and Metallurgical Engineering, 2005, 25(5): 33-35. (in Chinese)
[9] CASTRO S H, BALTIERRA L. Study of the surface properties of enargite as a function of pH [J]. Int J Miner Process, 2005, 77: 104-115.
[10] LI Chong-de, SUN Chuan-yao. Floatability of chalcopyrite and pyrite by using new collector PAC in Chinese [J]. Nonferrous Metals: Mineral Processing Section, 2002, 6: 33-35.
[11] ZHANG Dong-chen, ZHANG Ming-xu, CHEN Qing-ru. Research on SEM/TEM and XRD of germ oxidizing pyrite surface in coal [J]. Journal of China University of Mining and Technology, 2005, 34(6): 761-765. (in Chinese)
[12] XIONG Dao-ling, CHEN Xiang-qing, JIANG Yu-ren. Effect of calcium containing compounds on the flotation behavior of chalcopyrite and pyrite [J]. Hunan Nonferrous Metals, 2004, 20(6): 8-10. (in Chinese)
[13] LUO Xian-ping, QIU Ting-sheng, FANG Xi-hui. Study on selection and mechanism of effective organic depressants of pyrite in low alkaline medium [J]. Jiangxi Science, 2001, 19(2): 79-83. (in Chinese)
[14] LI Jie, ZHONG Hong, LIU Guang-yi. The research progress of collectors for flotation of copper sulfide ore [J]. Copper Engineering, 2004(4):15-18. ( in Chinese)
[15] SUN Zhi-jian, CHENG Xin-chao. Research on the preferential flotation for chalcopyrite by using new collector BK-330 [J]. Mining and Metallurgy, 2007, 16(2): 5-8. ( in Chinese)
[16] RICHARD R K. Optimizing the industrial flotation performance of sulfide minerals having some natural floatability [J]. Int J Miner Process, 2000, 58: 77-84.
[17] LIU Guang-yi. Research on the comprehensive utilization for copper sulfide ores with new collectors [D]. Changsha: Central South University, 2004. (in Chinese)
[18] NAGARAJ D R, BRINEN J S. SIMS study of adsorption of collectors on pyrite [J]. Int J Miner Process, 2001, 63: 45-57.
[19] LIU Guang-yi, ZHONG Hong, DAI Tai-gen. Investigation of the effect of N-substituents on performance of thionocarbamates as selective collectors for copper sulfides by ab initio calculations [J]. Minerals Engineering, 2008, 21: 1050-1054.
Foundation item: Project(50674102) supported by the National Natural Science Foundation of China
Received date: 2009-05-25; Accepted date: 2009-08-23
Corresponding author: GU Guo-hua, PhD, Professor; Tel: +86-731-88877867; E-mail: guguohua@126.com
(Edited by CHEN Wei-ping)