J. Cent. South Univ. Technol. (2007)04-0500-04
DOI: 10.1007/s11771-007-0097-x 
Effect of quaternary ammonium salts on flotation behavior of aluminosilicate minerals
ZHAO Sheng-gui(赵声贵), ZHONG Hong(钟 宏), LIU Guang-yi(刘广义)
(School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
___________________________________________________________________
Abstract: The electrokinetic properties and flotation of diaspore, kaolinite, pyrophyllite and illite with quaternary ammonium salts collectors were studied. The results of flotation tests show that the collecting ability of quaternary ammonium salts for the four minerals is in the order(from strong to weak) of octadecyl dimethyl benzyl ammonium chloride(ODBA), cetyl trimethyl ammonium bromide(CTAB), dodecyl trimethyl ammonium chloride(DTAC). Under the condition of alkalescence, it is possible to separate the diaspore from the silicate minerals such as kaolinite, illite and pyrophyllite using quaternary ammonium salts as collector. Isoelectric points (IEP) of diaspore, kaolinite, pyrophyllite and illite are pH=6.0, 3.4, 2.3 and 3.2, respectively. Quaternary ammonium salts can change ζ-potential of the aluminosilicate minerals obviously. The flotation mechanisms were explained by ζ-potential and Fourier transform infrared spectrum (FT-IR) measurements. The results demonstrate that only electrostatic interaction takes place between aluminosilicate minerals (diaspore, kaolinite, pyrophyllite and illite) and quaternary ammonium salts.
Key words: aluminosilicate mineral; quaternary ammonium salt; flotation
___________________________________________________________________
1 Introduction
There are abundant bauxite resources in China, and more than 98% of them are diaspore-type that possesses special properties, such as higher grade of aluminum and silicon, but low mass ratio of Al2O3 to SiO2 usually (m(Al)/m(Si)=4-6)[1-3]. Because of the disadvantage, this type of bauxite is difficult to be treated directly by the Bayer process. Therefore, some complex flow sheets, such as sintering process and united process (sintering process combined with the Bayer process), have been developed and applied to produce the alumina from bauxite ore with m(Al)/m(Si)<8 in alumina industry of China[4-5]. Compared with the Bayer process, the sintering and the united processes have many disadvantages, such as high energy consumption and high cost of alumina produced, which result in poor market competing capacities. This is the key problem that restrains the further development of China alumina industry. In order to utilize the bauxite effectively, an economic and rational method should be taken to remove the silica from the bauxite, which can increase the m(Al)/m(Si) and get high quality feed for Bayer process[6].
For this reason, a new flotation desilication-Bayer method was developed to produce alumina[7]. Flotation desilication can be either direct flotation or reverse flotation. Direct flotation was proved to be an efficient method for desilication from diasporic-bauxite using oleic acid as collector of diaspore in the slurry pH of 9-10[8-10]. But in the direct flotation process high collector consumption is necessary to float about 80% of the feed materials, resulting in a high operating cost[11-12]. Considering the speciality of diasporic-bauxite that the content of valuable mineral diaspore is high and the content of associated gangue silicates such as kaolinite pyrophyllite and illite is low[13], reverse flotation desilication has more advantages than the direct flotation in several aspects[14].
Selective depression of diaspore and enhanced collection of silicates are the two fundamental technologies in reverse flotation desilication[15]. Therefore, it is important to find an effective cationic collector to separate diaspore from associated gangue silicates. In the present work, the flotation effect of three quaternary ammonium salts collectors, i.e. dodecyl trimethyl ammonium chloride(DTAC), cetyl trimethyl ammonium bromide(CTAB) and octadecyl dimethyl benzyl ammonium chloride(ODBA) on aluminosilicate minerals (diaspore, kaolinite, pyrophyllite and illite) was investigated. The interactions of quaternary ammonium salts with the four minerals were studied by ζ-potential and Fourier transform infrared spectrum (FT-IR) measurements.
2 Experimental
2.1 Materials
Diaspore, kaolinite, pyrophyllite and illite used were obtained from Xiaoguan (Henan), Pingdingshan (Henan), Qingtian (Fujian) and Ouhai (Zhejiang), respectively. The pure mineral lumps were crushed and ground using a porcelain ball mill and then sieved. The parts with size less than 76 μm were used in flotation tests. The size fraction of less than 5 μm was used in ζ-potential and FT-IR measurements. Quaternary ammonium salts and other reagents used in the study were of analytical grade. Chloride acid and sodium hydroxide were used as pH modifiers. Distilled water was used in all the tests.
2.2 Experimental methods
2.2.1 Flotation tests
Flotation tests were conducted in a 40 mL micro-flotation cel1 of XFG5-35 flotation machine. The impeller speed was fixed at 1 650 r/min. In each test, 3 g mineral samples and suitable amount of distilled water were added into the micro-flotation. Chloride acid and sodium hydroxide adjust pH to the desired values. The mineral pulp was stirred with quaternary ammonium salts collectors for 3 min. After flotation for 6 min, the concentrate and tailing fractions were separately filtered, dried and weighed.
2.2.2 ζ-potential measurement
ζ-potential was measured on a Delsa-440SX zeta potential instrument (Brookhaven Corporation, USA). The mineral sample was ground to be less than 5 μm with an agate mortar. The mineral suspension was prepared to contain 0.01% (mass fraction) solid content in distilled water. The suspension pH value was adjusted to the desired value. The agitated suspension was transferred to a square sample vessel. Then ζ-potential was measured. The measurement error was within 5 mV after at least three measurements.
2.2.3 Infrared spectra
The FT-IR spectra were obtained with AVATAR360 FT-IR (Nicolet Corporation, USA) to characterize the nature of the interaction between the collectors and the minerals. The mineral sample was ground to be less than 5 μm after contacting with the collector.
3 Results and discussion
3.1 Flotation behavior of aluminosilicates using quaternary ammonium salts as collectors
The flotation recoveries of diaspore, kaolinite, pyrophyllite and illite using DTAC as collector are shown in Fig.1. It can be seen that the recoveries of the four aluminosilicate minerals decrease with increasing pulp pH value. But the flotation recovery of diaspore decreases more rapidly at pH>8. The flotation responses of the aluminosilicate minerals as a function of pH using CTAB and ODBA as collectors are shown in Fig.2 and Fig.3, respectively. CTAB and ODBA exhibit a stronger collecting power for pyrophyllite than for kaolinite and illite. Moreover, the pyrophyllite exhibits good floatability in tested pH range. Just as using DTAC as collector, the diaspore has lower flotation recovery at pH>9 using CTAB and ODBA as collectors.

Fig.1 Flotation recovery of minerals as function of pH at c(DTAC)=0.2 mmol/L
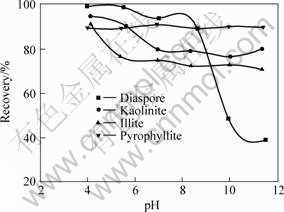
Fig.2 Flotation recovery of minerals as function of pH at c(CTAB)=0.2 mmol/L
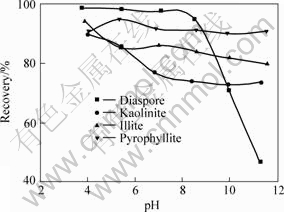
Fig.3 Flotation recovery of minerals as function of pH at c(ODBA) =0.2 mmol/L
It can be seen from Figs.1-3 that the collecting ability of quaternary ammonium salts for diaspore, kaolinite, pyrophyllite and illite is in the order (from strong to weak) of ODBA, CTAB, DTAC. There are high flotation recoveries of kaolinite, pyrophyllite and illite in the range of tested pH, however, the diaspore has poor floatability at pH>9. Therefore, it is possible to separate the diaspore from the silicate minerals, such as kaolinite, illite and pyrophyllite, using quaternary ammonium salts as collector in the pH range of 10-12.
3.2 ζ-potentials of aluminosilicate minerals
ζ-potentials of the aluminosilicate minerals in the absence of collectors were measured, and the results are shown in Fig.4. It can be seen that the negative potentials of the minerals are increased with the increase of pH value. The isoelectric points (IEP) of diaspore, kaolinite, illite and pyrophyllite are about 6.0, 3.4, 3.2 and 2.3, respectively. ζ-potentials of minerals in the presence of CTAB(c(CTAB)=0.2 mmol/L) are shown in Fig.5. Comparing Fig.5 with Fig.4, the positive ζ-potentials of minerals increase intensively in the tested pH range after they contacte with CTAB. The significant change of ζ-potentials suggests a strong electrostatic interaction of the aluminosilicate minerals with quaternary ammonium salts.

Fig.4 ζ-potential of minerals without collector as function of pH value

Fig.5 ζ-potential of minerals interacted with CTAB as function of pH value
3.3 FT-IR spectra
The FT-IR spectra of diaspore, kaolinite, pyrophyllite, illite and the four minerals contacted with CTAB are shown in Fig.6. It can be seen that, in the FT-IR spectra of kaolinite, pyrophyllite and illite that contact with CTAB, the peaks in the range of 2 800-3 000 cm-1 are due to the asymmetric and symmetric stretching vibrations of C—H bond[16]. This indicates the adsorption of CTAB on the three minerals. But there isn’t any shift of the peaks in the FT-IR spectra of the three minerals, so the adsorption of CTAB on kaolinite, pyrophyllite and illite may be mainly of physical interaction. As for the FT-IR spectrum of diaspore, there is a wider peak in the range of 2 800-3 000 cm-1, resulting in that the peaks of C—H bond are covered-up. In addition, other peaks in the FT-IR spectrum of diaspore have not any shift. This phenomenon demonstrates electrostatic interaction, not the chemical interaction, takes place between diaspore and quaternary ammonium salts.
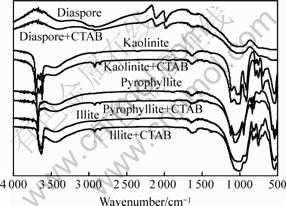
Fig.6 FT-IR spectra of minerals and CTAB
4 Conclusions
1) The collecting ability of quaternary ammonium salts for diaspore, kaolinite, pyrophyllite and illite is in the order (from strong to weak) of ODBA, CTAB, DTAC. There are high flotation recoveries of the four minerals at pH<8 using quaternary ammonium salts as collector. The diaspore has poor floatability at pH>9, while kaolinite, pyrophyllite and illite keep high flotation recoveries. If the effective depressor of the diaspore is found, quaternary ammonium salts will be suitable for bauxite reverse flotation.
2) The isoelectric points (IEP) of diaspore, kaolinite, illite and pyrophyllite are about 6.0, 3.4, 3.2 and 2.3, respectively. Quaternary ammonium salts can change ζ-potential of the aluminosilicate minerals obviously.
3) By ζ-potential and FT-IR measurement, the main action between quaternary ammonium salts and the aluminosilicate minerals is electrostatic interaction.
References
[1] LIU Zhong-fan, DU Ya-jun. Overall analysis of Chinese bauxite resource[J]. Light Metals, 2000(12): 8-13. (in Chinese)
[2] LU Yi-ping, ZHANG Guo-fang, FENG Qi-ming, et al. A novel collector RL for flotation of bauxite[J]. J Cent South Univ Technol, 2002, 9(1): 21-24.
[3] CHEN Jian-hua, ZHAO Qing-jie. Technical progress in last ten years and future development direction of Chinese alumina industry[J]. Light Metals, 2000(10): 3-7. (in Chinese)
[4] CHENG Dai, YAN Din-ou. Production actuality and development of bauxite industry in China[J]. Light Metals, 1997(1): l2-19. (in Chinese)
[5] CHEN Wan-kun, PENG Guan-cai. The Digestion Technology of Diasporic Bauxite[M]. Beijing: Metallurgical Industry Press, 1997. (in Chinese)
[6] HUANG Guo-zhi, FANG Qi-xue, GUI Ji-rang, Rang-jie, et al. The casting silicate means and studying evolvement of bauxite[J]. Light Metal, 1999(5): l6-20. (in Chinese)
[7] FANG Qi-xue, HUANG Guo-zhi. The character of bauxite and the technology of flotation desilicate in China[J]. Metallic Ore Dressing Abroad, 2000(5): 11-16.
[8] FENG Qi-ming, LIU Guang-yi, LU Yi-ping. The 90’s research and outlook of bauxite on impurity removing by mineral processing[J]. Light Metals, 1998(4): 9-13. (in Chinese)
[9] ZHANG Guo-fan. Theory and technology of flotation on bauxite desilicate[D]. Changsha: Central South University, 2001. (in Chinese)
[10] ZHANG Guo-fan, LU Yi-ping, OU Le-ming, et al. A new collector RL for flotation of bauxite[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(4): 712-715. (in Chinese)
[11] HU Yue-hua, XU Zhen-he. Role of crystal structure in flotation separation of dispore from koalinite, pyrophyllite and illite[J]. Minerals Engineering, 2003, 16: 219-227.(in Chinese)
[12] CAO Xue-feng, HU Yue-hua, JIANG Yi-ren. Flotation mechanism of aluminium silicate minerals with N-dodecyl-1, 3-diaminopropane[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(4): 696-700. (in Chinese)
[13] WANG Qiu-xia, ZHANG Ke-ren, CHEN Guo-ming, et al. Countermeasures for bauxite resources’ exploitation and conservation in China[J]. Conservation and Utilization of Mineral Resources, 2001(3): 49-54. (in Chinese)
[14] XU Zhen-he, PLITT V. Recent advances in reverse flotation of diasporic ores—A Chinese experience[J]. Minerals Engineering, 2004, 17: 1007-1015. (in Chinese)
[15] HU Yue-hua, JIANG Hao, QIU Guan-zhou, et al. Solution chemistry of flotation separation of diasporic bauxite[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(1): 125-130. (in Chinese)
[16] XU Shou-chang. Organic Chemistry[M]. Beijing: Higher Education Press, 1993. (in Chinese)
__________________________
Foundation item: Project(2005CB623701) supported by the National Key Fundamental Research and Development Program of China
Received date: 2006-11-12; Accepted date: 2007-02-28
Corresponding author: ZHAO Sheng-gui, Doctoral candidate; Tel: +86-731-8836309; E-mail: csuzsg@sina.com
(Edited by CHEN Wei-ping)