
Preparation and photocatalytic activity of BiOCl catalyst
SHI Zhu-qing1, WANG Yan1, 2, FAN Cai-mei1, WANG Yun-fang1, DING Guang-yue1
1. College of Chemistry and Chemical Engineering, Taiyuan University of Technology, Taiyuan 030024, China;
2. College of Chemistry and Bioengineering, Taiyuan University of Science and Technology, Taiyuan 030021, China
Received 3 November 2010; accepted 13 April 2011
Abstract: The BiOCl powders prepared by the hydrolysis method were investigated with the X-ray diffractometry (XRD), scanning electron microscopy (SEM) and differential thermal analysis (TG-DTA). The results show that the powders are of the tetragonal primitive crystal structure, composed of homogeneous particles of fine ferrite plates, and stable in the temperature range of 40-600 °C. In addition, the photocatalytic activity of BiOCl powders was evaluated by methyl orange (MO) in aqueous solution illuminated by xenon-lamp, and the effect of the BiOCl amount on the photocatalytic activity was investigated. Moreover, the photocatalytic properties of BiOCl and TiO2-P25 were also compared. The results show that the favorite amount of BiOCl powders is 1.0 g/L for the MO degradation,and the photocatalytic activity of the BiOCl catalyst is comparable to the TiO2-P25 catalyst under the same experiment condition.
Key words: BiOCl; photocatalysis; xenon lamp; methyl orange (MO)
1 Introduction
Heterogeneous photocatalysis of photocatalyst as an ideal “green” technology, has been attracting increasing interest because of many applications related with H2 production from water splitting [1], depuration of wastewater or air [2], and decomposition of organic compounds [3-4], especially TiO2 semiconductor photocatalysts. However, the recombination rate of photogenerated electron and hole is high, which limits the practical application of TiO2 photocatalysts. Therefore, noble metal deposition [5-6], ion doping [7-9] and compound semiconductor oxide [10-11] were applied to improving the photocatalytic efficiency of TiO2 photocatalyst, but the efficiency of improvement is very limited. In order to achieve high photocatalytic activity, it is indispensable to have some thoughts on new photocatalyst research and development.
Recently, BiOCl, an important chemical product, has drawn considerable attention due to its remarkable photocatalytic activities for degradation of organic compounds [12-13]. And prior to this, BiOCl was mainly used for pearly pigment [14-15], cosmetic of no toxicity [16], catalysts [17], etc. ZHANG et al [18] revealed that that BiOCl exhibited better performance on photocatalytic degradation of MO than TiO2-P25 after three cycles. Our group also revealed that BiOCl prepared by a hydrolysis method exhibited higher photocatalytic activity to MO degradation whether under UV irradiation or under xenon-lamp irradiation in 30 min. This phenomenon bandgap of BiOCl (Eg=3.3 eV) is wider than that of TiO2 (Eg=3.2 eV) perhaps because BiOCl has stronger oxidative ability due to VB potential of BiOCl more positive than that of TiO2.
In the present work, BiOCl photocatalyst was synthesized by a simple hydrolysis method by employing BiCl3 and hydrochloric acid as raw materials, which required only drying at low temperature instead of roasting, therefore, the process was conveniently operated with lower cost and shorter time than traditional methods. To understand the photocatalytic properties of BiOCl photocatalyst, its photocatalytic activity was evaluated by the degradation of MO solution under xenon-lamp irradiation, and relevant characterization was carried out by employing XRD, SEM, EDS and TG-DTA. In addition, a comparison of BiOCl and TiO2-P25 was presented in the treatment of organic compounds in water.
2 Experimental
2.1 Preparation of BiOCl photocatalyst
The BiOCl photocatalyst was prepared by a simple hydrolysis method. Firstly, in order to obtain a BiCl3-HCl aqueous system, the BiCl3 powders were completely dissolved in hydrochloric acid solution. Then Na2CO3 solution was gradually dropped into the stirring BiCl3-HCl aqueous solution, at the same time, a proper amount of hydrolytic agent was added and the pH of the aqueous solution was adjusted to 6-10. After stirring at room temperature for 30 min, the resulted precipitate was filtrated and washed with deionized water several times. Finally, the white BiOCl powders were obtained by drying the precipitate at 80 °C for 5 h. The reactions are Esq. (1) and (2).
Bi3++Cl-+2H2O → Bi(OH)2Cl + 2H+ (1)
Bi(OH)2Cl → BiOCl ↓ + H2O (2)
2.2 Analytical method
The crystal structure of BiOCl was characterized by X-ray diffractometry (XRD) on a Shimadu X-ray diffractometer at 40 kV and 30 mA with Cu Kα radiation; the TG-DTA analysis was carried out on a HCT thermal analyzer(Beijing, China) when the sample was heated from 40 to 900 °C with a raising ramp rate of 10 °C/min; the scanning electron microscopy (SEM) and energy- dispersive spectroscopic (EDS) measurements were performed on a JSM-6700F field-emission scanning electron microscope; the UV-vis absorption spectra and the concentration of the MO solution were measured by a Varian Cary 50 UV-vis spectrophotometer.
2.3 Photocatalysis
The photocatalytic activity of the BiOCl catalyst was evaluated by the degradation of 10 mg/L MO solution in a cylindrical quartz glass reactor with an effective vessel volume of 50 mL. A 500 W-xenon lamp with a refrigerating water circuit acted as a slide light source. During the reaction, the MO solution was irradiated by xenon lamp and was aerated by air through a mini-type pump to keep BiOCl in fluidized state. At given time intervals, the samples of MO solution were analyzed periodically by a Varian Cary 50 UV-vis spectrophotometer.
3 Results and discussion
3.1 XRD and TG-DTA analyses of BiOCl
In order to investigate the phase structure of as-prepared BiOCl powders, the XRD pattern is shown in Fig. 1. It clearly reveals that the tetragonal primitive crystal structure is coincide according to the standard JCPDS file of BiOCl(No.06—0249, a=3.891 ?, c=7.369 ?). So the synthesized powders are crystallized in BiOCl with a high purity.
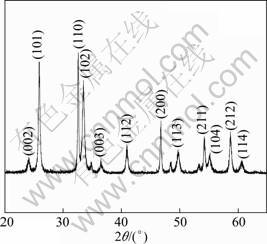
Fig. 1 XRD pattern of BiOCl powders
Figure 2 shows the DTA and TG curves of the BiOCl photocatalyst. The TG curve shows that no significant mass loss was recorded from 40 to 600 °C. The mass of BiOCl sharply decreased from 600 °C with an exothermic peak on the DTA curve, indicating the decomposition of BiOCl. It demonstrates that the BiOCl sample is steady below 600 °C. The DTA curve illustrates one big endothermic peak appearing below 150 °C, indicating that water molecules and hydrochloric acid were evaporated. And remarkable exothermic phenomenon is observed at 600-900°C, which may be caused by substance change.
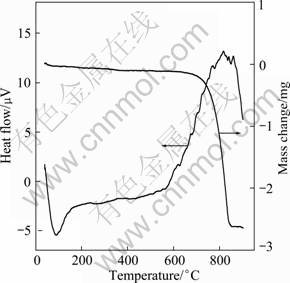
Fig. 2 TG-DTA curves of BiOCl powders
3.2 SEM and EDS analyses of BiOCl
Figure 3 shows the morphology and composition of the BiOCl powders characterized by SEM and EDS. It is clearly observed that the products are composed of homogeneous particles from low-magnification SEM images in Figs. 3(a) and (b). High-magnification SEM micrograph (Fig. 3(c)) further suggests that particles consist of fine ferrite plates with a thickness of about 100 nm, and are beneficial for the adsorption of the MO molecule.
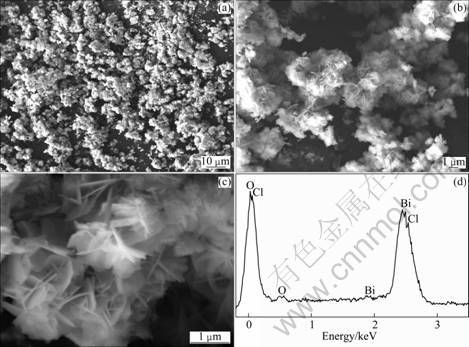
Fig. 3 SEM images (a, b, c) of BiOCl and EDS spectrum (d )
The composition of the BiOCl powders were also studied using EDS, and the molar ratios of Bi, Cl and O atoms from SEM are shown in Fig. 3(d). The analytical results from EDS are in reasonable agreement with the nominal mole ratio of Bi, Cl and O atoms (1:1:1).
3.3 Photocatalytic activity of BiOCl
In order to estimate the photocatalytic activity of BiOCl powders, UV-vis absorbance characteristics of methyl orange (MO) were investigated from 200 to 800 nm during the photocatalytic degradation process and the results are shown in Fig. 4. It can be seen a maximum absorbance peak at 464 nm. Under a simulant sunlight irradiation, the strong absorption peak of MO solution at 464 nm steadily decreased with increasing the light irradiation time, and the orange color of the solution turned gradually to colorless, suggesting that MO solution has been decomposed efficiently. The catalytic result confirms the photocatalytic activity of BiOCl powders.
To select an appropriate amount of catalyst affecting the photocatalytic degradation of MO, a series of tests were executed with different catalyst dosages of 0.4, 0.6, 0.8, 1.0 and 1.2 g/L, respectively. The experimental results are shown in Fig. 5. When the amount of BiOCl catalyst was less than 1.0 g/L, the degradation rate of MO increased with the increase of dosage of BiOCl, and the degradation efficiency of 1.0 g/L is the best for MO degradation. However, for the catalyst dosage of 1.2 g/L, the degradation efficiency rather decreased because smaller BiOCl particles can be sufficiently illuminated to produce photogenerated holes and electrons on the semi-conductor surface, while the amount of immobilized BiOCl is higher, part BiOCl powders aggregated together cannot absorb light effectively. So, 1.0 g/L is the optimum catalyst dosage for the MO photocatalytic reaction.
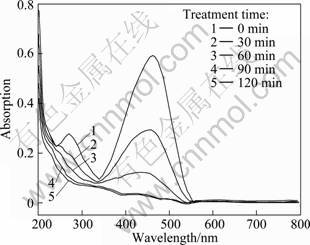
Fig. 4 UV-vis absorption spectra of MO solution after being treated with BiOCl at different time intervals

Fig. 5 MO degradation using different dosages of BiOCl sample
In order to distinguish the photolysis, adsorption and photocatalysis to MO degradation, we designed three experimental processes and the results are shown in Fig. 6. It can be seen that MO can be hardly degraded under the simulant sunlight irradiation without catalysts, indicating that MO is a stable substance and the photolysis can be ignored. The adsorption of BiOCl under dark condition is only nearly 10% after 2.5 h. While the photocatalytic degradation rate to MO under the simulant sunlight irradiation in the presence of BiOCl catalyst can reach 98.5%. Therefore, photocatalytic process is more efficient than adsorption and photocatalysis. At the same time, it also demonstrates that the BiOCl catalyst exhibits almost the similar photocatalytic activity as TiO2-P25 under same experiment condition.
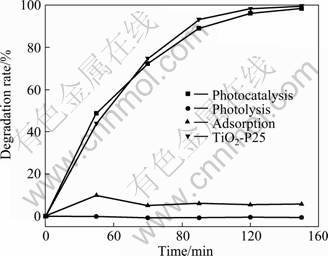
Fig. 6 Comparison of degradation rate of different degradation processes to MO solution
In order to make further comparison, MO was selected as pollutant for photocatalytic activity examination. And we measured photocatalytic activities of BiOCl and TiO2-P25 under UV irradiation (main wavelength of 365 nm) and the simulant sunlight irradiation (xenon lamp), the results are shown in Fig. 7. The BiOCl photocatalyst can acquire a higher activity than TiO2-P25 whether under UV irradiation or xenon lamp irradiation in 30 min, but the difference is very little. In general, it can be concluded that the catalytic activity of the BiOCl catalyst is comparable with the TiO2-P25 catalyst to MO degradation.
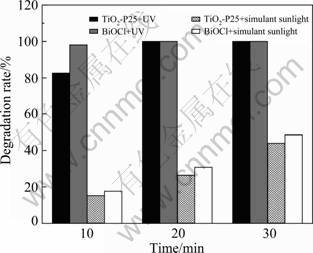
Fig. 7 Comparison of photocatalytic activities of BiOCl and TiO2-P25
4 Conclusions
1) A novel BiOCl photocatalyst was successfully prepared by a hydrolysis method with a high catalytic activity under simulated sunlight irradiation.
2) The as-prepared BiOCl sample has a tetragonal crystal structure and fine ferrite plates with thickness of about 100 nm.
3) The amount of BiOCl photocatalyst plays an important role in determining the photocatalytic activity, and the optimum dosage is 1.0 g/L.
4) The comparison experiments of photoactivity between BiOCl and TiO2-P25 show that the degradation rate of BiOCl is faster than that of TiO2-P25 whether under UV irradiation or xenon lamp irradiation in 30 min, but the difference is not very large, and the degradation rate of MO in aqueous solution all can achieve 98.5% after degradation for 150 min under xenon lamp irradiation.
References
[1] THAMMANOON S, CHOMPOONUCH J, SUMAETH C. Photocatalytic H2 production from water splitting under visible light irradiation using eosin Y-sensitized mesoporous-assembled Pt/TiO2 nanocrystal photocatalyst [J]. Journal of Power Sources, 2009, 190(2): 513-524.
[2] CARP O, HUISMAN C L, RELLER A. Photoinduced reactivity of titanium dioxide [J]. Progress in Solid State Chemistry, 2004, 32(1-2): 33-177.
[3] LI Ai-chang, WANG Li-na, FAN Hong-xian. Silver modification of TiO2/SnO2 thin films and their photocatalytic activity for methyl orange[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(3): 511-515. (in Chinese)
[4] MENG N C, BO J, CHRISTOPHER W K, CHRIS S. Recent developments in photocatalytic water treatment technology: A review [J]. Water Research, 2010, 44(10): 2997-3027.
[5] HOU X G, MA J, LIU A D, LI D J, HUANG M D, DENG X Y. Visible light active TiO2 films prepared by electron beam deposition of noble metals [J]. Nuclear Instruments and Methods in Physics Research Section B, 2010, 268(6): 550-554.
[6] WU Xiang-jiang, JIANG Yao-hui, PENG Zhen-shan, WEI Zong-yuan, DENG Qian, CAI Tie-jun. Preparation of Au/TiO2 catalyst and their performance for photo-catalytic elimination of light alcohols [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(1): 139-147. (in Chinese)
[7] MELTEM A, FUNDA S, ERTUGRUL A. Effect of Fe3+ ion doping to TiO2 on the photocatalytic degradation of Malachite Green dye under UV and vis-irradiation [J]. Journal of Photochemistry and Photobiology A, 2009, 203(1): 64-71.
[8] LI G Q, LIU C Y, LIU Y. Different effects of cerium ions doping on properties of anatase and rutile TiO2 [J]. Applied Surface Science, 2006, 253(5): 2481-2486.
[9] LUKAC J, KLEMENTOVA M, BEZDICKA P, BAKARDJIEVA S, SUBRT J, SZATMARY L, BASTL Z, JIRKOVSKY J. Influence of Zr as TiO2 doping ion on photocatalytic degradation of 4-chlorophenol [J]. Applied Catalysis B, 2007, 74(1-2): 83-91.
[10] ZHU Lei, DUAN Xue-chen, JIANG Bo, LIU Yang-lin, LIU Guo-cong, ZHANG Zhi-jian. Synthesis and characterization of ZnO/TiO2-nanotubes photocatalyst [J]. The Chinese Journal of Nonferrous Metals, 2010, 20(7): 1382-1389. (in Chinese)
[11] BESSEKHOUAD Y, CHAOUI N, TRZPIT M, GHAZZAL N, ROBERT D, WEBER J V. UV-vis versus visible degradation of acid orange II in a coupled CdS/TiO2 semiconductors suspension [J]. Journal of Photochemistry and Photobiology A, 2006, 183(1-2): 218-224.
[12] GHOSH R, MAITI S, CHAKRABORTY A. Facile catalyzed acylation of heteroatoms using BiCl3 generated in situ from the procatalyst BiOCl and acetyl chloride [J]. Tetrahedron Letters, 2004, 45(36): 6775-6778.
[13] WANG W D, HUANG F Q, LIN X P. xBiOI–(1-x)BiOCl as efficient visible-light-driven photocatalysts [J]. Scripta Mater, 2007, 56(8): 669-672.
[14] ZHOU S X, KE Y X, LI J M, LU S M. The first mesostructured bismuth oxychloride synthesized under hydrothermal condition [J]. Materials Letters, 2003, 57(13-14): 2053-2055.
[15] GENG J, HOU W H, LV Y N, ZHU J J, CHEN H Y. One-dimensional BiPO4 nanorods and two-dimensional BiOCl lamellae: Fast low-temperature sonochemical synthesis, characterization, and growth mechanism [J]. Inorganic Chemistry, 2005, 44(23): 8503-8509.
[16] AMPARO S. Analysis of cosmetic products [M]. Amsterdam and London: Elsevier science, 2007: 153-189.
[17] NORIHITO K, KOICHI M, MASAO S, TOMO O, TOMOHIRO K, HIROYUKI Y. Oxidative catalytic cracking of n-butane to lower alkenes over layered BiOCl catalyst [J]. Applied Catalysis A, 2001, 206(2): 237-244.
[18] ZHANG K L, LIU C M, HUANG F Q, ZHENG C, WANG W D. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst [J]. Applied Catalysis B, 2006, 68(3-4): 125-129.
BiOCl催化剂的制备及光催化性能
史竹青1, 王 艳1, 2, 樊彩梅1, 王韵芳1, 丁光月1
1. 太原理工大学 化学化工学院,太原 030024;
2. 太原科技大学 化学与生物工程学院,太原 030021
摘 要:通过水热法制备了一种新型光催化剂BiOCl粉末,并采用X射线衍射(XRD)、扫描电子显微镜(SEM)和热重-差热分析(TG-DTA)对其进行表征。测试结果表明,BiOCl粉末呈四方晶型结构,由均一的片状微粒组成。BiOCl粉末在40~600 °C的温度范围内结构保持稳定。以氙灯照射下的水中甲基橙为目标降解物,对BiOCl粉末的光催化活性进行研究,考察催化剂用量对光催化活性的影响,并将BiOCl与TiO2-P25的光催化性能进行比较。实验结果表明:BiOCl光催化剂的适宜用量为1.0 g/L,在相同的实验条件下BiOCl催化剂的催化活性与TiO2-P25的相当。
关键词:BiOCl;光催化;氙灯;甲基橙
(Edited by YANG Hua)
Foundation item: Project (20876104) supported by the National Natural Science Foundation of China; Project (20090311082) supported by Science and Technology Foundation of Shanxi Province, China
Corresponding author: FAN Cai-mei; Tel: +86-351-6018193; E-mail: fancm@163.com
DOI: 10.1016/S1003-6326(11)61004-2