铝合金高效节能微弧氧化的研究进展
来源期刊:中国有色金属学报(英文版)2017年第7期
论文作者:E. MATYKINA R. ARRABAL M. MOHEDANO B. MINGO J. GONZALEZ A. PARDO M. C. MERINO
文章页码:1439 - 1454
关键词:铝;阳极氧化;微弧氧化;磨损;腐蚀
Key words:aluminium; anodizing; plasma electrolytic oxidation; wear; corrosion
摘 要:为增强合金的摩擦腐蚀性能,减小微弧氧化能量损耗,研究了电压控制模式下各种工业用变形、重力铸造和流变铸造铝合金微弧氧化涂层。结果表明:采用传统多孔阳极膜前驱体,减小了微弧氧化能量损耗至50%;添加α-Al2O3 粒子的微弧氧化涂层后,6082合金的磨损比电解硬铬的低2倍。通过使用前驱体和疏水后处理,提高了包覆微弧氧化涂层A356合金的长期耐蚀性。
Abstract: Plasma electrolytic oxidation (PEO) coatings developed under voltage-controlled mode on various commercial wrought, gravity cast and rheocast aluminium alloys were discussed with respect to enhancement of their tribological and corrosion performance and minimization of the PEO energy consumption. It is demonstrated that use of conventional porous anodic film precursors reduces the PEO energy consumption by up to 50%. The wear of 6082 alloy with PEO coatings with added α-Al2O3 particles is two times lower compared with electrolytic hard chrome. The long-term corrosion resistance of the PEO-coated A356 rheocast alloy is enhanced via use of a precursor and hydrophobic post-treatment.
Trans. Nonferrous Met. Soc. China 27(2017) 1439-1454
E. MATYKINA1, R. ARRABAL1, M. MOHEDANO1, B. MINGO1, J. GONZALEZ2, A. PARDO1, M. C. MERINO1
1. Departamento de Ciencia de Materiales, Facultad de Ciencias  , Universidad Complutense, 28040 Madrid, Spain;
, Universidad Complutense, 28040 Madrid, Spain;
2. Helmholtz-Zentrum Geesthacht, Magnesium Innovation Centre, Institute of Materials Research, Max-Planck-Str. 1, D-21502 Geesthacht, Germany
Received 24 June 2016; accepted 10 October 2016
Abstract: Plasma electrolytic oxidation (PEO) coatings developed under voltage-controlled mode on various commercial wrought, gravity cast and rheocast aluminium alloys were discussed with respect to enhancement of their tribological and corrosion performance and minimization of the PEO energy consumption. It is demonstrated that use of conventional porous anodic film precursors reduces the PEO energy consumption by up to 50%. The wear of 6082 alloy with PEO coatings with added α-Al2O3 particles is two times lower compared with electrolytic hard chrome. The long-term corrosion resistance of the PEO-coated A356 rheocast alloy is enhanced via use of a precursor and hydrophobic post-treatment.
Key words: aluminium; anodizing; plasma electrolytic oxidation; wear; corrosion
1 Introduction
1.1 Fundamentals of PEO process
Plasma electrolytic oxidation (PEO) is variously known as micro-arc oxidation (MAO) [1], spark anodizing [2], anodic oxidation under spark discharge [3] and electrochemical microplasma process [4,5]. This terminology reflects several basic facts of PEO: 1) it is an oxidation process that occurs in aqueous electrolytes on the anode; 2) PEO initiates as conventional anodizing with growth of a barrier oxide film; 3) as the film thickness and hence the voltage increase, the dielectric breakdown of the film and ionization of the oxide material and accompanying gas (i.e., generation of plasma) occur, which are manifested as numerous short-lived discharges. The growth of the oxide film continues inside the discharge channels under the high temperature and pressure, which enable generation of crystalline phases, comprising the oxides of the substrate elements and new insoluble compounds which consist of the substrate and electrolyte species [6]; the latter is formed as a result of the electrolyte thermolysis and plasma chemical reactions occurring at the discharge sites.
PEO processing is most commonly used for aluminium [7], magnesium [8] and titanium alloys [9] but can be extended to any alloy that can be conventionally anodized, for instance, zirconium [10], tantalum [11] and niobium [12,13]. PEO coating can be generated by applying direct, alternating current, unipolar or bipolar pulsed current or voltage (Fig. 1(a)). The introduction of the negative pulse and a pause (“time off”) between the pulses facilitates quenching the microdischarges and prevents their transition into more powerful and destructive arc discharges. In aluminium alloys, the DC mode generates thinner coatings lacking the intermediate dense layer rich in α-Al2O3 [14], which is formed in AC and bipolar modes. The bipolar mode has the advantage of two DC power supplies separately controlling the positive and negative pulses so it is easy to vary the duty cycle and time off period between the pulses. From the industrial point of view, this mode of control permits achievement of higher power and frequencies up to several thousands Hz [15]. The PEO power supplies may differ with respect to the input signal control mode (Fig. 1(b)). There are some researches that use current as an input signal, in which the voltage increases in accordance with the increase of the coating resistance [16]. The other option is to input constant amplitude, peak-to-peak or rms voltage and limit the maximum current [17]. In this case, the current peak with the following current decay will be observed at the output as the impedance of the coating increases.
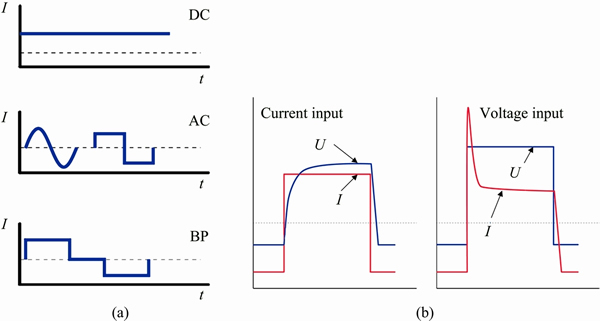
Fig. 1 Modes of PEO processing (a) and input signal control (b)
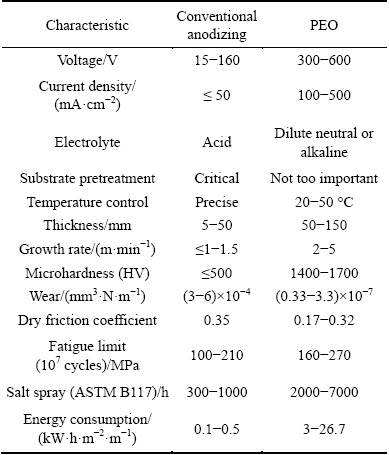
Table 1 summarizes the key characteristics of the coatings on aluminium alloys produced by PEO and conventional hard anodizing [15,18-21]. It is evident that PEO requires higher energy expenditure due to the considerably higher voltages and current densities applied. On the other hand, neutral and alkaline PEO electrolytes are environmentally friendly and easy to recycle compared with concentrated acids used in conventional anodizing.
High temperature phases convey some exceptional properties to the PEO coatings on Al alloys [22,23], the most important properties are high hardness, thermal and wear resistance, associated with the presence of α-Al2O3 in the coating. Hard anodizing, on the other hand, generates amorphous alumina, its high thickness (compared with other conventional anodic alumina films) being the only reason for its relatively high hardness. PEO coating growth rate can be 2-3 times higher than that of hard anodizing. However, with respect to energy expenditure PEO coatings can be 20-30 times more costly than conventional anodizing. Currently PEO is commercially used for niche applications in aerospace, gas and oil industry, tools, heavy machinery and transport applications, and can be particularly attractive as a greener alternative for the applications seeking to substitute heavy materials (e.g., steels) by lightweight ones with equivalent performance and, in case of transport applications, to reduce the mass and fuel consumption and CO2 emission. A further input (design and optimisation of PEO process parameters), is needed; however, in order to increase the energy efficiency of the PEO process and make it economically viable for mass production applications.
Table 1 Comparative characteristics of conventional anodizing and PEO of Al [15,18-22]
In order to understand why PEO needs more energy than conventional anodizing, one must look at the processes consuming energy in both cases. Porous and barrier anodic films can be grown in acid (e.g., chromic, sulphuric, oxalic, phosphoric and their mixtures) and alkaline (e.g., ammonium pentaborate, sodium phosphate) solutions, respectively. The followings are the processes involved in conventional porous film growth in acids.
A barrier film formation:
2Al+3O2-→Al2O3barrier+6e (1)
Dissolution of some of the barrier alumina regions:
Al2O3barrier+6H+→2Al3++3H2O (2)
Oxygen evolution:
2O2-→O2+4e (promoted by impurities) (3)
2H2O→O2+4H++4e (negligible) (4)
The barrier films grow at almost 100% current efficiency, as Eq. (1) is the only target reaction. The growth of porous film proceeds with inevitable loss of oxide material through chemical film dissolution Eq. (2), oxygen evolution Eqs. (3, 4) and field assisted dissolution of the substrate [24]. However, the oxygen evolution does not consume much current, since alumina has very low electron conductivity, the current flows mainly by ion, not electron, transfer, therefore reactions (3) and (4) are not facilitated.
In chromic acid or borax where anions practically do not incorporate into the film, the pores form by thermally enhanced field assisted dissolution, which results in current efficiency reduced by up to 50% (Table 2) [25]. In sulphuric or phosphoric acid, where anions are incorporated into the film, the pores grow by film flow due to electrostriction and mechanical stress [26,27], so only 25%-30% of energy is lost.
Table 2 Current efficiency of conventional anodizing and PEO [24-27]
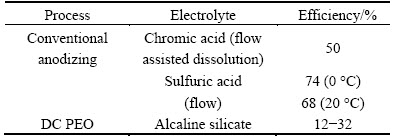
In the PEO process, the current efficiency can drop to 12%, depending on the charge passed and current density applied [28], the average value being ~25%. The losses are associated with dielectric breakdown of the film, which promotes electronic conductivity and hence the reactions (3) and (4). Additionally, much more oxygen is generated during PEO discharge than that corresponds to Faradaic process due to free radical reactions that occur in plasma and involve formation and following oxidation of H2O2 that takes place in the ionized gas [29].
To minimize the energy consumptions, the treatment time must be reduced to minimum and the coating growth rate must be high. Various DC and AC PEO process typically consume 4-30 (kW·h)/(m2·μm), depending on the coating thickness. It has been shown that high frequency bipolar processes are more energy efficient than AC process [15] and can offer the coating growth rate of ~3 m/min and energy consumption of 10-15 (kW·h)/(m2·m). Other strategies include wave form and cell geometry design, electrolyte design and the pre-anodizing approach. The most relevant features of each of them are described as follows.
1.2 Wave form design and cell geometry
Other approaches to minimize the energy consumption include tailoring the waveform, i.e., pulse sequence and duty cycle [30-32], cell [33] and cathode geometry design [34] and use of reactive resistances [35], all of which help to reduce the energy of the pulse and duration of the microdischarge so as to avoid the transition to destructive thermal arcs. For instance, DEHNAVI et al [36] studied the effect of duty cycle on the growth of PEO coatings formed in 6063 aluminium alloy and demonstrated that lower duty cycles (20%) lead to higher breakdown voltages and to a higher density of microdischarges of lower intensity compared with the ones obtained at higher duty cycles (80%) when working at constant frequency. Therefore, this higher density of microdischarges formed at lower duty cycles results in increased growth rates of the coating. Regarding the cathode geometry design, WEI et al [33] evaluated the influence of anode-cathode distance (5 and 25 cm) and the orientation of the cathode with respect to the anode, and states that these factors affect directly the uniformity of the coating thickness and surface properties. This is due to the diminution of the anode current when increasing the distance and when the cathode is not directly orientated towards the anode. Shorter distances and direct orientation result in coatings with improved corrosion and wear resistance. ZHANG et al [37] also studied the influence of the distance between anode and cathode on the efficiency of the process by using a cathode with grid shape, and concluded that shorter electrode distances reduce the voltage drop, reaching 25% of energy saving when working at <5 cm. However, very short distances may result in the induction of an arc between the specimen and cathode which damages both the cathode and the sample surface.
1.3 Electrolyte design
Electrolyte composition also influences the efficiency of the process. In fact, there are some additives (silicates and phosphates) that facilitate the metal passivation accelerating the dielectric breakdown of the oxide and, therefore, increasing the growth rate [38]. Modification of the electrolyte using complexing agents and particles [39] does not change the oxidation efficiency, but promotes formation of other phases in addition to the substrate element oxide, hence the coating efficiency increases. This approach is often employed in PEO of Mg and Ti alloys, especially for biomedical applications [40,41], and yields the coating growth rate of up to 10 m/min, but it is not always practical for Al alloys, as the applications usually require high content of α-Al2O3 to achieve the maximum hardness and wear resistance. There are, however, certain particles which are able to increase both coating efficiency and mechanical properties of PEO coatings on Al alloys. For instance, the incorporation of zirconia nanoparticles into PEO coatings on Al in a phosphate based electrolyte leads to an increase of the coating thickness and hardness [39]. LI et al [42] also found a positive response of the addition of TiO2 nanoparticles into PEO coatings formed in 6063 commercial aluminium alloy in a silicate-based electrolyte, which results in the formation of more compact coatings with improved hardness, adhesion and wear resistance. However, electrolyte design presents some limitations, as organic additives tend to decompose and particles tend to coalesce, so, technologically, such electrolytes could present a maintenance challenge.
1.4 Pre-anodizing approach
Another approach to reduction of energy consumption involves formation of a precursor film, typically by conventional anodizing, prior to subjecting the work piece to PEO treatment (Fig. 2).
The idea of a precursor is based on the understanding of the mechanism of microplasma discharge discussed in Refs. [43,44]. The micro- discharges that occur in the pores of the PEO coatings are thought to be a form of glow discharge, where the electrolyte essentially acts as a cathode and the electrons are injected into the gas from the electrolyte surface. The following particular aspects of PEO microdischarges should be mentioned.
1) The distance between the cathode and anode (the metal) continuously changes due to the increase of the coating thickness.
2) Depletion of electrolyte in charge carriers (mainly H+ and OH- ions, due to water electrolysis and gas evolution) results in increase of dielectric permittivity of the electrolyte up to ~80 (close to that of the water).
3) 90% of the electric field strength is concentrated in the anodic barrier layer.
4) Dielectric breakdown of the barrier layer and sharp increase in the electronic current results in copious O2 evolution, heating of the water, generation of vapour and build-up of the pressure. These processes lead to formation of gas-vapour bubbles above the pores (Fig. 2).
5) Dielectric permittivity of O2 and water vapour is close to 1, therefore the main part (90%) of voltage drop occurs in the gas-vapour bubble. If the field strength is sufficiently high, the breakdown of the gas occurs.
6) The temperature of the gas in the bubble increases, which leads to the expansion of the bubble, and hence the length of the discharge channel. The negative glow and positive column of the discharge are formed consecutively. The positive column increases with thickening of the oxide coating. At this stage, the plasma state of the discharge corresponds to a normal glow, but unlike normal glow with metallic cathode, it admits much higher range of current densities. The electron temperature is reported to be 104 K [45-48], but the gas temperature is much lower (~103 K), which is characteristic of a non-equilibrium plasma.
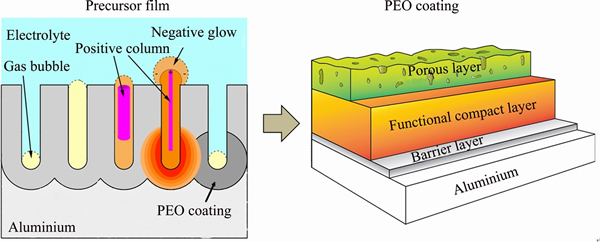
Fig. 2 Development of gas discharge and transformation of conventional anodic porous film precursor into PEO coating
7) Local overheating of the gas and increasing pressure lead to contraction of the positive column; the gas temperature in the positive column increases, the gap between the electron and gas temperature becomes smaller. This may possibly be accompanied by a hollow cathode effect, i.e., the overlapping of the negative glow areas on both sides of the positive column that is protruding above the oxide surface which results in further increase of the gas temperature. At this stage, the observed discharge is commonly called “soft sparking”, which is manifested by the absence of acoustic emission and bright white colour of the discharge. This form of microdischarge promotes fast formation of the functional layer (intermediate compact layer) of the PEO coating. When the gas bubble eventually collapses (at a d ~400 μm), the discharge ceases while others are initiated at new sites.
Typical morphology of the PEO coating comprises 3 layers (Fig. 2), of which the intermediate functional layer in a direct PEO process (i.e., without precursor film) may begin to grow uniformly after more than 1 h, when the “soft sparking” is eventually established everywhere on the surface (Fig. 3(a)). That moment is characterized by a transition from linear to continuous emission in the optical spectra acquired during PEO. Having a pore of right length and diameter (i.e., a porous precursor film) facilitates faster establishment of “soft sparking” (Fig. 3(b)); consequently, the overall PEO treatment time may be considerably reduced.
The objectives of the present work are to review the recent advances in use of precursor approach for PEO of different commercial alloys and to compare some of the resulting tribological and corrosion properties of the coatings.
2 Materials and methods
2.1 Test materials
Wrought AA1050-H18 and AA6082-T6, cast A356-F and rheocast A356-RC aluminium alloys were used as substrates for PEO coatings. Table 3 compiles the nominal composition of the studied alloys which was determined using a PANalytical Axios X-ray fluorescence instrument.
Specimens were gradually ground up to grade P1200 with silicon carbide abrasive paper, degreased in isopropyl alcohol and etched in 20% NaOH during 10 s. AA6082-T6 and A356-RC alloys were subsequently de-smutted in 70% nitric acid solution during 30 s. Finally, they were rinsed in deionized water and dried in warm air. The working area was limited to ~3 cm2 using a stop-off Lacquer 45(MacDermid plc). Conventional anodizing pretreatments to form 20 μm-thick anodic films were performed prior to PEO treatment in 24.5% sulphuric acid at 50 mA/cm2 for 600 s at 20 °C and in 0.4 mol/L phosphoric acid at 25 mA/cm2 for 2000 s at 20 °C. PEO coatings were obtained in a silicate based electrolyte under continuous stirring using a voltage- controlled EAC-S2000 power supply (ETsystems electronic) with a square electrical signal. A KUSB-3116 Keithley data acquisition card (500 kS/s) was used to record voltage-time and current-time dependencies. The electrolyte composition and the PEO electrical parameters are shown in Table 4.
After the treatment specimens were rinsed in deionized water and dried in warm air. The specific energy consumption was calculated by integration of the instantaneous voltage and current waveforms acquired periodically during the treatment by a two-channel oscilloscope TDS2012B (Tektronix). Post-treatment was carried out on A356-RC alloy following PEO. The sealing consisted in the immersion of the PEO-coated specimens during 1440 s in a solution containing 0.1672 g/L octadecylphosphonic acid in ethanol at 25 °C followed by cleaning with isopropyl alcohol and dried in warm air.
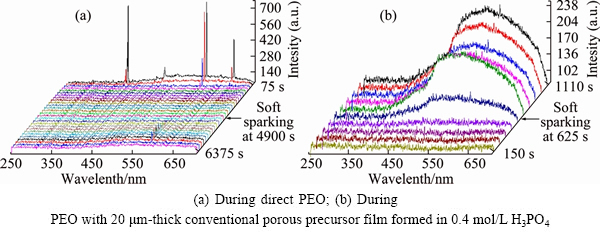
Fig. 3 Evolution of optical emission during development of “soft sparking” on AA1050 alloy
Table 3 Composition of alloys (mass fraction, %)

Table 4 Plasma electrolytic oxidation conditions
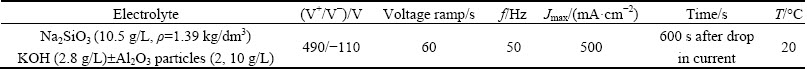
2.2 Specimen characterization
For metallographic characterization, un-coated materials were wet ground through successive grades of silicon carbide abrasive papers from P120 to P1200, followed by diamond finishing (0.1 μm) and etched with different solutions in order to reveal the microstructure (AA1050, Poulton reagent; AA6082 NaOH 20%; A356-F and A356-RC, Weck reagent). Cross-sections of coated specimens were polished to 1 μm diamond finish. Samples were examined using an optical (REICHERT MEF4 A/M) and scanning electron microscope (JEOL JSM-6400) equipped with Oxford Link energy dispersive X-ray (EDS) microanalysis hardware. Coating thickness was determined using an eddy-current meter ISOSCOPEFMP10 (Fischer) equipped with FTA3.3H probe. The presented values are the arithmetic average of 10 measurements done at randomly selected places. A Surtronic25 tester (Taylor Hobson Precision, UK) provided by TalyProfile software was used to measure the surface roughness of the coated specimens in five different locations applying a Gaussian filter of 0.25 mm. The micro-hardness of the coatings was measured on the cross-section specimens using an AKASHIMVK-E3 instrument applying 50 or 100 g load during 20 s. FTA1000 Drop Shape Analysis System (First Ten Angstroms) and accompanying software was used to measure static water contact angles (WCA) according to the sessile drop method. The measurements were carried out using deionized water at 20-25 °C and 30%-35% relative humidity. The angle values presented are the average of five measurements taken at different locations.
2.3 Wear tests
Tribological test were performed in dry conditions using a MT/60/NI ball-on-disc tester (MicroTest) at room temperature (~21 °C and ~35% RH) according to the ASTMG99-05 standard [49] using a WC ball of 6 mm in diameter and HV 1800 hardness as counterpart. The tests were carried out at different sliding distances (100, 200, 400, 600 and 1000 m) and normal loads (2, 5 and 10 N) with a rotational speed of 200 r/min and a wear track radius of 4 mm. Tested samples were examined by SEM with the aim of identifying the wear mechanisms and measuring the dimensions of the wear track. Specimens used in the sliding test were weighed prior and after each measurement in order to calculate the mass and volume loss. The steady-state wear rates were calculated from dividing the wear volume loss by the total sliding distance and load. The presented values are the average of three wear tests.
2.4 Corrosion test
Electrochemical impedance spectroscopy measure-ments were performed in an AUTOLAB- PGSTAT 30 computer-controlled potentiostat provided with a three-electrode cell. The specimen acted as the working electrode (~1 cm2), a graphite electrode was used as counter electrode and a silver/silver chloride (Ag/AgCl) electrode was used as reference electrode with a solution concentration inside the latter of 3 mol/L KCl, providing a potential of 0.210 V with respect to the standard hydrogen electrode. The tests were performed in a naturally aerated 3.5% NaCl solution at room temperature (22 °C) for immersion time from 1 h to 28 d using a sinusoidal perturbation of 10 mV amplitude with respect to OCP and a frequency sweep from 30 kHz to 0.01 Hz was applied. Tests were performed by triplicate in order to ensure repeatability. Electrochemical analysis software ZViewTM (Scribner Associates Inc.) software was used to analyze the impedance spectra.
3 Results and discussion
Figure 4 demonstrates that commercial Al alloys contain various second phase particles (such as Si in A356-F alloy) and intermetallics (AlFe, β-AlFeSi, π-AlFeSiMg, Mg2Si) with alloying elements, which are added for strength and corrosion resistance. The morphologies of the precursor films formed on some of the studied alloys are given in Fig. 5. As a rule, particles are obstructing and impeding the uniform growth of conventional anodic porous film, compared with pure aluminium or alloys with low content of intermetallics (Figs. 5(a)-(c)). Intermetallic particles in 6082 alloy often cause so called “burning”, or rupture, of the film (Figs. 5(d) and (e)), a well-known phenomenon for phosphoric acid anodizing, which occurs due to locally increased current density at the sites of the defects, inclusions etc. Si particles in A356-F alloy practically do not oxidize (Figs. 5(g)-(i)) and the film growth proceeds around them [50], leaving the particles occluded in the film and causing overgrowth and rupture of the film (Fig. 5(h)). These defects would compromise the functional properties of the film (corrosion resistance, in particular); however, they are not essential in a precursor that would be subsequently subjected to a PEO treatment.
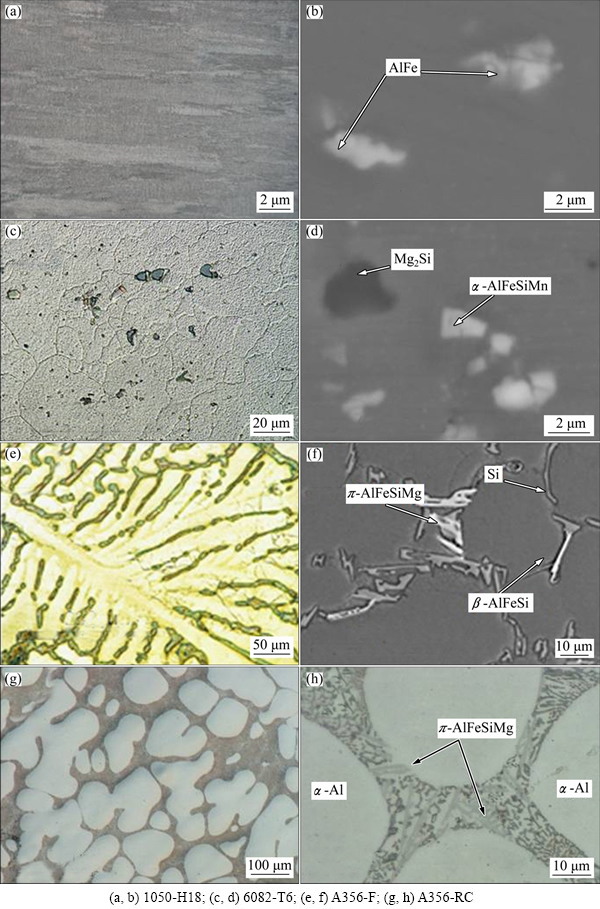
Fig. 4 SEM (b, d, f-h) and optical (a, c, e) micrographs of alloy
Figure 6 shows the transformation of the precursor on 1050 alloy into PEO coating. The PEO treatment in this case was stopped on purpose before the transformation was complete (Fig. 6(a)). It is evident that the precursor-to-PEO conversion initiates at the internal region of the precursor (Fig. 6(b)). This is in agreement with the previously described model (Fig. 2) which stated that initiation and contraction of the positive column of the microdischarge at the bottom part of the pore sustains temperatures high enough to melt alumina.
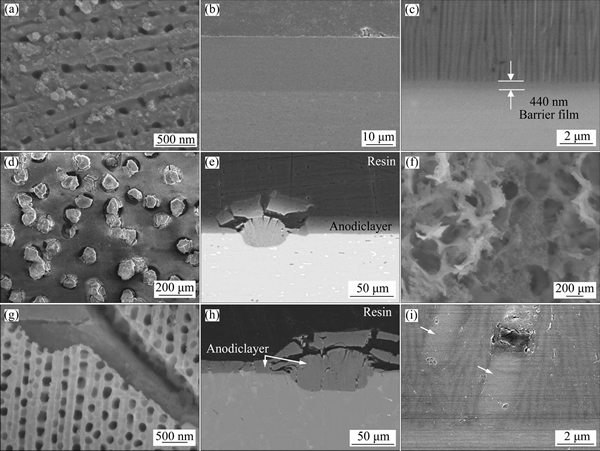
Fig. 5 Plan view (a, d, f, g) and cross-sectional (b, c, e, h, i) SEM micrographs of precursor porous film developed on 1050 alloy (a-c), 6082 alloy (d-f) and A356-F alloy (g-j)
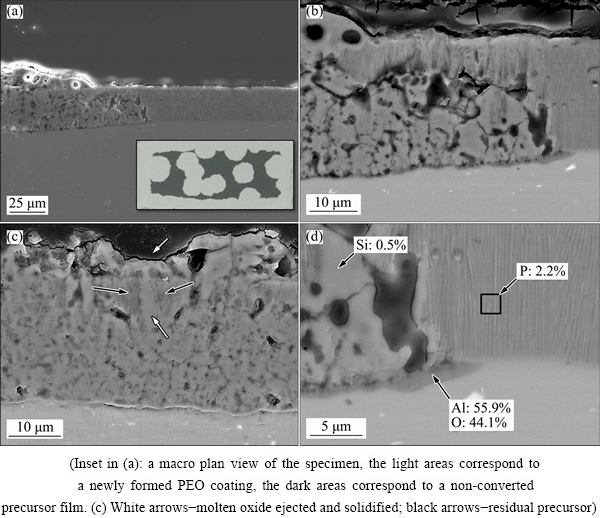
Fig. 6 Transformation of anodic precursor film into PEO film at 250 s of PEO treatment of 1050 alloy
The molten oxide material is often ejected outwards through the discharge channel and spills over the surface before it solidifies (Fig. 6(c)). New substrate material is also oxidized and incorporated into the coating, as is evident from recession of the coating/substrate interface with respect to the original precursor/substrate interface and a new barrier layer composed of pure Al2O3 is formed at the PEO coating/substrate interface (Fig. 6(d)). The inner region of the PEO coating also contains minor amount of Si; phosphorous originated from the precursor film becomes highly dispersed (<0.5% mole fraction) throughout the newly formed coating material.
At the moment the last portion of precursor film is converted into PEO coating, i.e., the soft sparking is uniformly established everywhere, a current drop is observed on a typical current-time curve (Fig. 7). This is related to an increase of the coating impedance, which attributed to an increased thickness and density of the coating over the entire specimen surface. In order to ensure the uniform thickness of the PEO coating everywhere on the surface, all the experiments in this work were stopped for 600 s after the current dropped. Figure 7(a) clearly demonstrates that the treatment time in case of direct PEO (i.e., without using a precursor film) may heavily vary depending on the alloy.
Precursor film, on the other hand, enables the same PEO treatment time for all the alloys in order to achieve equal thickness, which is a technological convenience (Fig. 7(b). Most importantly, the treatment time is 2-3 times shorter than that of direct PEO, which leads to energy savings.
The initial stages of the current-time curve are also strongly affected by the changing impedance of the system. At the beginning of the process, the current rapidly reaches the set limit; as the coating thickens and its impedance increases, the current decreases. The discharges at this stage, as mentioned before, are characterized by linear emission (Fig. 3(a)). Gradually, “soft sparking” is initiated around the edges of the specimen where local current density is higher. When “soft sparking” begins to spread towards the centre of the specimen, the current begins to rise since the area under discharge rapidly increases and the system impedance becomes smaller. Finally, when the area occupied by “soft” microdischarges becomes constant, the impedance change is more affected by the rapidly increasing oxide thickness, therefore a current drop is observed.
The presence of alloying elements influences the current response. The behaviour described above is predominantly observed for pure Al and AA1050 alloy and is less or not at all pronounced in the case of AA6082 and AA356 alloys. The current response at the initial stages of PEO depends mainly on the impedance of the barrier layer, since the rest of the coating is still highly porous, and on the intensity of the electric discharges. For instance, the barrier layer formed on pure Al and the AA1050 alloy is 400-500 nm-thick and highly uniform (Fig. 8(a)), whereas for AA6082 alloy the presence of secondary phases hinder the formation of a barrier layer (Fig. 8(b)), which is much thinner (100-150 nm) and irregular, therefore its impedance is not high enough to produce the current drop.
For all the pre-anodized specimens, the initial current drop is not observed (Fig. 7(b)), which could be associated with the presence of a thicker layer on the surface and fast establishment of the “soft sparking”.
The pore diameter of the precursor appears to affect the specific energy consumption during posterior PEO treatment (Fig. 9). PEO over a precursor formed in phosphoric acid requires about 30% less energy than PEO over sulphuric acid-formed precursor, but it should be born in mind that phosphoric acid anodizing in general requires longer time, higher voltage is more prone to burning than sulphuric acid anodizing.
Figure 10 summarizes the effect of precursor film approach on energy consumption during PEO: depending on the alloy it permits up to 50% improvement of process energy efficiency compared with direct PEO.
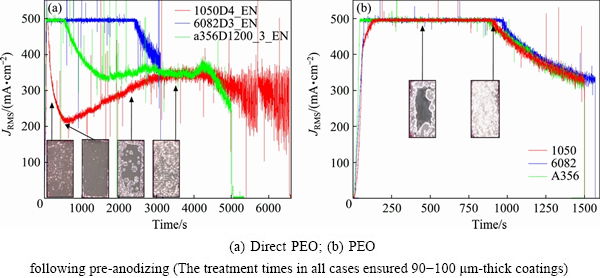
Fig. 7 Current-time curves demonstrating effect of precursor on PEO treatment time for different alloys

Fig. 8 Backscattered electron micrographs of metal/coating interface for 1050 alloy (a) and 6082 alloy (b), depicting thickness of barrier layer of PEO coating

Fig. 9 Current-time curves for PEO of 1050 alloy with precursor films formed in sulfuric and phosphoric acids (Etotal=Eprecursor+EPEO)

Fig. 10 Comparison of specific energy consumption values for direct PEO and PEO with precursors for different alloys
It has been shown elsewhere that PEO coatings with precursor films exhibit higher microhardness than direct PEO coatings [17] due to higher temperatures achieved by quickly established “soft sparking” and the resultant greater conversion of γ-Al2O3 into α-Al2O3. The properties further discussed in this work will only refer to pre-anodized (in phosphoric or sulphuric acid) PEO coatings. Figure 11(a) demonstrates the variation of coating microhardness for different alloys with the distance from the substrate. The outer regions of the coatings (average microhardness HV0.05 ~600) are about 4 times softer than the inner regions (HV0.05 ~1500), because of their higher porosity and composition, which is mainly constituted by SiO2 (Fig. 11(b)), originated from the electrolyte species. The average microhardness of the outer 20 μm of the coating can be increased up to HV0.05 ~800 by introducing α-Al2O3 particles into the electrolyte (Fig. 11(c)); the particles in this case do not undergo any phase transformation and simply act as pore fillers [51]. Comparison of the wear volume of 6082 alloy with and without PEO coatings versus that for electrolytic hard chrome as a function of sliding distance in ball-on-disk wear test (Fig. 12) reveals that the PEO coating increases the wear resistance of the alloy by ~40 times; the wear resistance of the PEO-coated alloy exceeds that of the hard chrome by ~2.5 times and the introduction of α-Al2O3 particles into the coating enables further reduction of the wear volume up to 4 times compared with the electrolytic chrome.
Surface preparation before PEO processing is generally considered as non-critical for PEO processing, meaning that an appropriate cleaning (by degreasing and, if necessary, etching) is sufficient, since PEO coating morphology, unlike that of conventional anodizing, practically does not depend on the surface roughness and composition (e.g., presence of intermetallics). Whereas, it is true that surface preparation for PEO does not need to be elaborated in order to achieve an adequate coating quality, the energy consumption of direct PEO may be affected by the surface roughness resultant from different preparation procedures (Table 5).
The current-time curves obtained for PEO of three different alloys (Fig. 13) reveal that surface roughness values do not significantly affect the process duration (in all cases treatment was terminated at 600 s following the current drop resulting in (95±5) μm-thick coatings).
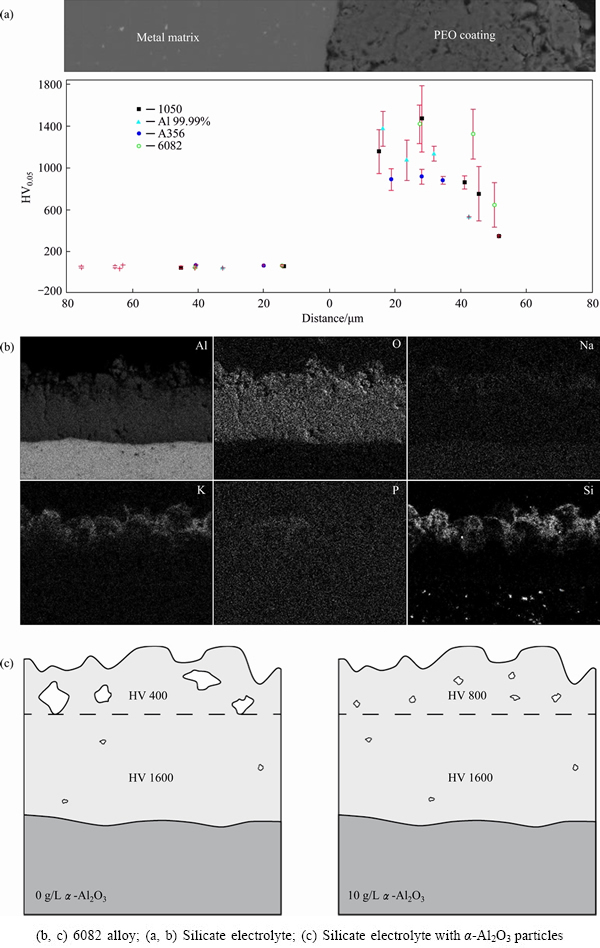
Fig. 11 Microhardness (a, c) and elemental profiles (b) of PEO coatings
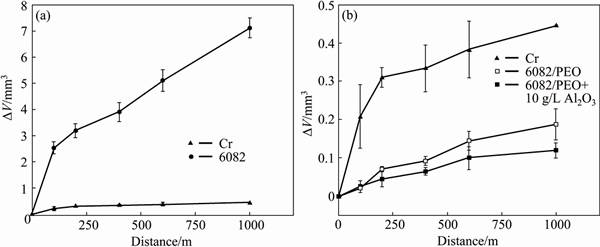
Fig. 12 Wear volume for electrolytic chrome vs 6082 alloy without (a) and with (b) PEO coatings (Normal load 10 N)
Table 5 Roughness following different surface preparations
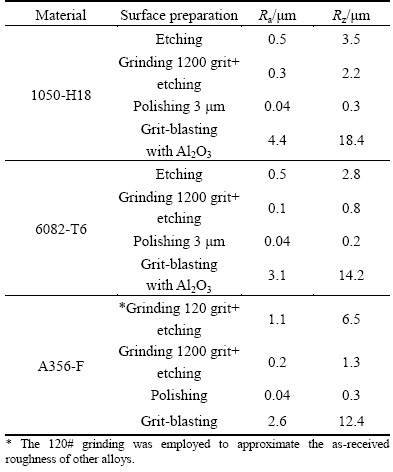
Grit blasting (Ra=2.6-4.4 μm, Rz=12.4-18.4 μm), on the other hand, reduces the direct PEO treatment time required to achieve the same thickness by up to ~50%, depending on the alloy. This is possibly related to the fact that the gas bubbles do not detach easily from rough grit-blasted surface (inset, Fig. 13(b)), i.e., the gas- vapour sheath that sustains the microdischarges is more stable, hence less total treatment time is required. Grit-blasting therefore may be a viable technological option for surface of simple geometry.
Regarding the corrosion resistance of PEO coatings, it has since long been established using electrochemical impedance spectroscopy that the outer and intermediate layers are permeable by the electrolyte and their contribution to the corrosion resistance of the system is much less significant compared with that of the inner barrier layer [19,52,53].
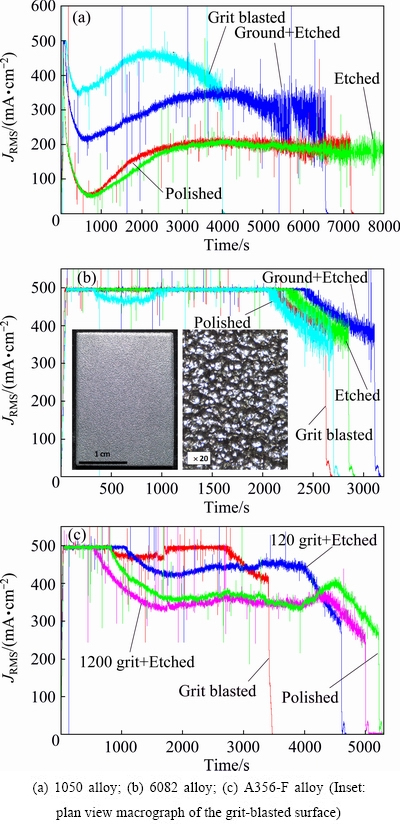
Fig. 13 Effect of surface preparation on direct PEO treatment time
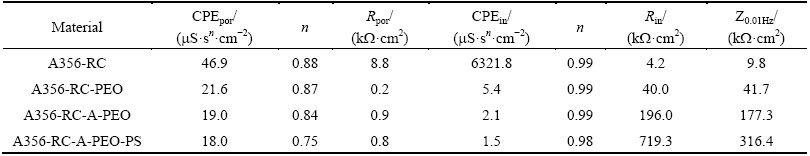
Figure 14 demonstrates the impedance of PEO/ A356-RC alloy systems following 28 d of immersion in 3.5% NaCl solution. Typically, a nested equivalent electrical circuit (inset, Fig. 14(a)) is used to model the EIS response of the inner and outer regions of the PEO coatings and to obtain their respective resistance values; the physical meaning and mathematical expression for the circuit parameters can be found elsewhere [19,52]. The electrical parameters obtained by fitting of the equivalent circuits are given in Table 6. The modelling discloses that the resistances of the outer porous part of the PEO coatings with and without precursor are equally low (Rpor <1 kΩ·cm2). On the other hand, the resistance of the barrier layer of the PEO coating with precursor (Rin ≈200 kΩ·cm2) is about 5 times greater than that of the PEO coating obtained directly (Rin ≈40 kΩ·cm2). The total impedance of the coating formed on A356-RC alloy with a precursor film (A356-RC-A-PEO, Table 6) is higher than that of a direct PEO coating (A356-RC-PEO) and can be further increased by sealing (A356-RC-A- PEO-PS). Octadecylphosphonic acid, employed for sealing, is known to adsorb on the surface forming mono-atomic layers [54,55]; in this instance, it increases the resistance of the inner layer of the PEO coating.

Fig. 14 Nyquist (a) and Bode (b) plots of EIS response of A356 rheocast alloy after 28 d of immersion in 3.5% NaCl, effect of direct PEO, precursor film and phosphonic acid sealing (c)
Sealing increases the resistance of the outer porous part of the pre-anodized PEO coatings (Rout ≈640 kΩ·cm2), which can be attributed to the hydrophobic film (WCA=120°) that phosphonic acid forms on initially hydrophilic (WCA=10°) surface of the PEO coating (Fig. 15).

Fig. 15 Water contact angle for A356-RC alloy with preanodizing and PEO (a) and with preanodizing, PEO and phosphonic acid sealing (b)
4 Conclusions
1) 20 μm-thick porous anodic film precursors enable 40%-50% reduction of energy consumption during AC PEO at 50 Hz.
2) PEO treatment time is not affected by the type of the alloy if precursor is used.
Table 6 Fitting parameters of EIS equivalent circuits obtained for coated and non-coated A356-RC alloy following 28 d of immersion in 3.5% NaCl
3) PEO coatings with precursor exhibit greater hardness than direct PEO coatings. PEO in α-Al2O3 particles-containing electrolyte improves the microhardness of the outer region of the coating.
4) The wear volume of PEO-coated Al 6082 alloy is two times lower than that of electrolytic hard chrome.
5) Precursor improves the resistance of the barrier layer of PEO and results in higher corrosion protection.
6) Sealing of the outer porous part of PEO coatings in phosphonic acid ensures excellent long-term corrosion resistance in 3.5% NaCl.
Acknowledgments
This work has been supported by Regional Government of Madrid and EU Structural Funds via Multimat Challenge Programme (S2013/MIT-2862-CM). M. Mohedano is grateful to Proyecto Retos Jovenes Investigadores Programme (MAT2015-73355-JIN) funded by MINECO, Spain.
References
[1] ZHANG Yi-chen, XU Hui, YANG Ying-fu. Study on the optimization of pulse frequency in the micro arc oxidation of aluminum alloys [C]//Proceedings of Vacuum Metallurgy and Surface Engineering. Beijing: Electronics Industry Press, 2007: 33-40.
[2] HABAZAKI H, ONODERA T, FUSHIMI K, KONNO H, TOYOTAKE K. Spark anodizing of [beta]-Ti alloy for wear-resistant coating [J]. Surface and Coatings Technology, 2007, 201: 8730-8737.
[3] KURZE P, KRYSMANN W. Anodic oxidation under spark discharge (ANOF) [J]. LEW-Nachrichten, 1989, 20: 38-42.
[4] BUTYAGIN P I, KHOKHRYAKOV Y V, MAMAEV A I. Microplasma systems for creating coatings on aluminium alloys [J]. Materials Letters, 2003, 57: 1748-1751.
[5] SONOVA A I, TERLEEVA O P. Morphology, structure, and phase composition of microplasma coatings formed on Al-Cu-Mg alloy [J]. Protection of Metals, 2008, 44: 65-75.
[6] YEROKHIN A L, NIE X, LEYLAND A, MATTHEWS A, DOWEY S J. Plasma electrolysis for surface engineering [J]. Surface and Coatings Technology, 1999, 122: 73-93.
[7] MATYKINA E, ARRABAL R, SKELDON P, THOMPSON G E. Investigation of the growth processes of coatings formed by AC plasma electrolytic oxidation of aluminium [J]. Electrochimica Acta, 2009, 54: 6767-6778.
[8] ARRABAL R, MATYKINA E, HASHIMOTO T, SKELDON P, THOMPSON G E. Characterization of AC PEO coatings on magnesium alloys [J]. Surface and Coatings Technology, 2009, 203: 2207-2220.
[9] MATYKINA E, ARRABAL R, SKELDON P, THOMPSON G E. Investigation of the growth processes of coatings formed by AC plasma electrolytic oxidation of aluminium [J]. Electrochimica Acta, 2009, 54: 6767-6778.
[10] CHENG Ying-liang, MATYKINA E, ARRABAL R, SKELDON P, THOMPSON G E. Plasma electrolytic oxidation and corrosion protection of zircaloy-4 [J]. Surface and Coatings Technology, 2012, 206: 3230-3239.
[11]  Characterization of oxide coatings formed on tantalum by plasma electrolytic oxidation in 12-tungstosilicic acid [J]. Applied Surface Science, 2011, 257: 10590-10594.
Characterization of oxide coatings formed on tantalum by plasma electrolytic oxidation in 12-tungstosilicic acid [J]. Applied Surface Science, 2011, 257: 10590-10594.
[12] SOWA M,  A, SOCHA R P, DERCZ G, MICHALSKA J. Modification of niobium surfaces using plasma electrolytic oxidation in silicate solutions [J]. Journal of Solid State Electrochemistry, 2014, 18: 3129-3142.
A, SOCHA R P, DERCZ G, MICHALSKA J. Modification of niobium surfaces using plasma electrolytic oxidation in silicate solutions [J]. Journal of Solid State Electrochemistry, 2014, 18: 3129-3142.
[13]  R. Anodic luminescence, structural, photoluminescent, and photocatalytic properties of anodic oxide films grown on niobium in phosphoric acid [J]. Applied Surface Science, 2015, 355: 912-920.
R. Anodic luminescence, structural, photoluminescent, and photocatalytic properties of anodic oxide films grown on niobium in phosphoric acid [J]. Applied Surface Science, 2015, 355: 912-920.
[14] MONFORT F, BERKANI A, MATYKINA E, SKELDON P, THOMPSON G E, HABAZAKI H. Development of anodic coatings on aluminium under sparking conditions in silicate electrolyte [J]. Corrosion Science, 2007, 49: 672-693.
[15] YEROKHIN A L, SHATROV A, SAMSONOV V, SHASHKOV P, PILKINGTON A, LEYLAND A. Oxide ceramic coatings on aluminium alloys produced by a pulsed bipolar plasma electrolytic oxidation process [J]. Surface and Coatings Technology, 2005, 199: 150-157.
[16] JASPARD-MECUSON F, CZERWIEC T, HENRION G, BELMONTE T, DUJARDIN L, VIOLA A. Tailored aluminium oxide layers by bipolar current adjustment in the plasma electrolytic oxidation (PEO) process [J]. Surface and Coatings Technology, 2007, 201: 8677-8682.
[17] MATYKINA E, ARRABAL R, PARDO A, MOHEDANO M, MINGO B,  I. Energy-efficient PEO process of aluminium alloys [J]. Materials Letters, 2014, 127: 13-16.
I. Energy-efficient PEO process of aluminium alloys [J]. Materials Letters, 2014, 127: 13-16.
[18] WALSH F C, LOW C T J, WOOD R J K, STEVENS K T, ARCHER J, POETON A R. Plasma electrolytic oxidation (PEO) for production of anodised coatings on lightweight metal (Al, Mg, Ti) alloys [J]. Transactions of the IMF, 2009, 87: 122-135.
[19] BARIK R C, WHARTON J A, WOOD R J K, STOKES K R, JONES R L. Corrosion, erosion and erosion-corrosion performance of plasma electrolytic oxidation (PEO) deposited Al2O3 coatings [J]. Surface and Coatings Technology Plasma Electrolysis, 2005, 199: 158-167.
[20] GODJA N, KISS N,  C, SCHINDEL A, GAVRILOVIC A, WOSIK J. Preparation and characterization of spark-anodized Al-alloys: Physical, chemical and tribological properties [J]. Tribology International, 2010, 43(7): 1253-1261.
C, SCHINDEL A, GAVRILOVIC A, WOSIK J. Preparation and characterization of spark-anodized Al-alloys: Physical, chemical and tribological properties [J]. Tribology International, 2010, 43(7): 1253-1261.
[21] LONYUK B, APACHITEI I, DUSZCZYK J. The effect of oxide coatings on fatigue properties of 7475-T6 aluminium alloy [J]. Surface and Coatings Technology, 2007, 201: 8688-8694.
[22] ZHUANG Jun-jie, SONG Ruo-xi, LU Xiao-ya, SU Xu-ping. Effects of current density on microstructure and properties of plasma electrolytic oxidation ceramic coatings formed on 6063 aluminum alloy [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 806-813.
[23] XIANG Nan, SONG Ren-guo, ZHAO Jian, LI Hai, WANG Chao, WANG Zhi-xiu. Microstructure and mechanical properties of ceramic coatings formed on 6063 aluminium alloy by micro-arc oxidation [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 3323-3328.
[24] ZHOU Fan-yu, MOHAMED AL-ZENATI A K,  A, CURIONI M, GARCIA-VERGARA S J, HABAZAKI H. Volume expansion factor and growth efficiency of anodic alumina formed in sulphuric acid [J]. Journal of the Electrochemical Society, 2011, 158: C202-C14.
A, CURIONI M, GARCIA-VERGARA S J, HABAZAKI H. Volume expansion factor and growth efficiency of anodic alumina formed in sulphuric acid [J]. Journal of the Electrochemical Society, 2011, 158: C202-C14.
[25] GARCIA-VERGARA S J, SKELDON P, THOMPSON G E, HABAZAKI H. A tracer investigation of chromic acid anodizing of aluminium [J]. Surface and Interface Analysis, 2007, 39: 860-864.
[26] GARCIA-VERGARA S J, SKELDON P, THOMPSON G E, HABAZAKI H. A flow model of porous anodic film growth on aluminium [J]. Electrochimica Acta, 2006, 52: 681-687.
[27] GARCIA-VERGARA S J, SKELDON P, THOMPSON G E, HABAZAKI H. Stress generated porosity in anodic alumina formed in sulphuric acid electrolyte [J]. Corrosion Science, 2007, 49: 3772-3782.
[28] SNIZHKO L O, YEROKHIN A L, PILKINGTON A, GUREVINA N L, MISNYANKIN D O, LEYLAND A. Anodic processes in plasma electrolytic oxidation of aluminium in alkaline solutions [J]. Electrochimica Acta, 2004, 49: 2085-2095.
[29] SNIZHKO L O, YEROKHIN A L, GUREVINA N L, PATALAKHA V A, MATTHEWS A. Excessive oxygen evolution during plasma electrolytic oxidation of aluminium [J]. Thin Solid Films, 2007, 516: 460-464.
[30] ZHAO Yu, YANG Wei, YANG Shi-yan. Design of parallel square-wave power pulse current source of microarc oxidation [J]. Transactions of China Electrotechnical Society, 2007, 22: 74-77.
[31] GE Yan-feng, JIANG Bai-ling, SHI Hui-ying. Effect of current pulse width on micro-arc oxidation process for aluminum alloy [J]. Transactions of Materials and Heat Treatment, 2013, 34: 165-169.
[32] YANG Wei, ZHAO Yu-feng, YANG Shi-yan. Effects of characteristic and parameters of power supply on micro-arc oxidation coatings property and energy consumption [J]. Journal of Materials Engineering, 2010: 86-90.
[33] WEI Chun-bei, TIAN Xiu-bo, YANG Shi-qin, WANG Xin, FU Ricky, CHU PAUL K. Anode current effects in plasma electrolytic oxidation [J]. Surface and Coatings Technology, 2007, 201: 5021-5024.
[34] ZHANG Xing-men, TIAN Xiu-bo, YANG Shi-qin, GONG Chun-zhi, FU Ricky, CHU PAUL K. Low energy-consumption plasma electrolytic oxidation based on grid cathode [J]. Review of Scientific Instruments, 2010, 81: 103504.
[35] GORDIENKO P S, KHARCHENKO U V, BULANOVA S B, PANIN E S, USOL'TSEV V K, DOSTOVALOV V A. Physicochemical properties of coatings formed on titanium by microarc oxidation with energy regulation in breakdown zones [J]. Protection of Metals, 2008, 44: 475-478.
[36] DEHNAVI V, LUAN B L, SHOESMITH D W, LIU Xing-yang, ROHANI S. Effect of duty cycle and applied current frequency on plasma electrolytic oxidation (PEO) coating growth behavior [J]. Surface and Coatings Technology, 2013, 226: 100-107.
[37] ZHANG Xing-men, TIAN Xiu-bo, YANG Shi-qin, GONG Chun-zhi, FU Ricky, CHU PAUL K. Low energy-consumption plasma electrolytic oxidation based on grid cathode [J]. Review of Scientific Instruments, 2010, 81(10): 103504.
[38] DEHNAVI V. Surface modification of aluminum alloys by plasma electrolytic oxidation [D]. Canada: The University of Western Ontario, 2014.
[39] MATYKINA E, ARRABAL R, SKELDON P, THOMPSON G E. Optimisation of the plasma electrolytic oxidation process efficiency on aluminium [J]. Surface and Interface Analysis, 2010, 42: 221-226.
[40] MOHEDANO M, MATYKINA E, ARRABAL R, PARDO A, MERINO M C. Metal release from ceramic coatings for dental implants [J]. Dental Materials, 2014, 30: e28-e40.
[41] MATYKINA E, ARRABAL R, MOHEDANO M, MINGO B, PARDO A, MERINO M C. Biodegradable magnesium implants treated by plasma electrolytic oxidation [C]//Proceedings of 14th International Conference on Plasma Surface Engineering. Garmisch-Partenkirchen, Germany, EFDS, 2014.
[42] LI Hong-xia, SONG Ren-guo, JI Zhen-guo. Effects of nano-additive TiO2 on performance of micro-arc oxidation coatings formed on 6063 aluminum alloy [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 406-411.
[43] BAKOVETS V V, POLYAKOV O V, DOLGOVESOVA I P. Plasma electrolytic anode treatment of metals [M]. Novosibirsk: Nauka, 1991.
[44] SUMINOV I V, EPELFELD A V, LYUDIN V B, KRIT B L, BORISOV A M. Microarc oxidation: Theory, technology, equipment [M]. Moscow: “Ekomet”, 2005. (In Russian)
[45] YEROKHIN A L, SNIZHKO L O, GUREVINA N L, LEYLAND A, PILKINGTON A, MATTHEWS A. Discharge characterization in plasma electrolytic oxidation of aluminium [J]. Journal of Physics D: Applied Physics, 2003, 36: 2110.
[46] YEROKHIN A L, SNIZHKO L O, GUREVINA N L, LEYLAND A, PILKINGTON A, MATTHEWS A. Spatial characteristics of discharge phenomena in plasma electrolytic oxidation of aluminium alloy [J]. Surface and Coatings Technology, 2004, 177-178: 779-783.
[47] HUSSEIN R O, NIE X, NORTHWOOD D, YEROKHIN O A, MATTHEWS A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process [J]. Journal of Physics D: Applied Physics, 2010, 43: 105203.
[48] HUSSEIN R O, NIE X, NORTHWOOD D O. Influence of process parameters on electrolytic plasma discharging behaviour and aluminum oxide coating microstructure [J]. Surface and Coatings Technology, 2010, 205: 1659-1667.
[49] ASTM. G99-05. Standard test method for wear testing with a pin-on-disk apparatus [S]. 2004.
[50] FRATILA-APACHITEI L, TICHELAAR F, THOMPSON G, TERRYN H, SKELDON P, DUSZCZYK J. A transmission electron microscopy study of hard anodic oxide layers on AlSi (Cu) alloys [J]. Electrochimica Acta, 2004, 49: 3169-3177.
[51] ARRABAL R, MOHEDANO M, MATYKINA E, PARDO A, MINGO B, MERINO M C. Characterization and wear behaviour of PEO coatings on 6082-T6 aluminium alloy with incorporated α-Al2O3 particles [J]. Surface and Coatings Technology, 2015, 269: 64-73.
[52] MOHEDANO M, MATYKINA E, ARRABAL R, MINGO B, PARDO A. PEO of pre-anodized Al-Si alloys: Corrosion properties and influence of sealings [J]. Applied Surface Science, 2015, 346: 57-67.
[53] VENUGOPAL A, PANDA R, MANWATKAR S, SREEKUMAR K, KRISHNA L R, SUNDARARAJAN G. Effect of micro arc oxidation treatment on localized corrosion behaviour of AA7075 aluminum alloy in 3.5% NaCl solution [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 700-710.
[54] ISHIZAKI T, TESHIMA K, MASUDA Y, SAKAMOTO M. Liquid phase formation of alkyl- and perfluoro-phosphonic acid derived monolayers on magnesium alloy AZ31 and their chemical properties [J]. Journal of Colloid and Interface Science, 2011, 360: 280-288.
[55] SATO H, FUJII T, TSUJI E, AOKI Y, SHIMIZU K, SKELDON P. Observation of self-assembled layers of alkyl phosphonic acid on aluminum using low-voltage scanning electron microscopy and AFM [J]. Surface and Interface Analysis, 2013, 45: 1441-1445.
E. MATYKINA1, R. ARRABAL1, M. MOHEDANO1, B. MINGO1, J. GONZALEZ2, A. PARDO1, M. C. MERINO1
1. Departamento de Ciencia de Materiales, Facultad de Ciencias  , Universidad Complutense, 28040 Madrid, Spain;
, Universidad Complutense, 28040 Madrid, Spain;
2. Helmholtz-Zentrum Geesthacht, Magnesium Innovation Centre, Institute of Materials Research, Max-Planck-Str. 1, D-21502 Geesthacht, Germany
摘 要:为增强合金的摩擦腐蚀性能,减小微弧氧化能量损耗,研究了电压控制模式下各种工业用变形、重力铸造和流变铸造铝合金微弧氧化涂层。结果表明:采用传统多孔阳极膜前驱体,减小了微弧氧化能量损耗至50%;添加α-Al2O3粒子的微弧氧化涂层后,6082合金的磨损比电解硬铬的低2倍。通过使用前驱体和疏水后处理,提高了包覆微弧氧化涂层A356合金的长期耐蚀性。
关键词:铝;阳极氧化;微弧氧化;磨损;腐蚀
(Edited by Xiang-qun LI)
Corresponding author: E. MATYKINA; E-mail: ematykin@ucm.es
DOI: 10.1016/S1003-6326(17)60166-3

