Microwave digestion and alkali fusion assisted hydrothermal synthesis of zeolite from coal fly ash for enhanced adsorption of Cd(II) in aqueous solution
来源期刊:中南大学学报(英文版)2018年第1期
论文作者:张覃 李显波 叶军建 刘志红 邱跃琴 李龙江 卯松 王贤晨
文章页码:9 - 20
Key words:microwave; coal fly ash; synthetic zeolite; adsorption; cadmium
Abstract: A novel microwave digestion and alkali fusion assisted hydrothermal method was proposed to synthesize zeolite from coal fly ash and the zeolite product was studied for removal of Cd(II) from aqueous solution through batch experiments. The adsorbent was characterized by X-ray diffraction, scanning electron microscopy, Fourier transform infrared spectroscopy, surface area analyzer and zeta potential measurement. The results show that the synthetic zeolite was identified as faujasite. The optimum conditions for removal of Cd(II) are found to be: adsorbent dose of 0.5 g/L, pH 6, contact time of 90 min and initial concentration of 20 mg/L, the removal rate of Cd(II) is 98.55%. The experimental kinetic data agree well with the pseudo second-order equation; the Langmuir isotherm model is found to be more suitable to explicate the experimental equilibrium isotherm results than Freundlich, Dubinin-Radushkevich and Temkin models, and the maximum adsorption capacity of Cd(II) is found to be 86.96 mg/g. The thermodynamic parameters such as ΔGΘ, ΔHΘ and ΔSΘ were evaluated and the results show that the adsorption of Cd(II) onto the as-synthesized zeolite is spontaneous, endothermic and feasible under studied conditions.
Cite this article as: LI Xian-bo, YE Jun-jian, LIU Zhi-hong, QIU Yue-qin, LI Long-jiang, MAO Song, WANG Xian-chen, ZHANG Qin. Microwave digestion and alkali fusion assisted hydrothermal synthesis of zeolite from coal fly ash for enhanced adsorption of Cd(II) in aqueous solution [J]. Journal of Central South University, 2018, 25(1): 9–20. DOI: https://doi.org/10.1007/s11771-018-3712-0.

J. Cent. South Univ. (2018) 25: 9-20
DOI: https://doi.org/10.1007/s11771-018-3712-0
Cd(II) in aqueous solution
LI Xian-bo(李显波)1, 2, 3, YE Jun-jian(叶军建)2, 3, 4, LIU Zhi-hong(刘志红)2, 3, 4, QIU Yue-qin(邱跃琴)2, 3, 4,
LI Long-jiang(李龙江)2, 3, 4, MAO Song(卯松)2, 3, 4, WANG Xian-chen(王贤晨)2, 3, 4, ZHANG Qin(张覃)2, 3, 4
1. College of Materials and Metallurgy, Guizhou University, Guiyang 550025, China;
2. Mining College, Guizhou University, Guiyang 550025, China;
3. National amp;Local Joint Laboratory of Engineering for Effective Utilization of Regional Mineral Resources from Karst Areas, Guiyang 550025, China;
4. Guizhou Key Lab of Comprehensive Utilization of Non-metallic Mineral Resources,Guiyang 550025, China
 Central South University Press and Springer-Verlag GmbH Germany,part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany,part of Springer Nature 2018
Abstract: A novel microwave digestion and alkali fusion assisted hydrothermal method was proposed to synthesize zeolite from coal fly ash and the zeolite product was studied for removal of Cd(II) from aqueous solution through batch experiments. The adsorbent was characterized by X-ray diffraction, scanning electron microscopy, Fourier transform infrared spectroscopy, surface area analyzer and zeta potential measurement. The results show that the synthetic zeolite was identified as faujasite. The optimum conditions for removal of Cd(II) are found to be: adsorbent dose of 0.5 g/L, pH 6, contact time of 90 min and initial concentration of 20 mg/L, the removal rate of Cd(II) is 98.55%. The experimental kinetic data agree well with the pseudo second-order equation; the Langmuir isotherm model is found to be more suitable to explicate the experimental equilibrium isotherm results than Freundlich, Dubinin-Radushkevich and Temkin models, and the maximum adsorption capacity of Cd(II) is found to be 86.96 mg/g. The thermodynamic parameters such as △GΘ, △HΘ and △SΘ were evaluated and the results show that the adsorption of Cd(II) onto the as-synthesized zeolite is spontaneous, endothermic and feasible under studied conditions.
Key words: microwave; coal fly ash; synthetic zeolite; adsorption; cadmium
Cite this article as: LI Xian-bo, YE Jun-jian, LIU Zhi-hong, QIU Yue-qin, LI Long-jiang, MAO Song, WANG Xian-chen, ZHANG Qin. Microwave digestion and alkali fusion assisted hydrothermal synthesis of zeolite from coal fly ash for enhanced adsorption of Cd(II) in aqueous solution [J]. Journal of Central South University, 2018, 25(1): 9–20. DOI: https://doi.org/10.1007/s11771-018-3712-0.
1 Introduction
Nowadays, heavy metal contamination is one of the major issues because of its toxicity, bioaccumulation and persistence in natural environment [1]. Even trace amount of heavy metals are harmful to the human body, especially cadmium (Cd) is well-known as one of the most toxic metals even at low concentrations due to the low excretion rate of body. However, waste waters containing heavy metals are still discharged by many industries such as mineral processing, metal plating, smelting and tanneries [2], so removal of heavy metals from waste waters have been extensively studied. The conventional methods such as chemical precipitation [3], ion-exchange [4], electrochemical treatment [5], membrane filtration [6], reverse osmosis [7] and adsorption are used for removal of heavy metal ions from aqueous solutions. Among these methods, adsorption is considered to be the most promising technique because it is relatively low-cost, highly efficient and easy to implement [8]. Various adsorbents have been used for removal of metal ions such as activated carbon [9], carbon nanotubes [10], magnetite chitosan films [11] and zeolites [12]. Among which, zeolites have been proved to be a promising adsorbent because of its high capacity and stability.
Coal fly ash (CFA) is a by-product generated in large quantities from coal-fired power plants. The stockpiling of CFA occupies a lot of land and causes environmental pollution. Therefore, the effective utilization of CFA has become a global environmental issue [13]. Usually, CFA can be used as cement additive, soil conditioner and backfill material. Furthermore, quartz and mullite are the major crystalline phases in CFA, which can be used as the silicon (Si) and aluminum (Al) sources for synthesis of zeolites. The products are expected to be high-value industrial materials for adsorbent and ion exchanger, etc.
Natural zeolites are restricted by their pore sizes and adsorption capacity [14], whereas CFA- based synthetic zeolites possess a variety of pore structures and are potential and probably economically viable adsorbent for removal of heavy metals from waste water [15]. Some synthetic methods of zeolites from CFA have been reported previously such as conventional hydrothermal method [16–18], alkali fusion assisted hydrothermal method, microwave-assisted hydrothermal method [19–21] and molten salt method. The conventional methods for zeolite preparation employ traditional heating. Although zeolites can be synthesized from CFA by the conventional hydrothermal method in alkaline solution at a low temperature (about 90– 100 °C), the shortcomings are the long preparation period above 48 h and low conversion rate of Si and Al because of the low reactivity of mullite and quartz [18, 22]. HOLLMAN et al [23] adopted hydrothermal synthesis of zeolite Na-P1 by mixing 500 g of CFA in 1.25 L of 2 mol/L NaOH followed by 96 h of heat treatment at 90 °C. Alkali fusion prior to the hydrothermal treatment could shorten synthesis time to about 12 h, and all of the inert mineral phases such as mullite and quartz present in CFA were dissolved after fusion and ageing steps in the alkali solution, thus ultimately increasing the conversion rate of CFA [24]. Unfortunately, the alkali fusion process needs heating at a higher temperature above 800 °C and continuing for 2 h [25], resulting in high energy consumption.
Recently, microwave (MW) has been reported as a novel heating technique to influence chemical reactions [26]. The mechanism of energy transfer due to MW is distinctly different from conventional heating, thus giving it a unique advantage over conventional synthesis. The energy transfer is due to the interaction of dielectric molecules with MW [24]. MW is absorbed directly into dielectric molecules and causes dipole rotation, so it can heat an object up rapidly, selectively and directly [21]. It was reported that the activation time of zeolite synthesis from CFA using MW heating was reduced from 24–48 h to 30 min or less. Besides, compared to conventional heating only, when MW was applied in the course of hydrothermal treatment, zeolitization was promoted obviously in the early- stage irradiation for 15 min [19]. The short-time zeolitization is preferable for efficient industrial production because the long-time reaction consumes more energy. Nonetheless, MW digestion and alkali fusion assisted hydrothermal synthesis of zeolite from CFA has not been reported based on the available literature.
The aim of this study is to synthesize zeolite from CFA in a shorter preparation period using a novel MW digestion and alkali fusion assisted hydrothermal method and to investigate its adsorption characteristics toward Cd(II) from aqueous solution. The effects of various parameters such as pH, adsorbent dose, contact time and initial concentration on removal of Cd(II) were examined. Besides, the adsorption kinetics and adsorption isotherms were also systematically evaluated. Finally, the thermodynamic parameters such as the free energy, enthalpy and entropy were investigated as well to provide insights to the adsorption reactions and mechanisms.
2 Materials and methods
2.1 Materials
The CFA used in this study originated from a thermal power plant located in Guizhou Province, China. The loss on ignition (LOI) is 5.72% and the BET specific surface area is 4.66 m2/g. The results of X-ray diffraction (XRD) analysis (Figure 1(a)) revealed that the crystalline phases of the CFA are primarily quartz, mullite and hematite. The chemical composition of the ash was analyzed by using X-ray fluorescence (XRF, PANalytical Axios mA×4 KW), and the results of which are presented in Table 1.
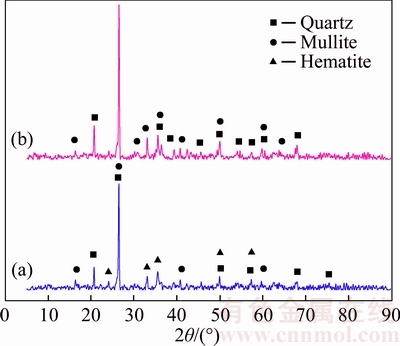
Figure 1 XRD patterns of CFA (a) and pretreated CFA (b)
Table 1 Chemical composition of CFA (mass fraction, %)

Solution of Cd(II) was prepared by dissolving predefined amounts of CdSO4·8/3H2O in deionized water, so as to achieve concentrations of 1000 mg/L in each flask. Different initial concentrations of Cd(II) from 10 to 90 mg/L were prepared by diluting the stock solution. The initial pH of the solutions was adjusted by a pH-meter using 0.1 mol/L HNO3 and 0.1 mol/L NaOH solutions to achieve the desired values. All the chemicals employed in this study were of analytical grade. CdSO4·8/3H2O was obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China), while HNO3 and NaOH were purchased from Chongqing Chuandong Chemical (Group) Co., Ltd. (Chongqing, China).
2.2 Adsorbent synthesis
2.2.1 Pretreated by acid solution
The CFA was ground and sieved into a uniform particle size of 75 μm before the experiments, then underwent an acid (15 wt.% HCl) hydrothermal treatment at 80 °C for 1 h (solid/ liquid ratio=1:5), whose purpose is to remove impurity of hematite in CFA and increasing the amount of Si and Al (Figure 1(b)). After that, CFA pretreated by HCl was filtered and washed with deionized water until its pH value was neutral, and then dried.
2.2.2 Synthesis of zeolite
Zeolite was synthesized by a novel MW alkali fusion of the ash instead of conventional muffle furnace heating. 5 g of pretreated CFA was mixed with 6 g of solid NaOH and then the mixture was ground into a fine powder. The powder was transferred into a crucible and then placed in a microwave box-type high-temp reactor with the temperature set to 450 °C for 15 min. The solids, after MW alkali fusion treatment, were ground into powder and mixed with 70 mL of deionized water, the molar ratio Si/Al was adjusted by adding NaAlO2 in the reaction medium. The slurry was stirred for 4 h with a magnetic stirrer at room temperature to allow extraction of the Si and Al species from the fused ash into solution, and then the suspension was heated to 90 °C for 12 h without stirring. The resulting sample was finally filtered, washed repeatedly with deionized water until its pH value became neutral, and then dried at 105 °C for 4 h. Finally, the zeolite product was ground into powder and stored in a desiccator for further adsorption experiments.
2.3 Characterization methods
Mineralogical composition of the adsorbent was examined by XRD (PANalytical X'pert Powder). The operating conditions were 40 kV and 40 mA. Cu and Kα were used as radioactive sources. The sample was scanned within the 2θ angle range of 5°–90°. Identification of mineral phases was based on the JCPDS-ICDD database. The surface morphology of the adsorbent was observed by using a scanning electron microscopy (SEM, ZEISS) operated at 5 kV.
The specific surface area of the adsorbent was measured using N2 adsorption isotherm at 77 K with a surface area analyzer (ASAP2020M). The total pore volume (Vt) was calculated at a relative pressure of 0.99. The specific surface area (SBET) was calculated by Brunauer-Emmett-Teller method, applying to adsorption branch within the relative pressure p/p0 range of 0.05–0.30. The t-plot method was applied to calculate the micropore area. The average pore diameter (Da) was determined by the Barrette-Joyner-Halenda method.
The surface functional groups were examined with a Fourier transform infrared spectroscopy analyzer (FTIR, Nicolet iS50). The sample was mixed with KBr and determined within the wavenumber range of 4000–400 cm–1.
The pH of point of zero charge (pHpzc) was determined by zeta potential and particle size analyzer (DelsaNanoC). Atomic absorption spectrophotometer (AAS, 240FS AA) was used to determine the Cd(II) concentrations.
2.4 Adsorption experiments
All batch experiments were carried out in a thermostated shaker (TS-100C) at 180 r/min using conical flasks containing 50 mL solution. To optimize the experimental conditions of Cd(II) removal, different parameters such as the effects of initial pH (3–10), adsorbent dose (0.02–3 g/L), contact time (3–180 min) and the initial concentration (10–90 mg/L) on the removal rate of Cd(II) from 50 mL solution have been taken into consideration. Absorption kinetics and absorption isotherms were also evaluated in this study. At the end of each predetermined time interval, the suspension was filtered and the concentration of residual Cd(II) was determined by AAS method. The removal rate (R) of Cd(II) and equilibrium adsorption capacity (qe) were calculated using the following equations:
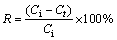 (1)
(1)
 (2)
(2)
where Ci (mg/L) and Ce (mg/L) represent the initial concentration and equilibrium concentration of Cd(II) solution, respectively; Ct (mg/L) is the concentration at time t; V (L) is the solution volume; m (g) is mass of the adsorbent.
3 Results and discussion
3.1 Characterizations of adsorbent
The XRD pattern of the product is shown in Figure 2. The most intense phases were identified as faujasite. The diffraction peaks of quartz and mullite disappeared after MW digestion and alkali fusion assisted hydrothermal synthesis. The crystallinity of the zeolite is about 93%. Besides, the XRD analysis reveals that the product still contained trace amounts of amorphous phases.
The surface morphology of the zeolite product is presented in Figure 3. As shown in Figure 3, a clear shape of octahedron particles can be observed in the image, which shows that the zeolite is faujasite. This observation is consistent with the results shown in Figure 2. However, there are some amorphous aluminosilicate geopolymers on the surface of zeolite grains, which resulted in the low crystallinity of zeolite.
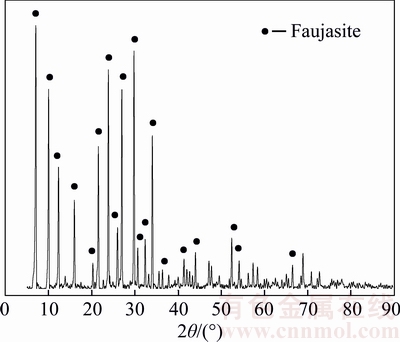
Figure 2 XRD pattern of zeolite product
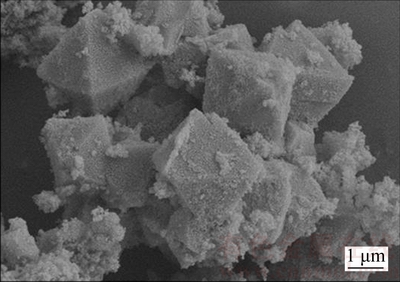
Figure 3 SEM image of zeolite product
Figure 4 presents the FTIR spectrum of the zeolite product, the band located at about 3440 cm–1 and 1637cm–1 are assigned to O—H stretching vibration of adsorbed and coordinated water, respectively [27]. The absorption bands at 1030 cm–1 and 669 cm–1 are assigned to the asymmetric stretching vibration and the symmetric stretching vibration of T—O bonds in TO4 tetrahedra (where T=Si or Al), respectively [20, 28]. A sharp peak is observed at 1030 cm–1 for faujasite which may be formed due to the presence of Al atoms in the tetrahedral forms of silica framework.

Figure 4 FTIR spectrum of zeolite product
The N2 adsorption-desorption isotherm is shown in Figure 5. The porous structure was observed from the isotherm shape according to the International Union of Pure and Applied Chemistry (IUPAC) classification that this isotherm matches well the type IV isotherm with hysteresis loop and is typical of mesoporous materials [29].
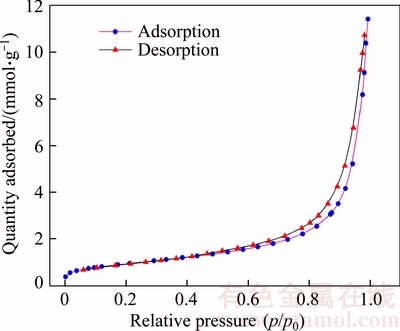
Figure 5 Nitrogen adsorption isotherm at 77 K on zeolite product
The porous structure parameters of the product are listed in Table 2. Compared with the SBET value of CFA, the SBET value of the synthetic zeolite has been increased to 75.72 m2/g from 4.66 m2/g, which is highly beneficial for catalytic or adsorption reaction. The Smicro is only 1.26 m2/g, which shows the low microporosity of the synthetic zeolite. Generally, pores of solid materials are classified into three categories [30]: micropores (d<2 nm), mesopores (2 nm
Table 2 Characteristics of porous structure of zeolite product

3.2 Effect of pH
The solution pH plays an important role in the whole adsorption process and particularly on the removal rate. The initial pH range of 3–10 was used to study the effect of the solution pH on removal of Cd(II). Figure 6 shows that the removal rate of Cd(II) is found to gradually increase with the initial pH. Note that the adsorption was depressed at pH lower than 4, and the Cd(II) was precipitated above pH 7. Thus, the effective removal of Cd(II) is in the initial pH range of 4–7. The difference in removal efficiency under acidic conditions is largely related to the change of surface charge of adsorbent during adsorption process.
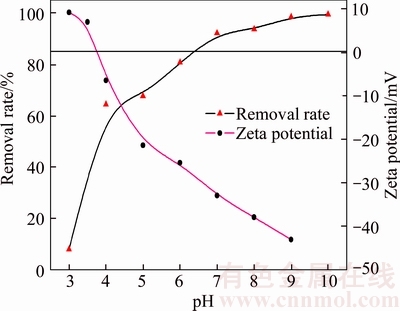
Figure 6 Effect of pH on removal rate of Cd(II)(Initial Cd(II) concentration: 20 mg/L; adsorbent dose: 0.25 g/L; temperature: 298 K; contact time: 90 min) and zeta potential of synthetic zeolite
As shown in Figure 6, the zeta potential significantly decreases with the increasing pH, and the pHzpc of the synthetic zeolite has been found to be 3.80, so the zeolite surface has a positive charge at pH<pHzpc due to the adsorption of hydrogen ions. The competitive adsorption between Cd(II) and H+ on the adsorbent surface impeded the adsorption of Cd(II).
3.3 Effect of adsorbent dose
Figure 7 shows the effect of adsorbent dose on the removal of Cd(II). It can be noticed that the removal rate of Cd(II) sharply increases with an increase in adsorbent dose due to the increase in the total available surface area and the number of active sites for adsorption. When the adsorbent dose is 0.5 g/L, the removal rate and adsorption capacity are 98.55% and 39.42 mg/g, respectively. A further increase in the adsorbent dose does not affect the removal rate of Cd(II). Therefore, an adsorbent dose of 0.5 g/L was fixed for the rest of the experiments.
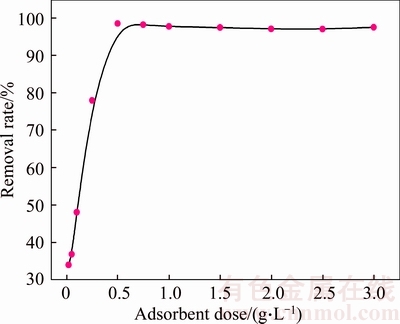
Figure 7 Effect of adsorbent dose on removal of Cd(II) (Initial Cd(II) concentration: 20 mg/L; initial pH value: 6.0; temperature: 298 K; contact time: 90 min)
3.4 Effect of contact time
The effect of contact time on Cd(II) removal rate with three different initial concentrations (20, 30 and 40 mg/L) is shown in Figure 8. The removal rates increase very rapidly at the initial stages and then become lower gradually until nearly adsorption equilibrium is reached after 90 min. The removal rates of Cd(II) are 98.27%, 94.00% and 77.50% at 180 min with initial concentrations of 20, 30 and 40 mg/L, respectively.
3.5 Adsorption kinetics
Kinetics is usually conducted to analyze the time for reaching the adsorption equilibrium. The pseudo first-order kinetics (Eq. (3)) and pseudo second-order kinetics (Eq. (4)) models were employed to fit the experimental kinetics data [31]. They are expressed as follows:
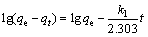 (3)
(3)
 (4)
(4)
where qe (mg/g) is the equilibrium adsorption capacity; qt (mg/g) is the adsorption capacity at any time t; k1 (min–1) and k2 (g/(mg·min)) are the adsorption rate constants of corresponding kinetics equations.
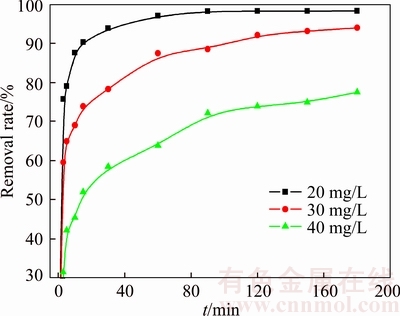
Figure 8 Effect of contact time on removal of Cd(II) (Adsorbent dose: 0.5 g/L; initial pH value: 6.0; temperature: 298 K; initial Cd(II) concentration: 20, 30 and 40 mg/L)
The adsorption capacity of the synthetic zeolite was fitted with the pseudo first-order (Figure 9(a)) and pseudo second-order (Figure 9(b)) models and the kinetic parameters are presented in Table 3. Compared with the pseudo first-order model, the values of correlation coefficients (R2) for the pseudo second-order model are all higher than 0.99. Besides, the theoretical qe values calculated from the pseudo second-order kinetic model are closer to the experimental qe values than those calculated from the pseudo first-order kinetic model at three tested concentrations. Therefore, the pseudo second-order model describes the adsorption process better than the pseudo first-order model.
In order to further investigate the controlling mechanism of the adsorption process, the intra- particle diffusion model (Eq. (5)) was proposed to evaluate whether intraparticle diffusion was the rate limiting step.

Figure 9 Pseudo first-order kinetics (a), pseudo second- order kinetics (b) and intra-particle diffusion (c) models fitting of Cd(II) adsorption onto synthetic zeolite
qt=kintt0.5+C (5)
where kint (mg/(g·min0.5)) is the intraparticle diffusion rate constant and C is a constant related to the boundary layer thickness. If the rate limiting step is only controlled by intraparticle diffusion, the plot of qt versus t0.5 should be a straight line through the origin (C=0). Otherwise, if the data present multi-linear plots, the intraparticle diffusion is not the only rate limiting step [32].
As shown in Figure 9(c), the straight lines obtained from fitting experimental data are not linear over the whole time range and do not pass through the origin, indicating that the pore diffusion is not the only rate limiting step during the adsorption process. This can be attributed to the fact that adsorption was due to boundary layer diffusion in the initial stages, and to intraparticle diffusion in the later stages [32].
3.6 Adsorption isotherms
The analysis of the isotherm data is extremely important to determine the adsorption capacity of the adsorbent. The adsorption isotherms of Cd(II) onto the synthetic zeolite at different temperatures from 25 to 45 °C are presented in Figure 10. The equilibrium adsorption capacities increase with their equilibrium concentrations under the same temperature. Furthermore, the adsorption capacities elevate significantly with temperature increasing from 25 to 45 °C, meaning that the adsorption process is endothermic.
In order to evaluate the effect of temperature on the adsorbent equilibrium capacity for removal of Cd(II) from aqueous solution, the Langmuir, Freundlich, Dubinin-Radushkevich (D-R) and Temkin isotherm models are always employed to describe the equilibrium adsorption data. The Langmuir isotherm model assumes that energies of adsorption sites on the adsorbent surface are homogeneous and monolayer adsorption of adsorbate takes place at specific homogeneous sites [33]. The Langmuir adsorption isotherm equation is given as follows:
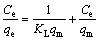 (6)
(6)
Table 3 Kinetic parameters for adsorption of Cd(II) onto synthetic zeolite
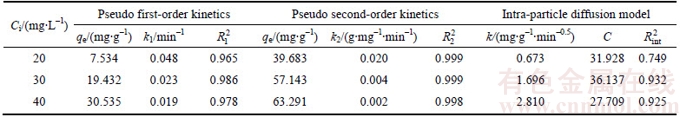
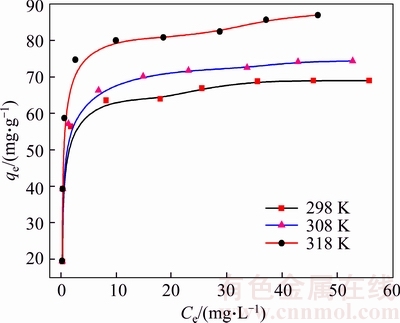
Figure 10 Adsorption isotherms of Cd(II) onto synthetic zeolite at different temperatures (Adsorbent dose: 0.5 g/L; contact time:180 min; initial pH value: 6.0)
where qm (mg/g) and KL (L/mg) are the Langmuir constants in relation to the maximum monolayer capacity and energy of adsorption, respectively.
The Freundlich model can be used to describe multilayer adsorption on heterogeneous surface, and the equation is expressed as follows [12]:
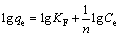 (7)
(7)
where KF (mg·g–1) and 1/n are the Freundlich constants related to adsorption capacity and surface heterogeneity, respectively.
The D-R isotherm model assumes a uniform porefilling adsorption and can predict the free adsorption energy change, the equation can be described as [34, 35]:
lnqe=lnqm–βε2 (8)
where qm (mg/g) is the maximum adsorption capacity; β (mol2/J2) is the model constant which is related to mean adsorption energy; ε is the Polanyi potential which is related to the equilibrium concentration as follows:
ε=RTln(1+1/Ce) (9)
where R (8.314 J/(mol·K)) is molar gas constant and T (K) is the thermodynamic temperature.
The mean free energy of adsorption E is obtained by:
E=(2β)–0.5 (10)
The Temkin equation proposes a linear reduction in adsorption energy when the degree of completion of an adsorbent is raised. As a relationship between adsorbent and adsorbate, when the coverage is increased, the energy released by adsorption along the layer would decrease [2]. The linear form of the Temkin isotherm is described as follows:
qe=BlnKT+BlnCe (11)
where B=RT/b; KT is the Tempkin constant.
The results of Langmuir, Freundlich, D-R and Temkin fitting of Cd(II) onto the synthetic zeolite are shown in Figure 11 and the isotherms fitting parameters are given in Table 4. Based on the linear correlation coefficients (R2), it is obvious that the adsorption of Cd(II) onto the synthetic zeolite obeys Langmuir adsorption isotherm at three tested temperatures very well. Besides, the RL2 values are higher than 0.999, indicating a preferable mathematical fit. The good agreement of the Langmuir model with the experimental data indicates a monolayer coverage of Cd(II) onto the zeolite surface. The maximum adsorption capacity for Cd(II) is found to be 86.96 mg/g.
The essential characteristic and practicability of Langmuir isotherm can be evidenced by a dimensionless constant called constant separation factor (RL) and the equation is expressed as follows [36]:
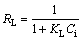 (12)
(12)
where KL is the Langmuir constant; Ci (mg/L) is the initial Cd(II) concentration. The RL values indicate the type of isotherm to be irreversible (RL=0), favorable (0
As shown in Table 4, all RL values for adsorption of Cd(II) onto the synthetic zeolite lie between 0 and 1 and decrease with initial Cd(II) concentrations, indicating that the adsorption process is favorable at all studied temperatures. Moreover, an increase in initial concentrations is favorable for Cd(II) adsorption.
3.7 Adsorption thermodynamics
Thermodynamic parameters for adsorption process include the Gibbs free energy change (△GΘ), enthalpy change (△HΘ) and entropy change (△SΘ), and the direction and complexity of adsorption reaction can be judged by these parameters. Since the KL is essentially equilibrium constant, the variation of KL with temperature can be used to estimate the enthalpy change accompanying adsorption [37]. The values of △GΘ, △HΘ and △SΘcan be calculated using the following equations:
△GΘ=–RTlnKL (13)
 (14)
(14)
where KL is the Langmuir isothermal adsorption constant; T (K) is the absolute temperature; △HΘ (J·mol–1) and △SΘ (J·mol–1·K–1) can be obtained from the slope and intercept of the line plotted by lnKL versus 1/T. The values of these parameters are listed in Table 5.
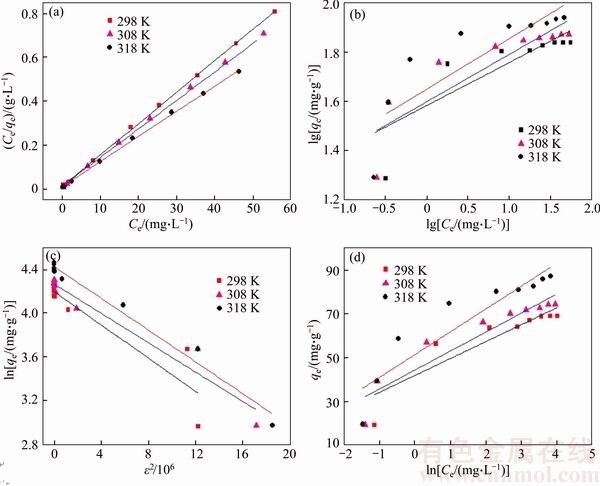
Figure 11 Langmuir isotherm (a), Freundlich isotherm (b), D-R isotherm (c) and Temkin isotherm (d) for adsorption of Cd(II) onto synthetic zeolite at various temperatures
Table 4 Isotherm parameters for adsorption of Cd(II) onto synthetic zeolite
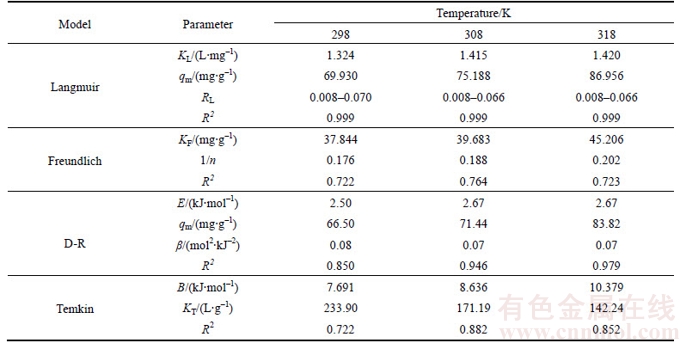
Table 5 Thermodynamic parameters for Cd(II) adsorption onto synthetic zeolite
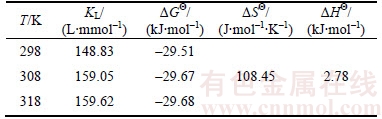
Generally, the values of △GΘ within the range of –20 to 0 kJ/mol correspond to spontaneous physical processes, while those with values in the –80 to –400 kJ/mol range correspond to chemisorption [30]. In this work, the negative values of △GΘ indicate that the spontaneous and feasible nature of the adsorption process at three tested temperatures. The △GΘ values are around –29 kJ/mol, which mean that the absorption belongs to both physisorption and chemisorption. The positive value of △HΘ reveals that the adsorption process is endothermic, so higher temperature is more favorable for the Cd(II) adsorption. The positive value of △SΘ shows an increase in disorder of solid/liquid interface during adsorption.
3.8 Comparison of Cd(II) adsorption capacity with other zeolites
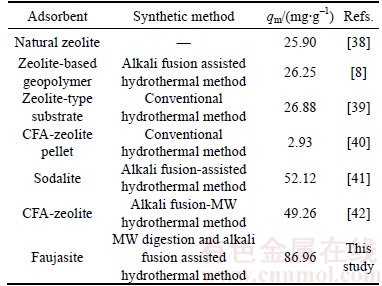
The maximum Cd(II) adsorption capacity of the synthetic zeolite from CFA by MW digestion and alkali fusion assisted hydrothermal method was compared with other zeolites reported in the literature. The values are listed in Table 6. As shown in Table 6, the synthetic zeolite has an obvious advantage in adsorption capacity of Cd(II). Therefore, the MW heating is considered to be an effective and energy-saving method to synthesize zeolite from CFA.
Table 6 Comparison of Cd(II) adsorption capacity with other zeolites
4 Conclusions
1) A novel MW digestion and alkali fusion assisted hydrothermal method was proposed to synthesize zeolite from CFA, the synthesis conditions are MW alkali fusion for 15 min at 450 °C, suspension stirring for 4 h at room temperature and then crystallization for 12 h at 90 °C. The product was identified as faujasite.
2) The optimum conditions for removal of Cd(II) from aqueous solution are adsorbent dose 0.5 g/L, pH 6, contact time 90 min and initial concentration 20 mg/L, the removal rate is 98.55%.
3) The experimental kinetic data agree well with the pseudo second-order equation. The Langmuir isotherm model is proved to be the best in fitting the adsorption process, and the maximum adsorption capacity of Cd(II) is found to be 86.96 mg/g.
4) The thermodynamic parameters △GΘ, △HΘ and △SΘ values show that the adsorption of Cd(II) onto the synthetic zeolite is spontaneous, endothermic and feasible in nature.
5) Comparative studies show that the synthetic zeolite is a more efficient adsorbent for removal of Cd(II) from wastewater. The MW digestion and alkali fusion assisted hydrothermal method is proved to be an effective and energy-saving method to synthesize zeolite from CFA.
References
[1] SUN Wei-ling, JIANG Bo-feng, WANG Fei, XU Nan. Effect of carbon nanotubes on Cd(II) adsorption by sediments [J]. Chemical Engineering Journal, 2015, 264: 645–653.
[2] MUBARAK N M, SAHU J N, ABDULLAH E C, JAYAKUMAR N S, GANESAN P. Microwave assisted multiwall carbon nanotubes enhancing Cd(II) adsorption capacity in aqueous media [J]. Journal of Industrial and Engineering Chemistry, 2014, 24: 24–33.
[3] FU Feng-lian, XIE Li-ping, TANG Bing, WANG Qi, JIANG Shu-xian. Application of a novel strategy-advanced Fenton-chemical precipitation to the treatment of strong stability chelated heavy metal containing wastewater [J]. Chemical Engineering Journal, 2012, 189–190: 283–287.
[4] ZEWAIL T M, YOUSEF N S. Kinetic study of heavy metal ions removal by ion exchange in batch conical air spouted bed [J]. Alexandria Engineering Journal, 2015, 54(1): 83–90.
[5] HUNSOM M, PRUKSATHORN K, DAMRONGLERD S, VERGNES H, DUVERNEUIL P. Electrochemical treatment of heavy metals (Cu2+, Cr6+, Ni2+) from industrial effluent and modeling of copper reduction [J]. Water Research, 2005, 39(4): 610–616.
[6] BESSBOUSSE H, RHLALOU T, VERCHERE J F, LEBRUN L. Removal of heavy metal ions from aqueous solutions by filtration with a novel complexing membrane containing poly(ethyleneimine) in a poly(vinyl alcohol) matrix [J]. Journal of Membrane Science, 2008, 307(2): 249–259.
[7] OZAKI H, SHARMA K, SAKTAYWIN W. Performance of an ultra-low-pressure reverse osmosis membrane (ULPROM) for separating heavy metal: Effects of interference parameters [J]. Desalination, 2002, 144(1–3): 287–294.
[8] JAVADIAN H, GHORBANI F, TAYEBI H, ASI S H. Study of the adsorption of Cd(II) from aqueous solution using zeolite-based geopolymer, synthesized from coal fly ash; kinetic, isotherm and thermodynamic studies [J]. Arabian Journal of Chemistry, 2015, 8(6): 837–849.
[9] ANIRUDHAN T S, SREEKUMARI S S. Adsorptive removal of heavy metal ions from industrial effluents using activated carbon derived from waste coconut buttons [J]. Journal of Environmental Sciences, 2011, 23(12): 1989–1998.
[10] GOUDA A A, AL GHANNAM S M. Impregnated multiwalled carbon nanotubes as efficient sorbent for the solid phase extraction of trace amounts of heavy metal ions in food and water samples [J]. Food Chemistry, 2016, 202: 409–416.
[11] LASHEEN M R, EL-SHERIF I Y, TAWFIK M E, EI-WAKEEL S T, EI-SHAHAT M F. Preparation and adsorption properties of nano magnetite chitosan films for heavy metal ions from aqueous solution [J]. Materials Research Bulletin, 2016, 80: 344–350.
[12] VISA M. Synthesis and characterization of new zeolite materials obtained from fly ash for heavy metals removal in advanced wastewater treatment [J]. Powder Technology, 2016, 294: 338–347.
[13] WANG Jian-cheng, LI De-kui, JU Feng-long, HAN Li-na, CHANG Li-ping, BAO Wei-ren. Supercritical hydrothermal synthesis of zeolites from coal fly ash for mercury removal from coal derived gas [J]. Fuel Processing Technology, 2015, 136: 96–105.
[14] IZIDORO J D C, FUNGARO D A, ABBOTT J E, WANG Shao-bing. Synthesis of zeolites X and A from fly ashes for cadmium and zinc removal from aqueous solutions in single and binary ion systems [J]. Fuel, 2013, 103(1): 827–834.
[15] KOSHY N, SINGH D N. Fly ash zeolites for water treatment applications [J]. Journal of Environmental Chemical Engineering, 2016, 4(2): 1460–1472.
[16] NASCIMENTO M, SOARES P S M, SOUZA V P D. Adsorption of heavy metal cations using coal fly ash modified by hydrothermal method [J]. Fuel, 2009, 88(9): 1714–1719.
[17] ZHANG Bao-ping, CHEN Yun-lin, WEI Lin, ZU Zhi-nan. Preparation of molecular sieve X from coal fly ash for the adsorption of volatile organic compounds [J]. Microporous and Mesoporous Materials, 2012, 156(8): 36–39.
[18] KOUKOUZAS N, VASILATOS C, ITSKOS G, MITSIS I. Removal of heavy metals from wastewater using CFB-coal fly ash zeolitic materials [J]. Journal of Hazardous Materials, 2010, 173(1–3): 581–588.
[19] INADA M, TSUJIMOTO H, EGUCHI Y, ENOMOTO N, HOJO J. Microwave-assisted zeolite synthesis from coal fly ash in hydrothermal process [J]. Fuel, 2005, 84 (12, 13): 1482–1486.
[20] TANAKA H, FUJII A, FUJIMOTO S, TANAKA Y. Microwave-assisted two-step process for the synthesis of a single-phase Na-A zeolite from coal fly ash [J]. Advanced Powder Technology, 2008, 19(1): 83–94.
[21] FUKUI K, KANAYAMA K, YAMAMOTO T, YOSHIDA H. Effects of microwave irradiation on the crystalline phase of zeolite synthesized from fly ash by hydrothermal treatment [J]. Advanced Powder Technology, 2007, 18(4): 381–393.
[22] ITSKOS G, KOUTSIANOS A, KOUKOUZAS N, VASILATOS C. Zeolite development from fly ash and utilization in lignite mine-water treatment [J]. International Journal of Mineral Processing, 2015, 139: 43–50.
[23] HOLLMAN G G, STEEBRUGGEN G, JANSSEN- JURKOVICOVA M. A two-step process for the synthesis of zeolites from coal fly ash [J]. Fuel, 1999, 78(10): 1225–1230.
[24] BUKHARI S S, BEHIN J, KAZEMIAN H, ROHANI S. Conversion of coal fly ash to zeolite utilizing microwave and ultrasound energies: A review [J]. Fuel, 2015, 140: 250–266.
[25] CUI Xing-yu, ZHANG Xu-ning, CHEN Sun-wei, FAN Bin-bin, MA Jing-hong, LI Rui-feng. Synthesis of zeolite 4A from coal fly ash [J]. Journal of Taiyuan University of Technology, 2012, 43(5): 539–543. (in Chinese)
[26] ONUTAI S, JIEMSIRILERS S, THAVORNITI P, KOBAYASHI T. Fast microwave syntheses of fly ash based porous geopolymers in the presence of high alkali concentration [J]. Ceramics International, 2016, 42(8): 9866–9874.
[27] LIU Yi, YAN Chun-jie, QIU Xiu-mei, LI Dan, WANG Hong-quan, ALSHAMERI A. Preparation of faujasite block from fly ash-based geopolymer via in-situ hydrothermal method [J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 59: 433–439.
[28] THUADAIJ P, NUNTIYA A. Preparation and characterization of faujasite using fly ash and amorphous silica from rice husk ash [J]. Procedia Engineering, 2012, 32: 1026–1032.
[29] LEOFANTI G, PADOVAN M, TOZZOLA G, VENTURELLI B. Surface area and pore texture of catalysts [J]. Catalysis Today, 1998, 41(1–3): 207–219.
[30] LIU Hai-cheng, CHEN Wei, CUI Biao, LIU Cheng. Enhanced atrazine adsorption from aqueous solution using chitosan-modified sepiolite [J]. Journal of Central South University, 2015, 22(11): 4168–4176.
[31] CHEN Wei, LIU Hai-cheng, Adsorption of sulfate in aqueous solutions by organo-nano-clay: Adsorption equilibrium and kinetic studies [J]. Journal of Central South University, 2014, 21(5): 1974–1981.
[32] MIRETZKY P, MUNOZ C. Enhanced metal removal from aqueous solution by Fenton activated macrophyte biomass [J]. Desalination, 2011, 271(1–3): 20–28.
[33] LI Xin, WANG Guang-zhi, LI Wei-guang, WANG Ping, SU Cheng-yuan. Adsorption of acid and basic dyes by sludge-based activated carbon: Isotherm and kinetic studies [J]. Journal of Central South University, 2015, 22(1): 103–113.
[34] HRITCU D, HUMELNICU D, DODI G, POPA M I. Magnetic chitosan composite particles: Evaluation of thorium and uranyl ion adsorption from aqueous solutions [J]. Carbohydrate Polymers, 2012, 87(2): 1185–1191.
[35] WANG Yan, TANG Xiao-wu, WANG Heng-yu. Characteristics and mechanisms of Ni(II) removal from aqueous solution by Chinese loess [J]. Journal of Central South University, 2015, 22(11): 4184–4192.
[36] LIN Li-dan, LIN Yan, Li Chun-jie, WU De-yi, KONG Hai-nan. Synthesis of zeolite/hydrous metal oxide composites from coal fly ash as efficient adsorbents for removal of methylene blue from water [J]. International Journal of Mineral Processing, 2016, 148(1): 32–40.
[37] FERNANDES A N, ALMEIDA C A P, DEBACHER N A, SIERRA M M D S. Isotherm and thermodynamic data of adsorption of methylene blue from aqueous solution onto peat [J]. Journal of Molecular Structure, 2010, 982(1–3): 62–65.
[38] TAAMNEH Y, SHARADQAH S. The removal of heavy metals from aqueous solution using natural Jordanian zeolite [J]. Applied Water Science, 2017: 7(4): 2021–2028.
[39] VISA M. Synthesis and characterization of new zeolite materials obtained from fly ash for heavy metals removal in advanced wastewater treatment [J]. Powder Technology, 2016, 294: 338–347.
[40] CHEN Ju. Evaluation of synthesized fly ash-zeolite pellets as potential adsorbents for Cd(II) ion in wastewater [D]. Nanjing: Nanjing University of Science and Technology, 2010. (in Chinese)
[41] HE Kuang, CHEN Yuan-cai, TANG Zheng-hua, HU Yong-you. Removal of heavy metal ions from aqueous solution by zeolite synthesized from fly ash [J]. Environmental Science and Pollution Research, 2016, 23(3): 2778–2788.
[42] YANG Wen-huan, CHEN A-hui, LI Wei-ping, ZHANG Xue-feng, GUO Jun-wen. Synthesization of fly-ash zeolite by alkali fusion- microwave crystallization process and adsorption to Cd(II) [J]. Environmental Protection of Chemical Industry, 2015, 35(5): 547–551. (in Chinese)
(Edited by HE Yun-bin)
中文导读
粉煤灰微波碱熔辅助水热合成沸石及其对水溶液中Cd(II)的强化吸附作用
摘要:针对传统水热法合成沸石存在碱熔温度高、反应时间长的问题,提出了一种新的粉煤灰微波碱熔辅助水热合成沸石的方法,并将沸石产品用于吸附水溶液中的 Cd(II)。其合成工艺是将粉煤灰与氢氧化钠混合物置于微波箱式高温反应器中以450 °C反应15 min,碱熔产物加水在常温下磁力搅拌4 h,再在90 °C下静置12 h,过滤、洗涤、干燥得到沸石产品。采用X射线衍射分析、扫描电镜分析、红外光谱分析、比表面积分析和zeta电位分析表征吸附剂。结果表明:合成的沸石为八面沸石,含量约为93%,比表面积为75.72 m2/g,pHzpc为3.80。从水溶液中去除Cd(II),优化的吸附剂用量为0.5 g/L, pH 6, 吸附时间为90 min,初始浓度为 20 mg/L。在此条件下,Cd(II)的去除率达98.55%。Cd(II)在沸石表面的吸附符合伪二级动力学模型,Langmuir等温吸附模型比Freundlich模型、Dubinin-Radushkevich模型和Temkin模型更适合于描述等温吸附过程,最大的Cd(II)吸附量为86.96 mg/g。热力学计算表明:Cd(II)在合成沸石表面的吸附为自发、吸热过程。
关键词:微波;粉煤灰;合成沸石;吸附;Cd(II)
Foundation item: Projects(2013BAC15B01, 2013BAB07B03) supported by the National Key Technology Research and Development Program of the Ministry of Science and Technology of China; Project(Qian Ke He JZ [2014] 2009) supported by the Key Foundation of Science and Technology of Guizhou Province, China
Received date: 2016-05-16; Accepted date: 2017-12-01
Corresponding author: ZHANG Qin,PhD, Professor;Tel: +86–851–88292081;E-mail:qzhang@gzu.edu.cn; ORCID: 0000-0003-1253- 8652

