
Optimization of separation processing of copper and iron of
dump bioleaching solution by Lix 984N in Dexing Copper Mine
LIU Qing-ming(刘清明)1, 2, YU Run-lan(余润兰)1, 3, QIU Guan-zhou(邱冠周)1, 3,
FANG Zheng(方 正)1, 2, CHEN Ai-liang(陈爱良)4, ZHAO Zhong-wei(赵中伟)4
1. Key Laboratory of Biometallurgy, Ministry of Education, Central South University, Changsha 410083, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
3. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
4. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 3 September 2007; accepted 19 December 2007
Abstract: The effects of the concentration of Lix 984N, phase ratio, initial pH value of aqueous phase and extraction time on the extraction of copper and iron under the condition of low Cu2+ /Fe3+ ratio in dump bioleaching solution of Dexing Copper Mine were explored. The optimal conditions of extraction are as follows: the concentration of Lix 984N 10%; the phase ratio (O/A) 1?1; the initial pH value of aqueous phase 1.5 and the mixing time 2 min. The stripping experiments show that H2SO4 solution could efficiently recover copper from the organic phase under the optimal conditions.
Key words: copper solvent extraction; processing optimization; Lix 984N; bioleaching solution
1 Introduction
Bioleaching—solvent extraction—electrowinning (BL-EX-EW) has become a key process for recovering copper from the bioleaching solution of low-grade ores in the last two decades[1]. The separation of copper and iron with solvent extraction is basic in biological metallurgy. In recent years, a lot of studies on copper solvent extraction in acid or ammoniacal medium using Lix series extractants have been reported[2-15]. Now, Lix 984N and M5640 are widely used as extractive reagents. Lix 984N is a mixture of Lix 860N and Lix 84 in kerosene. The active ingredients are 2-hydroxy-5- nonylacetyloxime and 2-hydroxy-5-dodecalkyl salicyl- aldoxime. The extractive reactions are as follows[16]:
Cu2++2HR0=CuR20+2H+ (1)
Cu2++4HR0=CuR22HR0+2H+ (2)
In dump bioleaching solution of Dexing Copper Mine, the concentration of Cu2+ was designed as 1 g/L. Now it maintains about 0.3 g/L for several years. The concentration ratio of Cu2+ to Fe3+ is very low. The accumulated concentration of Fe3+ in the electrowinning solution is up to 6-8 g/L. Therefore, it is necessary to study the separation behaviors of copper and iron from the bioleaching solution with low Cu2+/ Fe3+ ratio by Lix 984N by optimization of the extractive parameters.`
2 Experimental
2.1 Raw material and chemical reagents
The aqueous phase was prepared by CuSO4·5H2O, Fe2(SO4)3 and H2SO4 (all in AR grade) according to the composition of solution of Dexing Copper Mine. The solution contained Cu2+ 10 g/L, Fe3+ 20.4 g/L and its pH is about 2. The copper extractive reagent was Lix 984N (Germany Henkel Corporation) with kerosene as the diluent reagent. The stripping reagent was 180 g/L H2SO4 solution.
2.2 Analytical method
The concentration of copper and iron in the aqueous phase was measured by 3510 atomic absorption spectrophotometer. The concentration of copper and iron in the organic phase was calculated based on the mass balance.
2.3 Condition experiments
The experiments were carried out in a separatory funnel. Before each run, the funnel was washed with kerosene. The aqueous phase and the organic phase were added into the funnel. Then the cone was vibrated under 200 r/min at (25±1) ℃. The phases were disengaged after vibrating for a certain minutes. The concentration of Cu2+ and Fe3+ in the aqueous phase was measured and that in the organic phase was calculated. The extraction rate and the separation coefficient of copper and iron were determined under different conditions of extractive reagent, phase ratio, pH value and extractive time, respectively. The organic phase was further stripped by 180 g/L H2SO4 solution. The conditions of counter extraction were similar to the extraction experiments.
3 Results and discussion
3.1 Solvent extraction behaviors of copper and iron
Fig.1 shows the change in the extraction rate of copper and iron with Lix 984N concentration. The extraction rate of copper increases as the concentration of extractive reagents enhances. When the concentration of Lix 984N is 10%, the extraction rate of copper reaches about 98.5%, then remains stable. Simultaneously, the extraction rate of iron stays very low, and keeps constant over the concentration of 30% Lix 984N. The extraction rate of iron slightly increases as the concentration of Lix 984N enhances. The largest separation coefficient of Cu/Fe occurs in the Lix 984N range from 10% to 12.5%. The optimal concentration of Lix 984N is suggested to be about 10%.
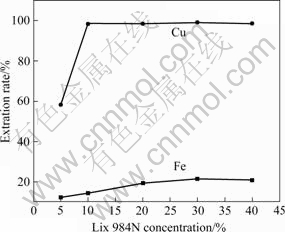
Fig.1 Effect of Lix 984N concentration on separation of copper and iron (Initial pH value of aqueous phase 1.5; time 2 min; O/A=1?1; 298 K)
Fig.2 shows the effect of the phase ratio (O/A) on the separation of copper and iron. When the phase ratio(O/A) is lower than 1?1, the extraction rates of both copper and iron ascend rapidly with the increase of the phase ratio, reaching about 96.3% for copper. Then, they maintain only a slight increase over the phase ratio of 1?1. Basically, the phase ratio almost does not influence the separation coefficient of Cu/Fe. The optimal phase ratio is suggested to be 1?1.
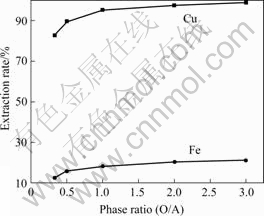
Fig.2 Effect of phase ratio on extraction rates of copper and iron (Time 2 min; 10% Lix 984N; 298 K)
The effect of the initial pH value of aqueous phase on the extraction rates of copper and iron is shown in Fig.3. There is an obvious increase in the extraction rate of copper with the increase of initial pH value. During the extraction process, reaction (1) happens between Cu2+ and Lix 984N, so the initial pH value of the aqueous phase plays an important role in the reaction. The extraction rate of iron also slightly increases with the increase of the pH value. However, it begins to decrease rapidly when pH is over 2.0. The reason is that the aqueous phase becomes turbid because of the hydration of Fe3+ as follows.
Fe3++H2O=Fe(OH)2++H+ (3)
Fe(OH)2++H2O=Fe(OH)2++H+ (4)
Fe(OH)2++H2O=Fe(OH)3+H+ (5)
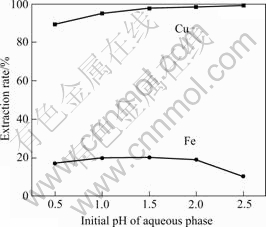
Fig.3 Effect of initial pH value of aqueous phase on extraction rate of copper and iron (Time 2 min; 10% Lix 984N; O/A=1?1; 298 K)
Although the separation coefficient of Cu/Fe keeps ascending with the increase of the initial pH value, the hydration of Fe3+ is accelerated at the high pH value, resulting in the formation of the third phase. So the optimal initial pH value of aqueous phase is 1.5. If the third phase can be treated by other method, the higher initial pH value of bioleaching solution is advantageous in the selective separation for Cu/Fe and the extraction rate of Cu.
Fig.4 shows the effect of mixing time on the extraction rate of copper and iron. The extraction rate of copper increases with the prolonging of time. The reaction is so rapid that it almost finishes within 1 min. Meanwhile, the extraction rate of iron is 20%. The separation coefficient of Cu/Fe does not change a lot after 2 min. The optimal mixing time is 2 min.

Fig.4 Effect of mixing time on extraction rate of copper and iron (Initial pH =1.5; 10% Lix 984N; O/A=1?1; 298 K)
3.2 Stripping behaviors of copper and iron
As shown in Fig.5, the stripping rate of copper goes up with the rise of H2SO4 concentration from 1 to 1.5 mol/L, and reaches 97.8% when H2SO4 is 1.5 mol/L. But the stripping rate of iron also increases and almost attains the maximum at 2.5 mol/L H2SO4, then it is stable over the H2SO4 concentration. Only about 60% of iron is stripped into the aqueous phase during stripping. Obviously, the behavior of iron differs from that of copper in the stripping process. The appropriate H2SO4 concentration for the stripping is from 1.5 to 2.0 mol/L.

Fig.5 Effect of H2SO4 concentration on stripping rate of copper and iron (Reaction time 2 min; O/A=1?2; 298 K)
As illustrated in Fig.6, the stripping rate of copper increases sharply with prolonging the time, and reaches 94.2% in 2 min. Then it increases slightly. The optimal time is 2 min.
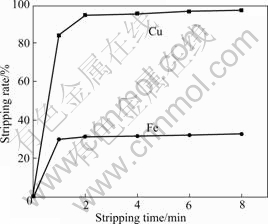
Fig.6 Effect of stripping time on stripping rate of copper and iron (H2SO4 concentration 1.5 mol/L; O/A=1?2; 298 K)
Fig.7 shows that the stripping rate of copper increases with the phase ratio increasing. When the ratio is below 0.5, the rates of both copper and iron increase rapidly. The highest value is 97.8% when the phase ratio is 1?2. At the same time, the stripping rate of iron remains very low. Thus, the proper phase ratio (O/A) is 1?2.
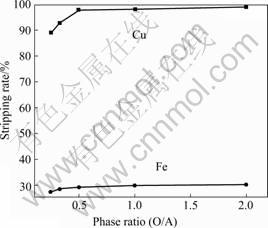
Fig.7 Effect of phase ratio on stripping rate of copper and iron (H2SO4 concentration 1.5 mol/L; time 2 min; 298 K)
4 Conclusions
1) The extraction rate of copper increases with the increase of the Lix 984N concentration. It reaches 98.5% at the optimal Lix 984N concentration of 10%. When the proper phase ratio(OA) is 1?1, the extraction rate of copper can reach a high value with a low cost of production. The initial pH value of aqueous phase obviously affects the extraction rate of copper and the separation of copper from iron. The optimal initial pH value for the extraction is 1.5. The extraction can be completed in 1 min and the extraction rate of copper is 96.5%.
2) H2SO4 solution is high-efficient stripping reagent. The optimal condition of stripping of copper is O/A=1?2 with 1.5 mol/L H2SO4 for 2 min.
References
[1] WU Wen-jian, JIANG Han-ying, YANG Song-qing. Extractive separation of copper and nickel with Lix 984N [J]. Mining and Metallurgical Engineering, 1995, 15(4): 43-47. (in Chinese)
[2] LIU Jian-she, GE Yu-qing, QIU Guan-zhou, WANG Dian-zuo. Selectively extract copper from copper, iron and zinc acid solution [J]. Copper Engineering, 2002, 18(1): 18-21. (in Chinese)
[3] BARTOS P J. SX-EW copper and the technology cycle [J]. Resources Policy, 2002, 28: 85-94.
[4] LIU Xiao-rong, QIU Guan-zhou, HU Yue-hua, YANG Jun-he, JIN Ming-lin. Effect of Lix 984N content on phase disengagement dynamics in copper-SX [J]. Trans Nonferrous Met Soc China, 2003, 13(4): 963-967.
[5] MARTIN T P, DAVIES G A. The extraction of copper from dilute aqueous solutions using a liquid membrane process [J]. Hydrometallurgy, 1977, 2: 315-334.
[6] SZYMANOWSKI J, TONDRE C. Kinetic and interfacial phenomena in classical and micellar extraction systems [J]. Solvent Extraction and Ion Exchange, 1994, 12(4): 873-905.
[7] WANG Ping-rui, LANG Shu-ling, LIU Du. Recent progress in copper solvent extraction [J]. Study of Nonferrous Chemical Engineering, 1991, 2: 18-21. (in Chinese)
[8] de SAN MIGUEL E R, AGUILAR J C, BERNAL J P, BALLINAS M L, RODRIGUEZ M T J, de GYVES J, SCHIMMEL K. Extraction of Cu2+, Fe3+, Ca2+, Ni2+, In3+, Co2+, Zn2+ and Pb2+ with Lix984 dissolved in n-heptane [J]. Hydrometallurgy, 1997, 47: 19-30.
[9] ALGUACIL F J. Recovery of copper from ammoniacal/ammonium carbonate medium by Lix 973N [J]. Hydrometallurgy, 1999, 52: 55-61.
[10] NIINAE M, KOYANAKA S, NAKAHIRO Y, WAKAMATSU T. A study of interfacial properties in solvent extraction [J]. Transactions of the Mining and Metallurgical Association, 1992, 21(8): 572-579.
[11] SZYMANOWSKI J, PROCHASKA K. The surface excess isotherms and mechanism of copper extraction by hydroxyoxime extractants [J]. Journal of Colloid and Interface Science, 1988, 125(2): 649-666.
[12] ZHU Tun. Modern Copper Hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 1986(4): 15-20. (in Chinese)
[13] FENG Bai-hua. Chemical engineering handbook (2) [M]. Beijing: Chemical Industry Press, 1989: 5-54. (in Chinese)
[14] KONGOLO K, MWEMA M D, BANZA A N, GOCK E. Cobalt and zinc recovery from copper sulphate solution by solvent extraction [J]. Minerals Engineering, 2003, 16: 1371-1374.
[15] YANG X J, FANE A G. Performance and stability of supported liquid membranes using LIX 984N for copper transport [J]. Journal of Membrane Science, 1999, 156: 251-263.
[16] ATA O N. Modelling of copper ion transport through supported liquid membrane containing LIX 984 [J]. Hydrometallurgy, 2005, 77: 269-277.
Foundation item: Project (2004CB619200) supported by the National Basic Research Program of China
Corresponding author: Yu Run-lan; Tel: +86-731-8836943; E-mail: yrl715@sina.com
(Edited by YUAN Sai-qian)