
Effect of cobalt on chemical segregation and solution process in Re-containing single crystal superalloys
WANG Wen-zhen(王文珍)1, 2, JIN Tao(金 涛)1, ZHAO Nai-ren(赵乃仁)1, WANG Zhi-hui(王志辉)1,
SUN Xiao-feng(孙晓峰)1, GUAN Heng-rong(管恒荣)1, HU Zhuang-qi(胡壮麒) 1
1. Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China;
2. School of Graduate, Chinese Academy of Sciences, Beijing 100039, China
Received 28 July 2006; accepted 15 September 2006
Abstract: The effect of cobalt on chemical segregation and solution process in three nickel base single crystal superalloys was investigated. Three alloys containing the mass fraction of cobalt of 12% (named Alloy 1), 3% (named Alloy 2) and 0 (named Alloy 3), respectively were studied, in which the contents of other elements were same. The results show that the segregation extent of W, Re, Ta, Al between dendrite and interdendritic region rises with the increase of cobalt content. The incipient melting points decrease by 10 ℃ and 20 ℃ respectively when the content of cobalt increases from 0 through 3% to 12%. During solid solution at 1 340 ℃, the solid solution of large gamma prime in interdendritic region and the dissolution of eutectic in alloy 1 become easier than in other two alloys. After heat-treatment at 1 340 ℃ for 8 h, the segregation extent of elements in alloy 1 decreases dramatically, while in alloy 2 and alloy 3, the segregation ratios decrease slowly. It suggests that the higher content of cobalt can accelerate the diffusion process at high temperature.
Key words: single crystal superalloy; cobalt; chemical segregation
1 Introduction
In modern Ni-base superalloys, more and more alloying elements were added to enhance the solid solution strengthening effects, especially the refractory elements, such as Re, W, Ta, Mo[1-2]. The contents of refractory elements (Re+W+Mo+Ta) of representative first (CMSX-2), second (CMSX-4) and third generation (CMSX-10) alloys were increased from approximately 14% to 16.5% and then to greater than 20%[2-4]. The creep strength was improved significantly with rhenium addition; on the other hand, some drawbacks have induced: 1) Increase in density; 2) Increased propensity for precipitation of brittle intermetallic phase know as topologically close packed (TCP) phase; and 3) Increase in cost. Therefore it is significant and essential to develop the alloys having lower levels of rhenium and their properties comparable to those of the third generation single crystal superalloys. It is also practical by taking advantage of the effect of rhenium and other refractory element completely and utilizing the interaction of alloying elements. It is interesting to note that the element cobalt plays a subtle role in superalloys. Cobalt in particular has been studied in wrought nickel-base superalloy[5] and first generation single crystal superalloy MAR-M247[6-9]. However some of the effects of cobalt are controversial and the reasons are unclear. When more and more alloying elements, especially the rhenium, were added to the single crystal superalloys, only a few articles referred to the effect of cobalt. In the two representative commercial third generation single crystal superalloys CMSX-10 [3] and Rene N6[10], the contents of cobalt were 3%(mass fraction) and 12%, respectively. ERICKSON[3] limited the Co level at 3% in CMSX-10 claming that it reduced the tendency to form TCP phase. WALSTON et al[10] recommended a high level of 12.5% Co in N6 in order to improve phase stability. The effects of cobalt in single crystal superalloy are still unclear. The interaction between Re and Co has not been reported. In this study, three alloys were tested. The content of rhenium was 4%, and the nominal contents of other elements remained identical. The only difference was the content of cobalt in order to investigate its effects on chemical segregation, heat treatment process and elemental diffusion and the interaction with Re. The effect of cobalt on mechanical properties was investigated.
2 Experimental
The single crystal bars with gauge size of 16 mm× 200 mm were produced by means of crystal selection method in a directional solidification vacuum furnace under a high thermal gradient. The deviation of axis orientation from [001] was within 10?. The samples were sectioned into d16 mm×10 mm to test the heat treatment conditions. The as-cast and heat treated samples were etched with 25 mL HCl+5 g CuSO4+20 mL H2O. The microstructures were characterized by Leica optical microscope and JSM-6301 scanning electron microscope(SEM) equipped with energy dispersive spectrometer (EDS). The chemical segregation was determined by electron microprobe techniques. Dendrite core and interdendritic areas were examined in each as-cast and heat treated samples. The compositions of at least three of each area were determined for each sample and the average values were reported. The nominal compositions of three alloys are listed in Table 1.
Table 1 Nominal composition of three alloys(mass fraction,%)

3 Results
3.1 Segregation ratio of as-cast materials
In comparison with the bulk alloy composition, the dendrite cores were significantly enriched in W and Re and depleted in Ta and Al, as shown in Table 2. Conversely, the interdendritic region of the as-cast microstructure was enriched in Al and Ta, while lower levels of W and Re were observed. Because the elements including Co, Cr and Mo do not exhibit any significant preference partitioning to either the dendrite core or the interdendritic regions during solidification and heat treatment process, only W, Ta, Re and Al are considered. The partitioning ratios of contents at dendrite core to contents at interdendritic region for these elements are listed in Table 2.
It can be seen that the extents of segregation of these four elements especially the rhenium rise obviously with the increase of Co content.
Table 2 Segregation ratios for three as-cast alloys

3.2 Incipient melting point
The incipient melting points were determined by metallographical methods. The incipient melting points for alloy 1, alloy 2 and alloy 3 are 1 345 ℃, 1 355 ℃and 1 365 ℃, respectively, as shown in Fig.1.
The incipient melting points decrease by 10℃ and 20℃ respectively with the increase of content of cobalt from 0 through 3% to 12%.
3.3 Solution process
The purpose of the solution heat treatment is to both dissolve the large gamma prime (g?) in interdendritic region and the eutectic g/g?, and to reduce or eliminate the chemical segregation. In order to investigate the effect of cobalt on solution process and elemental diffusion, the three alloys were solution heat treated at
1 340 ℃ for 1, 2, 4 and 8 h, respectively. Examinational result of the microstructure of the heat-treated samples indicates that the eutectic of alloy 1 is dissolved completely after 1 h, while for alloy 2 and alloy 3,few eutectics are dissolved. The majority of the cooling g? of alloy 2 goes into solution after 8 h at 1 340℃, and for alloy 3 many large g? phases are still persisted at interdendritic region even if the holding time is in excess of 8 h (see Fig.2). It can be seen from above observation that higher contents of Co facilitate the solution of g? and the dissolution of g/g? eutectic.
During the solution process, the degree of degradation of the chemical segregation varies with contents of cobalt. Fig.3 shows the dependence of the segregation ratio of Re and Al on solution time for the three alloys. It indicates that the ratio of segregation is degraded more rapidly for the alloy with 12% Co than the two others with less Co, which suggests cobalt accelerates the diffusion of Re, W, Ta and Al. It is interesting to note that the segregation of Co does not show any significant change with extending solution time, and varies surrounding with unity. The change of the segregation ratio of W with the solution time has the same tendency with Re. So does Ta with Al.
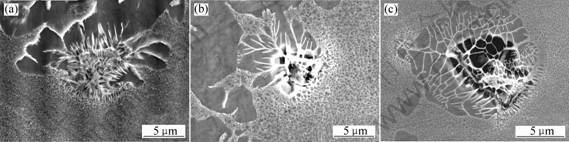
Fig.1 SEM images of incipient melting structure: (a) Alloy 1 at 1 345 ℃, (b) Alloy 2 at 1 355 ℃; (c) Alloy 3 at 1 365 ℃
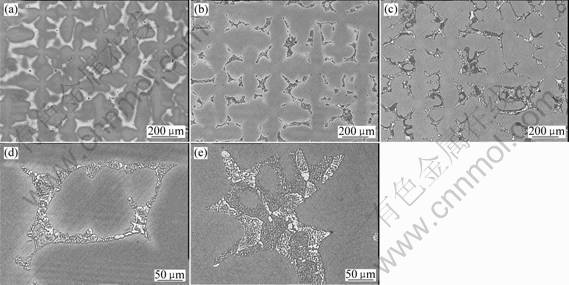
Fig.2 Microstructures of three alloys at 1 340 ℃ holding for different times: (a) Alloy 1 after 1 h; (b) and (d) Alloy 2 after 8 h; (c) and (e) Alloy 3 after 8 h
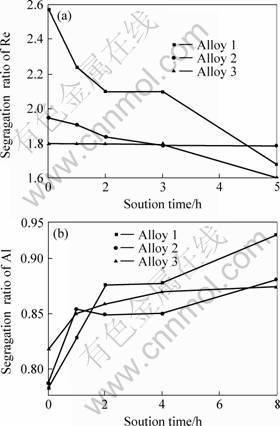
Fig.3 Dependence of segregation ratios of Re(a) and Al (b) on solution time
4 Discussion
The dissolution of the g/g′ eutectic, the solid solution of g′, the reduction or elimination of chemical segregation are all controlled by diffusion of elements. A number of degradation mechanisms are usually mediated in diffusion process. For example, creep deformation occurs at a rate dependent upon diffusion rearrangements at dislocation cores[11-12]. Directional coarsening of the g′ phase (the so-called rafting effect) requires mass transport on the scale of the periodicity of the g′ precipitates[11,13]. Diffusion is the most important mechanism controlling the performance of superalloy. The effects of elements on diffusion are different.
In addition to forming rhenium cluster and hindering the movement of dislocation[14-15], Re also causes the development of directional and incompressible Ni-Re bond by alloying with Ni which does not favor solute-vacancy exchanges[11,16], thus decreasing diffusion rates. (The activation energy of diffusion of Re in Ni is about 330 kJ/mol[11]). As for cobalt, because its magnetic moment is large (1.7mB), and the barrier energy for diffusion is low (The activation energy of diffusion of Co60 in Ni is 270.48 kJ/mol at 850-1 370 ℃[17]), so the activation energy of diffusion is small and favor diffusion[11].
In nickel-base superalloys, there are interactions among elements. Between Re and Co, there is a competition on bonding with Ni. Co promotes diffusion while Re restrains diffusion. The atomic number and radius of Co are very close to Ni, and the solubility of Co in Ni is 100%, therefore Co alloying with Ni is easier than Re. For the alloy containing cobalt, it is difficult for rhenium to form directional and incompressible Ni-Re bond and the effect of Re is only the ordinary solid solution strengthening, then the effect of Re decreasing the diffusion rates will be reduced. Therefore Co plays the role of a buffering agent in alloys and balances out the effects of other elements.
For alloys with high contents of refractory metals and low levels of or no cobalt, it is expected that the creep rupture life of such alloy should be long and the microstructures would be unstable. The diffusion rate will be slow because of the less cobalt, which causes the local supersaturation of refractory metals and the deleterious phases will occur[18].
When the levels of cobalt are enough high, the deleterious phase will be eliminated. RAE et al[19] pointed out that cobalt suppressed the nucleation of s phase and increase of the level of cobalt would eliminate s phase completely. WALSTON et al[10] recommended a high level of 12.5%Co in N6 and in a fourth generation single crystal superalloy, and the levels of Co were increased to 20% in order to improve phase stability[20]. MATSUOKA et al[21] reduced secondary reaction zone(SRZ) on a fourth generation single crystal superalloy by additional Co and/or Ru coating. It is believed that diffusion played a key role on eliminating deleterious phase. Beyond all doubt, Co could accelerate diffusion. Above examples could be taken as some indirect proofs of Co to be beneficial for diffusion.
It can be inferred that the alloys with moderate levels of cobalt and rhenium would achieve an excellent balance between mechanical performance and microstructural stability.
5 Conclusions
1) The incipient melting point decreased from 1 365℃ to 1 345 ℃ as cobalt content increases from 0 to 12%.
2) Higher levels of cobalt accelerate the solution process and the reduction or elimination of chemical segregation.
References
[1] CARON P, KHAN T. Third generation superalloys for single crystal blades[C]// Materials for Advanced Power Engineering, volume 5 of Proceedings of the 6th Liége Conference, partⅡ. Jülich: Forschungszentrum Jülich Gmbh Publishers, 1998: 897-912.
[2] FUCHS G E. Solution heat treatment response of a third generation single crystal Ni-base superalloys[J]. Materials Science and Engineering A, 2001, A300: 52-60.
[3] ERICKSON G L. The development and application of CMSX-10[C]// Superalloys. Warrendale, Pennsylvania: The Minerals, Metals & Materials Society, 1996: 35-41
[4] FUCHS G E. Improvement of creep strength of a third generation , single crystal Ni-base superalloy by solution heat treatment[J]. Journal of Materials Engineering and Performance, 2002, 11(1): 19-25
[5] JARRETT R N, KTIEN J. Effect of cobalt on structure, microchemistry and properties of a wrought nickel-base superalloy [J]. Metallurgical Transactions A, 1982, 13 A: 1021-1032.
[6] NATHAL M V, MAIER R D, EBERT L J. The influence of cobalt on the tensile and stress-rupture properties of the nickel-base superalloy MAR-M247[J]. Metallurgical Transactions A, 1982, 13A: 1767-1774
[7] NATHAL M V, MAIER R D, EBERT L J. The influence of cobalt on the microstructure of the nickel-base superalloy MAR-M247[J]. Metallurgical Transactions A, 1982, 13 A: 1775-1783.
[8] NATHAL M V, EBERT L J. The influence of cobalt, tantalum and tungsten on the microstructure of single crystal nickel-base superalloys[J]. Metallurgical Transactions A, 1985, 16A: 1849-1862.
[9] NATHAL M V, EBERT L J. The influence of cobalt, tantalum and tungsten on the elevated temperature mechanical properties of single crystal nickel-base superalloys[J]. Metallurgical Transactions A, 1985, 16 A: 1863-1870.
[10] WALSTON W S, O’HARA K S, ROSS E W, POLLOCK T M. MURPHY W H. Rene N6: third generation single crystal superalloy[C]// Superalloys. Warrendale, Pennsylvania: The Minerals, Metals & Materials Society, 1996: 27-34.
[11] FU Chong-long, REED R, JANOTTI A, KRCMAR M. On the diffusion of alloying elements in the nickel-base superalloys[C]∥ Superalloys. Warrendale, Pennsylvania: The Minerals, Metals & Materials Society, 2004: 867-876
[12] POLLOCK T M, ARGON A S. Creep resistance of CMSX-3 nickel base superalloy single crystals[J]. Acta Metallurgical et Materialia, 1992, 40: 1-30.
[13] MATEN N, COX D C, RAE C N F, REED R C. On the kinetics of rafting in CMSX-4 superalloy single crystals[J]. Acta Materialia, 1999, 47: 2031-2045.
[14] BLAVETTE D, CARON P, KHAN T. An atom probe investigation of the role of rhenium additions in improving creep resistance of Ni-base superalloys[J]. Scripta Metallurgica, 1986, 20: 1395-1400.
[15] BLAVETTE D, CARON P, KHAN T. An atom probe study of some fine-scale microstructural features in Ni-based single crystal superalloy[C]// Superalloys. Warrendale, Pennsylvania: The Metall- urgical Society, 1988: 305-314.
[16] KARUNARATNE M S A, REED R C. Interdiffusion of the platinum-group metals in nickel at elevated temperatures[J]. Acta Materialia, 2003, 51: 2905-2919.
[17] LECLAIRE A D. Smithells Metals Reference Book, Chapter 13[M]. 6 ed. London: Butter Worth & Co(Publishers) Ltd, 1983: 13-20.
[18] ACHARYA M V, FUCHS G E. The effect of long-term thermal exposures on the microstructure and properties of CMSX-10 single crystal Ni-base superalloys[J]. Materials Science and Engineering A, 2003, 381: 143-153.
[19] RAE C M F, REED R C. The precipitation of topologically close-packed phased in rhenium-containing superalloys[J]. Acta Materialia, 2001, 49: 4113-4125.
[20] WALSTON S, CETEL A, MACKAY R, O’HARA K, DUHL D, DRESHFIELD R. Joint development of a fourth generation single crystal superalloy[C]// Superalloys. Warrendale, Pennsylvania: The Minerals, Metals & Materials Society, 2004: 15-24.
[21] MATSUOKA Y, AOKI Y, MATSUMOTO K, SUZUKI A, CHIKUGO K, MURAKAMI K. The formation of SRZ on a fourth generation single crystal superalloy applied with aluminide coating[C]// Superalloys. Warrendale, Pennsylvania: The Minerals, Metals & Materials Society, 2004: 637-642.
(Edited by YANG You-ping)
Corresponding author: WANG Wen-zhen; Tel: +86-24-23971767; E-mail: wzhwang@imr.ac.cn