
Adsorption and removal of cadmium (Ⅱ) from aqueous solutions by bio-formulation
CHAI Li-yuan(柴立元) , CHEN Yun-nen(陈云嫩), SHU Yu-de(舒余德),CHANG Hao(常 皓), LI Qing-zhu(李青竹)
Department of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 26 October 2006; accepted 20 March 2007
Abstract: A novel sorbent, bio-formulation(BF), was prepared and employed for the adsorption of cadmium(Cd) ion from aqueous system. The process of adsorption follows the pseudo second-order kinetic equation and the adsorption behavior is fitted with a Freundlich isotherm. The removal rate of Cd is slightly dependent on the initial pH value over a wide range of 4-11. The addition of Zn2+ and Cu2+ ions hardly affects the Cd adsorption, whereas the coexisting Pb2+ ion greatly interferes with the adsorption. The adsorption mechanism is supposed as a cation-exchange process between Cd2+ and calcium and Mg2+ present on the surface of BF, and somewhat as adsorption in a hydrolyzed form. The adsorbed Cd ions are desorbed effectively by a 0.1 mol/L HCl solution.
Key words: cadmium; bio-formulation(BF); adsorption; desorption
1 Introduction
Release of large quantities of heavy metals into the natural environment has resulted in a number of environmental problems[1]. Cadmium is a metal widely used in industries such as electroplating, paint pigments, plastics manufacturing, mining and metallurgical processes[2]. It is a non-essential and non-beneficial element to plants and animals, and is extremely toxic in relatively low dosages[3]. Diseases such as renal damage, anemia and hypertension are associated with excess cadmium[4]. The main anthropogenic pathway through which cadmium enters the environment is via wastewater. At present there are a number of different technologies for treating cadmium-bearing waste streams, including chemical precipitation, filtration, ion exchange, adsorption using active carbon and membrane processes. Chemical precipitation processes have several disadvantages, which include incomplete metal removal and sludge generation[5]. The other technologies are generally expensive when cadmium is present in the wastewater at low concentrations, or when a very low concentration of cadmium in treated water is required[6].
In the last decade, due to its potential, bio-sorption is one of the alternative means of treating wastewaters containing low levels of cadmium by various biological materials[7-8]. Recovery of cadmium from solution would be beneficial since cadmium may be cumulated by the biological materials and recovered for reusage, and release of potentially toxic metals into the environment would be restricted[9]. Bio-sorption involves the use of biomass[10] or natural substrates such as agricultural residues[11], microorganisms[12], and sugar beet pulp[13].
BF is agricultural waste modified by acid and base. In the present study, BF was used as a biosorbent for the removal of cadmium from aqueous solutions. The objective of this study is to explore the feasibility of using the BF for the removal of toxic heavy metals from wastewaters. Batch experiments were carried out to investigate metal biosorption properties of BF. The factors affecting the adsorption of cadmium, such as pH, initial concentration, contact time, coexisting ions were studied.
2 Experimental
The agricultural waste was obtained from a local agricultural working factory located in Changsha, China. The waste was sun dried and ground to be less than 0.355 mm. Preliminary studies using the waste treated with acid and base were carried out in order to optimize the adsorption of cadmium. Maximum adsorption was obtained by treating the waste with calcium hydroxide (Ca(OH)2) saturated solution at room temperature for 10 h. And the BF was gained after the materials were dried at 80 ℃.
The effect of pH on metal removal was carried out with 3 g/L of Ca(OH)2-treated BF in 100 mL solution of cadmium. The pH of the solution was adjusted with 0.1 mol/L HCl or NaOH solution. The mixture was agitated with a magnetic stirrer in a 100 mL Bunsen beaker at room temperature ((28±2) ℃). Each beaker was removed after the required reaction time and the solution was filtered through qualitative filter paper, and the supernatant was analyzed for its metal ions using an atomic absorption spectrophotometer (WFX 120). Controls were also performed with untreated BF.
All other experiments were run in the solution of pH 8. The adsorption isotherm for Ca(OH)2-treated BF was conducted with cadmium ion concentrations of 0.05-0.20 mmol/L. In the contact time experiment, samples were withdrawn at pre-determined intervals and Cd removal was analyzed.
Each removal experiment was run in triplicate and the mean values were reported.
3 Results and discussion
3.1 Effect of chemical treatment on adsorption
The effect of treatment of BF on the adsorption of Cd is listed in Table 1. Treatment with Ca(OH)2 greatly enhances metal adsorption whereas BF treated by HCl shows lower adsorption than the control material. Ref.[14] reported that sodium hydroxide treated waste products showed greater adsorption of Cd compared with the unmodified materials. Other work[15] also showed that NaOH treated soybean and cottonseed hulls improved adsorption capacity for Zn as compared with the untreated materials. However, in our study the BF treated by Ca(OH)2 has adsorption capacity for Cd greater than that treated by NaOH. It can be explained by an increase in the amount of hydroxyl functional groups.
Table 1 Adsorption of Cd with various chemically modified BF

3.2 Kinetic study
Effect of time on adsorption of Cd onto BF can be seen in Fig.1(a). An aqueous solution (100 mL) of 0.1 mmol/L Cd was contacted with 0.1, 0.2, 0.3 g of BF at room temperature. It reveals that the adsorption is rapid in the first 30 min and then slows down considerably as the reaction reaches the equilibrium. The adsorption rate is found to accelerate with an increase in the amount of BF.
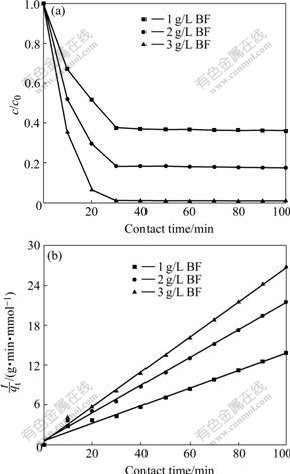
Fig.1 Time dependence of Cd ion adsorption onto BF: (a) Time course; (b) Kinetics curves (Cd initial concentration: 0.1 mmol/L; BF: 1, 2 and 3 g/L, respectively.)
Various adsorption kinetic equations have been used to describe the removal of metals. Recently, Ref. [16] reported that most of the adsorption systems followed a pseudo second-order kinetic equation that can be expressed as
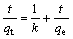 (1)
(1)
where t is the contact time (min); qt and qe are the quantities of sorbate sorbed at time t and equilibrium (mmol/g); k is the rate constant (mmol?g-1?min-1). Plots of t/qt vs t for Cd-BF systems are shown in Fig.1(b). It can be seen that the adsorption process for Cd on BF follows the pseudo second-order kinetics.
3.3 Effect of pH on Cd adsorption
pH is a key parameter, along with metal concentration and solution composition, in determining biosorption levels. The objective of this study was to determine the effects of pH (2-11) on removal levels. Fig.2 shows the effect of initial solution pH on the removal of Cd2+ by BF. The results show excellent removal capacities for Cd2+. The Cd2+ removal increases from 79.7% at pH 2 to 99.4% at pH 8. Then the removal keeps almost constant until pH 10. At higher values of pH cadmium ions precipitate Cd(OH)2[17]. And the Cd2+ shows a decrease in adsorption with pH less than 3 and greater than 10.
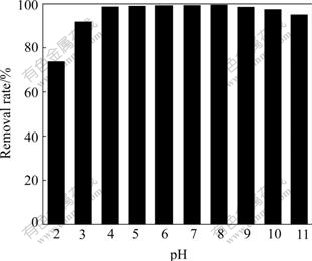
Fig.2 Removal rate of Cd2+ by BF with different pH values (Cd initial concentration: 0.1 mmol/L; BF: 3 g/L; Contact time: 30 min)
The dependence of metal removal rate on pH is related to both the surface functional groups present on the biomass and the metal chemistry in solution. At low pH, the surface ligands are closely associated with the hydronium ions (H3O+) and restrict the approach of metal cations as a result of the repulsive force[18-19]. Furthermore, the metal ions removal rate by BF dependent on pH can also be justified by the association-dissociation of certain functional groups, such as the carboxyl and hydroxyl groups present on the biomass. In fact, it was known that at low pH value, most of the carboxyl groups were not dissociated and could not bind the metal ions in solution, although they took part in complexation reactions[20]. Based on these results, the following experiments were performed at pH 8.
3.4 Adsorption capacity
In order to investigate the adsorption capacity, the adsorbent BF concentration of 3 g/L was agitated with the aqueous solution of Cd varied from 0.05-0.20 mmol/L at room temperature for 30 min. After removal of the adsorbent, the Cd2+ concentration was determined. It is found from the results that the adsorption rate decreases with the increase of the Cd2+ concentration. The data for the Cd2+ concentration of 0.05-0.20 mmol/L were analyzed in the light of the Freundlich mode of adsorption. The Freundlich isotherm was tested with the following linear form:
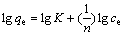 (2)
(2)
where qe is the amount of Cd2+ adsorbed at equilibrium (mmol/g); ce is the equilibrium concentration of Cd2+ in solution (mmol/L); K and 1/n are the Freundlich constants.
As shown in Fig.3, with very high regression correlation coefficients (0.999 9), the plot of lgqe vs lg ce gives straight lines for BF, which supports the applicability of the Freundlich isotherm model to the present study. The values of Freundlich constants K and 1/n represent the adsorption capacity (mmol/g) and adsorption intensity of these sorbents, respectively. The constants K and 1/n are evaluated from the intercept and the slope of the straight lines using a least-square fit program and are found to be 0.328 mmol/g and 0.997 for BF.
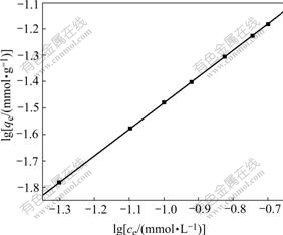
Fig.3 Adsorption capacity of Cd2+ by BF (pH value: 8; Cd initial concentration: 0.05-0.20 mmol/L; BF: 3 g/L; Contact time: 30min)
3.5 Influence of coexisting cations upon adsorption
Fig.4 shows the influence of coexisting cations upon the adsorption capacity of Cd, which was tested by batch operation at various initial molar ratios of these cations to Cd ions. All Cd2+ solutions with initial concentrations of 0.1 mmol/L were used. The initial molar ratio varies between 0 (no coexisting cations) and 20. In the binary metal solutions, lead ion dominates the competitive binding. The addition of zinc, copper ions hardly affects the Cd adsorption.
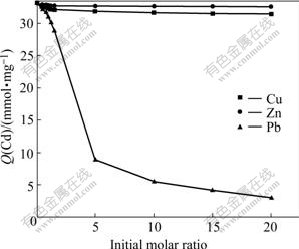
Fig.4 Cd adsorption capacity Q(Cd) at different initial molar ratios of coexisting cations to Cd ion by BF (Coexisting cations: Pb, Zn, Cu; pH: 8; BF: 3 g/L; Contact time: 30 min)
3.6 Desorption of Cd and regeneration of BF
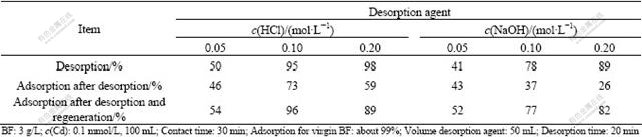
As shown in Fig.2, low adsorption for Cd is presented in extremely pH range especially in strong acidic pH, which implies that adsorbed Cd ion can be desorbed from BF by both acidic and alkaline media. Therefore, desorption tests were carried out using sodium hydroxide and hydrochloride acid solution in batch operation. As demonstrated in Table 2, 0.1 mol/L HCl appears to be the most suitable desorption agent. After desorption operation, the adsorption rate of Cd onto the BF is reduced to about 73%. However, the adsorption capacity of BF is favorably restored to 96% by regeneration operation, that is, it is loaded again with Ca(OH)2 saturated solution.
Table 2 Desorption and regeneration of BF
3.7 Adsorption mechanism study
The Fourier transform infrared spectroscopy(FTIR) has been considered as a kind of direct means for investigating mechanisms of metals adsorption on biomass. FTIR of BF unmodified, modified by Ca(OH)2and after Cd binding adsorption at pH 8 are shown in Fig.5. The absorption at 3 700-3 000 cm-1, found in the three spectra, is —OH associated with hydrogen bond stretch vibration or amide, but the three adsorption bands are all broad, which is not accord with the rule of adsorption peak amide[21-22]. On the other hand, the —OH associated with hydrogen bond stretch vibration in the three spectra indicates that —OH is always on the BF, so the adsorption mechanism may be cation exchange effect.
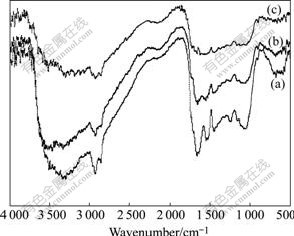
Fig.5 FTIR spectra of unmodified BF (a), BF modified by Ca(OH)2 (b) and modified BF after Cd adsorption (c)
From the curves (b) and (c) of Fig.5, the wave number shifting from 3 310 cm-1 to 3 440 cm-1 implies that the hydroxyl group maybe changes from high aggregate to low one. This illustrates that the adsorption of Cd lowers the aggregate of hydroxyl group, which maybe supplies more opportunities for Cd2+ to bond with hydroxyl group.
In order to understand the cadmium trend of adsorption on BF, consideration of the types of associations among adsorbing metal ions and Ca—OH functional groups may be helpfu1.
Ca—OH+Mn+→M—OH(n-2)++Ca2+ (3)
Heavy metal cations Mn+ may also hydrolyze in aqueous solution and adsorb in a hydrolyzed form according to the following reaction:
Mn++H2O→M—OH(n-1)++H+(Hydrolysis) (4)
And from Fig.4, it can be seen that the sorption of lead is superior to that of cadmium. The reason of higher value for Pb2+ is that BF adsorbs it a bit strongly as Pb2+ can easily be hydrolyzed in water[23], which in turn favors its chemisorptions on BF. It is, therefore, can be deduced that adsorption and hydrolysis are somewhat correlated. If the heavy metal ion is easily hydrolysable, its sorption on BF is relatively higher as that in the case of Pb2+.
Spectral energy distributions of the modified BF before and after Cd adsorption are shown in Fig.6. By comparison of the two figures, the concentration of calcium and magnesium falls after Cd adsorption. The adsorption peaks of the two substances are almost absence, and a peak of cadmium appears. From this, the cation-exchange adsorption occurs at the adsorption process of Cd on BF. That is also confirmed by Eqn.(3).
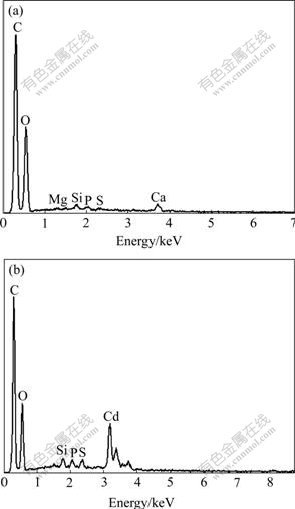
Fig.6 Spectral energy distributions (Accelerating voltage: 20 kV; Light spot: 52; Working range: 11 mm.): (a) Modified BF before Cd adsorption; (b) Modified BF after Cd adsorption
4 Conclusions
1) BF is an effective adsorbent for the removal of Cd(Ⅱ) from aqueous systems like drinking water. Cd(Ⅱ) ion adsorption on BF follows pseudo second-order kinetics and the adsorption mode is of a Freundlich isothermal nature. The adsorption of Cd(Ⅱ) is just slightly dependent on the initial pH over a wide range (4-11) and the BF can be directly and effectively employed to common water systems without pH adjustment.
2) BF presents a quite high Cd(Ⅱ) adsorption ability, and seems to be especially suitable for removal of Cd(Ⅱ) in low concentration. BF is quite selective towards Cd(Ⅱ). The coexisting cations, except lead, hardly interfere with the adsorption of Cd(Ⅱ) ions. The adsorbed Cd(Ⅱ) ions are effectively desorbed by a 0.1 mol/L HCl solution.
3) In order to realize its full potential as a commercial sorbent, removal of metals under continuous condition with industrial wastewater containing such toxic metals must be evaluated. Currently such an investigation is being undertaken.
References
[1] NRIAGU J O. A silent epidemic of environmental metal poisoning [J]. Environ Pollution, 1988, 50: 139-161.
[2] FORSTNER V, WITTMAN G T W. Metal pollution in the aquatic environment [J]. Springer-Verlag, Heidelberg, 1981, 2(5): 126-132.
[3] DRASH G A. Increase of cadmium body burden for this century [J]. Sci Total Environ, 1993, 67: 75-89.
[4] FRIBERG L, PISCAOR M, NORDBERG G F, KJELLSTROM T. Cadmium in the environment (2nd Ed) [M]. Cleveland, OH: CRC Press, 1974.
[5] KRISHNAN E R, UTRECHT P W, PATKAR A N, DAVIS J S, POUR S G, FOERST M E. Recovery of metals from sludges and wastewaters [M]. NJ: Noyes Data Corporation, 1992.
[6] CHONG K H, VOLESKY B. Description of two metal biosorption equilibria by Langmuir type models [J]. Biotechnol, 1995, 47: 451-460.
[7] WILDE E W, BENEMANN J R. Bio-removal of heavy metals by the use of micro-algae [J]. Biotech Adv, 1993, 11: 781-812.
[8] WILLIAMS C J, ADERHOLD D, EDYVEAN R G J. Comparison between biosorbents for the removal of metal ions from aqueous solutions [J]. Water Res, 1998, 32: 216-224.
[9] GADD G M, WHITE C. Microbial treatment of pollution [J]. Biotechnol Tech, 1993, 11: 353-360.
[10] LU Y, WILKINS E. Heavy metal removal by caustic treated yeast immobilized in alginate [J]. Hazard Mater, 1996, 49: 165-179.
[11] KUMAR P, DARA S. Utilization of agricultural wastes for decontaminating industrial/domestic wastewaters from toxic metals [J]. Agric Wastes, 1982, 4: 213-223.
[12] BRADY D, STOLL A, DUNCAN J R. Biosorption of heavy metal cations by nonviable biomass [J]. Environ Technol, 1994, 15: 429-438.
[13] DRONNET V M, RENARD C M. Binding of divalent metal cations by sugar beer pulp [J]. Carbohydr Polym, 1997, 34: 73-82.
[14] AZAB M S, PETERSEN P J. The removal of cadmium from water by the use of biological sorbents [J]. Water Sci Technol, 1989, 21: 170-176.
[15] MARSHALL W E, JOHNS M M. Agricultural by-products as metal adsorbents: Sorption properties and resistance to mechanical abrasion [J]. Chem Tech Biotechnol, 1996, 66: 192-198.
[16] HO Y S, MCKAY G. Pseudo second-order model for sorption process [J]. Process Biochem, 1999, 34: 451-465.
[17] SCORZELLI I B. Removal of cadmium and zinc from very dilute solutions by ion flotation [D]. Brazil: Catholic University of Rio de Janeiro, 1999.
[18] AKSU Z. Equilibrium and kinetic modelling of cadmium(II) biosorption by C. vulgaris in a batch system: Effect of temperature [J]. Separation and Purification Technology, 2001, 21: 285-294.
[19] SHENG P X, TING Y P, CHEN J P, HONG L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms [J]. Journal of Colloid and Interface Science, 2004, 275: 131-141.
[20] CHUBAR N, CARVALHO J R, NEIVA M J. Cork biomass as biosorbent for Cu(II), Zn(II) and Ni(II) [J]. Colloids and Surfaces B: Biointerfaces, 2004, 230: 57-65.
[21] KAPOOR A, VIRARAGHAVAN T. Heavy metal biosorption sites in Aspergillus Niger [J]. Bioresource Technology, 1997, 61: 221-227.
[22] GADD G M, LIN S, RAYSON G D. Chemical modification and metal binding studies of datura innoxia [J]. Environ Sci Technol, 1996, 30: 110-114.
[23] MURRAY B M. Environmental chemistry of soils [M]. UK: Oxford University Press, 1994.
Foundation item: Projects(20477059, 50508044) supported by the National Natural Science Foundation of China; Project(05SK1003-1) supported by Key Program of Science and Technology Plan of Hunan Province, China
Corresponding author: CHEN Yun-nen; Tel: +86-797-8312140; E-mail: cyn70yellow@yahoo.com.cn
(Edited by LI Xiang-qun)