J. Cent. South Univ. Technol. (2011) 18: 1408-1412
DOI: 10.1007/s11771-011-0854-8
Heavy metals accumulation effect in rabbit body fluids after smoking
ZHONG Kuang-biao(钟狂飚)1, GUI Ming(桂明)1, ZHU Li-yong(朱立勇)1,
LI Wei(李维)1, GUAN Cha-xiang(管茶香)1, GUO Fang-qiu(郭方遒)2
1. Urology Surgery of The Third Xiangya Hospital, Central South University, Changsha 410083, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract: Concentration of heavy metals in blood and urine of rabbit after inhaling three different kinds of cigarette was studied through the animal passive smoking pattern. The samples were prepared by nitric acid solution digestion and determination of seven kinds of heavy metals including Hg, Se, Sn, Pb, Cd, Ni and Cr was performed by inductively coupled plasma-atomic emission spectrometry (ICP-AES). The ICP-AES method was established with good precision and accuracy, relative standard deviation (n=6) was between 2.9% and 5.9%, and the recovery was in the range of 95.0%-104.2%. Concentration of six heavy metals increases in some extent in blood and urine after period of smoking and the increasing of heavy metals in blood and urine all shows time dependence. Significantly higher heavy metal levels are observed in the blood and urine of the cigarette inhaling rabbits in the exposed group. The concentration of six kinds of heavy metals in the blood of the rabbit increases after 16 weeks exposing to cigarette smoking. Three times of Hg, ten times of Se and trace amount of Pb, Cd, Ni and Cr are detected in the blood after 16 weeks of smoking. For urine samples, about three times of Hg, two times of Se, five times of Pb and trace amount of Cd are detected after 16 weeks of inhalation of cigarette. Comparatively, higher concentration of heavy metals are detected after inhaling of Nise cigarette.
Key words: inductively coupled plasma-atomic emission spectrometry; blood; urine; heavy metal element
1 Introduction
Tobacco smoking is a worldwide problem with 1.3 billion people currently smoking cigarettes and one person in every six dies of tobacco related illnesses [1]. Smoking has been linked to cancers of various organ systems, non-cancer lung disease and heart disease. These diseases result from the biological impact of the inhalation of a concentrated mixture of chemical substances that are generated by pyrosynthesis, direct transport or distillation of the chemical components from tobacco. Heavy metals are presented in tobacco smoke and have long been associated with various diseases. Many heavy metals, including cadmium, lead and thallium, are efficiently extracted from the soil where tobacco plants. Researchers have determined heavy metal of lead, cadmium and manganese in the blood of volunteers for years, and the results showed that smoking can aggravate accumulation of these heavy metals in blood, and these heavy metals can damage and affect the amendment and recovery of damaged DNA in cell [2]. Tobacco is a rich source of toxic heavy metals as metals get preferentially enriched in the tobacco leaves during plant growth [3]. Cut tobacco absorbs cadmium salt from soil, which leads to the content of cadmium about 3-4 times higher than the allowable content [4-5]. In general, tobacco plants accumulate heavy metals like Pb, Cd, and Zn preferentially [6]. Tobacco smoking is one of the significant sources of toxic metals in both human body and environment and smoking has apparent effect on elements as copper, zinc, iron, lead and cadmium in blood. It is reported that the concentration of cadmium in the blood of smoker increases obviously than non-smoker, and has positive correlation with the amount of smoking [7]. Metal ion, especially nickel and cadmium, can accumulate in kidney organ. Analysis of heavy metal in body fluids such as blood and urine has already applied clinically to get information about toxicity of heavy metal, professions, etc. but there are few researches about the relation between accumulation of heavy metal in blood and urine of animal and tobacco smoking. This work focused on the research of accumulating effect of heavy metals related to cigarette exposing.
There are a variety of instrumental methods for accurate analysis of trace metal element, such as atomic absorption spectrometry [8], X-ray fluorescence spectrometry [9] and inductively coupled plasma mass spectrometry [10]. Compared to the classical flame, arc spark spectrum analysis, atomic absorption method and inductively coupled plasma-atomic emission spectrometry (ICP-AES) have been established as powerful analytical techniques with 4-5 wider linear range, high sensitivity, small matrix effect, quick analysis rate and can provide the ability of simultaneous multi-elemental analysis [11-15]. It has been widely used in the determination of trace elements of biological samples. In this work, the content of several kinds of heavy metal elements in rabbit blood and urine before and after smoking were determined with the help of ICP-AES, and the accumulation effect of heavy metal elements in the body fluid through smoking was also discussed. The results are useful for the further biological mechanisms of injury of these heavy metals to the health of animals.
2 Experimental
2.1 Reagents and standard solutions
All reagents were of analytical grade unless otherwise stated. Double distilled water was used in the preparation of the solutions. Nitric acid and hydrochloric acid were of ultrapure quality (Merck). The laboratory glassware was kept overnight in a 5% nitric acid solution. Before use, the glassware was washed with deionized water and dried in a dust-free environment. The concentrations of Hg, Cr, Pb, Ni, As and Cd of mixed standard stock solution purchased from National Standard Material Center were all 100 μg/mL.
2.2 Instrument
OPTIMA 5300DV inductively coupled plasma atomic emission spectrometer (ICP-AES, PerkinElmer Corporation of USA), solid-state CCD detector projection optical system, RYTON material atomizer, 40.68 MHz solid-state high-frequency self-excited generator and MAS microwave digestion system (CEM Corporation of USA) were employed in the present work. The ICP-AES operating parameters were listed in Table 1.
Table 1 ICP-AES operating parameters

2.3 Standard curve and detection limit
The standard stock solution was diluted with 5% nitric acid step by step, and mixed standard solutions of Hg, Cr, Ni, Sn, Se, Cd and Pb were prepared by the gradient of 0, 0.01, 0.05, 0.10, 0.50, 1.00 and 2.00 μg/mL. Standard curves were obtained with standard solution and light intensity. Reagent blank was used to get the detection limit.
2.4 Animal, smoke generation and exposure
Common grade experimental rabbits were purchased from experimental animal feed factory of Ningxiang, Hunan Province, China, with body mass ranging from 1 500 g to 2 000 g. They were raised for 20 d before smoking intervene experiment. Rabbits were randomly divided into three experimental and control groups. The rabbits were housed in stainless steel wire cages except during smoke exposure and fed with standard rabbit food. Three brands of cigarette, which were Baisha group, Nise group and fined Baisha group, were used in the study. Each experimental group of rabbits (n=6) was exposed to cigarette smoke for 2 h each day over 16 consecutive weeks using a smoking machine. The controlled animals (n=6) were restrained in identical chambers but only exposed to room air.
The smoke exposure system consists of three glass chambers, fans, and pumps. Centrifugal fans are attached to all chambers of the smoking machine. Pumps were used to push the air through the burning cigarette into the generation chamber and then to the dilution and exposure chambers respectively. One volume of cigarette smoke was mixed with nine volumes of room air in the dilution chamber using rotating fans. During smoke exposure, rabbits were placed in the exposure chamber and inhaled 10% of the cigarette smoke. Each filter cigarette was burned for approximately 8-10 min and a total number of 12-15 cigarettes were used every day. Immediately after exposure, a number of experimental and controlled animals were anaesthetised with ether and cardiac blood samples and urine samples were collected into heparinized tubes.
2.5 Sample preparation
Aliquots of blood and urine were taken and treated with 67% ultrapure nitric acid in 1:3 (volume ratio) dilution for 24 h and centrifuged at 4 000 r/min for 15 min, then the supernatants were taken to be determined by ICP-AES.
3 Results and discussion
3.1 Detection of wavelength and detection limit
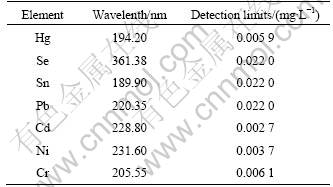
The spectral lines, which have little spectral interference and high precision, were selected to detect the wavelength and detection limit, as shown in Table 2.
Table 2 Elemental analysis of wavelength and detection limits (n=6)
3.2 Standard curves, precision and recovery test
Each element in standard curves showed favorable linearity between the concentration of 0 and 2.0 μg/mL, and the correlation coefficients were greater than 0.999. To ensure the precision of the experiment, the urine samples after 16 weeks smoking of fined Baisha cigarette were selected to detect the precision and recovery with six parallel samples. The relative standard deviations (RSD) range from 2.9% to 5.9%, and the recoveries range from 95.5% to 105.5%, as listed in Table 3, which proved this method was accurate and precise.
3.3 Heavy metals in blood and urine after period of smoking
Determination of the heavy metals in blood and urine of rabbits in the four groups after 1 week, 8 weeks and 16 weeks was carried out by the previous developed and validated methods, and the results were shown in Table 4. Concentrations of seven heavy metals except Sn increased in some extent in blood and urine after period of smoking. The increasing of heavy metals in blood and urine all showed time dependence. For the blood samples, Pb, Cd, Ni and Cr were not detected in blank samples, but three times of Hg, ten times of Se and trace amount of Pb, Cd, Ni and Cr were detected in the blood after 16 weeks of smoking. For urine samples, about three times of Hg, two times of Se, five times of Pb and trace amount of Cd were detected after 16 weeks of inhalation of cigarette. The amount of Ni and Cr kept almost the same after 16 weeks Baisha and fined Baisha inhaling, but for the Nise cigarette, the amounts of Ni and Cr were doubled.
Three different kinds of cigarettes were also investigated, and the results were shown in Fig.1 and Fig.2. From Fig.1, it can be seen that the concentration of Hg, Sn, Cd, Ni and Cr increased through inhaling of three kinds of cigarettes, especially, Hg, Sn and Cr increased significantly after taking the Nise cigarette. The similar result was obtained from the urine samples. The concentration of Pb in blood and urine showed different distribution. Pb was not detected in blood because of the detection limit, but it showed high concentration in urine. It is reported that Pb can be absorbed through respiratory and gastrointestinal tract, and lead poisoning is mainly from respiratory absorption. The result also indicated that Pb can be accumulated in kidney and excreted from urine. Seven kinds of heavy metals can all be accumulated in kidney, and excreted through urine over time. The excretion of Cd is very slow and the biological half-life is longer than 15 a, so it showed lower concentration in urine.
The concentration of heavy metals in blood and urine through period of inhaling of three different brands of cigarettes was also compared. They all showed the same trend of accumulation but with different amounts. The concentration of heavy metals after inhaling Baisha and fined Baisha showed the same distribution. The concentration of seven metals was higher after inhaling of Nise cigarette than the other two brands of cigarettes except Se in blood. The concentrations of Hg, Se, Sn, Pb, Cd, Ni and Cr reached to 0.121, 0.115, 0.032, 0, 0.012, 0.023 and 0.044 μg/mL in blood, and 0.904, 1.060 3, 0.804, 0.698, 0.034. 0.220 and 0.104 μg/mL in urine, respectively. Compared to the blank group, the variations of Sn, Ni and Cr were smaller than other metals, which indicated that lower concentrations of these three heavy metals existed in the smoke of the three cigarettes, especially in Baisha and fined Baisha.
In order to demonstrate whether the accumulation of heavy metal is from smoking or from other kind of sources, a research report is cited in this work which was done by some researchers from USA and Canada. Concentration of heavy metals of almost all common brands cigarette of China market was detected for three sequential years. The results showed that the average content of As is 0.82 μg/g (standard value 0.3-3.3), Cd is 3.21 μg/g (2.0-5.4), Pb is 2.65 μg/g (1.2-6.5) and Cr is 0.55 μg/g (0.0-1.0), respectively. It is obvious that higher concentration of heavy metals in cigarette leads to accumulation of heavy metals in the body fluid of animals and organs.
Table 3 Precision and recovery of present method (n=6)
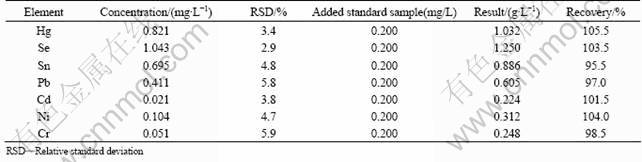
Table 4 Concentrations of metal ions in blood and urine of rabbit
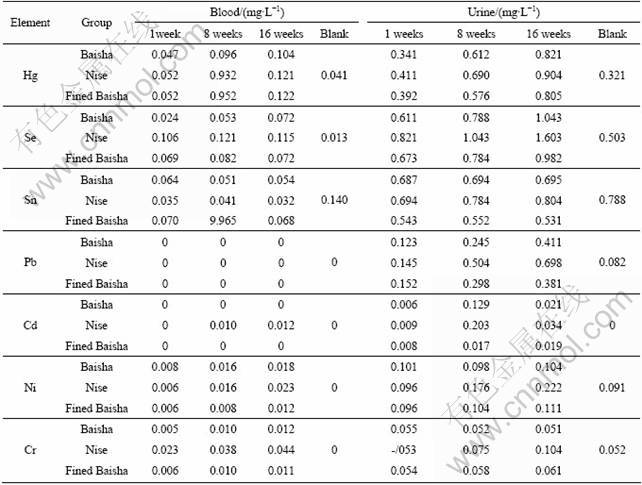
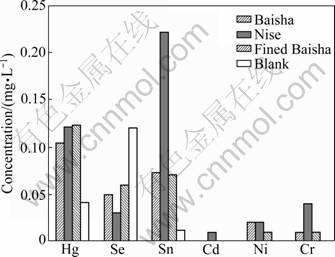
Fig.1 Concentrations of heavy metal elements in blood of rabbit after smoking different kinds of cigarettes
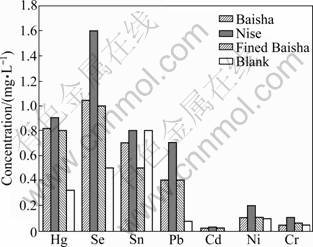
Fig.2 Concentrations of heavy metal elements in urine of rabbit after smoking different kinds of cigarettes
4 Conclusions
1) The method of determination of heavy metals in the blood and urine of rabbit through ICP-AES is established. The established method shows good precision and accuracy and low detection limit. It is proved to be an efficient tool for the determination of trace amount of heavy metals in the body fluids of animals.
2) Concentration of six heavy metals increases in some extent in blood and urine after period of smoking and the increasing of heavy metals in blood and urine all shows time dependence.
3) The concentration of six kinds of heavy metals in the blood of rabbit increases after 16 weeks exposing to cigarette smoking. Three times of Hg, ten times of Se and trace amount of Pb, Cd, Ni and Cr are detected in the blood after 16 weeks of smoking. For urine samples, about three times of Hg, two times of Se, five times of Pb and trace amount of Cd are detected after 16 weeks of inhalation of cigarettes, comparatively, higher concentration of heavy metals is detected after inhaling of Nise cigarette.
4) Cigarette smoke exposing to animal leads to higher concentration of heavy metals in blood and urine, which is potentially harmful to health. However, more research is needed to ascertain biological mechanisms of injury of these heavy metals to the health of animals.
References
[1] Geneva: World Health Organization [EB/OL]. 2008-04-04. http:// www.who.int/dietphysicalactivity/publications/facts/chronic/en.
[2] XI Zhu-ge, CHAO Fu-huan, SUN Yong-mei, YANG Feng-dan, ZHANG Hua-shan, LI Guan-xian, LI Yuan. Study on the mechanism of oxidative damage of DNA induced by reactive oxygen species due to metal ions [J]. Acta Scientiae Circumstantiae, 2003, 23(5): 662-667.
[3] SCHNEIDER G, KRIVAN V. Multi-elemental analysis of tobacco and smoke condensate by instrumental neutron activation analysis and atomic absorption spectrometry [J]. Int J Environ Anal Chem, 1993, 53(2): 87-100.
[4] ZHU Zhi-guo, WANG Gui-xian, CHEN Jing-hua. Determination of potassium, calcium, manganese, chromium and cadmium in 10 kinds of cigarettes by flame atomic absorption spectrophotometer [J]. Spectroscopy and Spectral Analysis, 1999, 19(2): 210-211.
[5] VERMA S, YADAV S, SINGH I. Trace metal concentration in different Indian tobacco products and related health implications [J]. Food and Chemical Toxicology, 2010, 48(8/9): 2291-2297.
[6] ANGELOVA V, IVANOV K, IVANOVA R. Effect of chemical forms of lead, cadmium and zinc in polluted soils on their uptake by tobacco [J]. Journal of Plant Nutrition, 2004, 27(5): 757-773.
[7] QIU Xiao-hui, WEI De-ren. Effect of content of metal elements in blood serum [J]. Journal of Public Health, 1994, 10(1): 20-24.
[8] GUPTA J P. Determination of yttrium and rare-earth elements in rocks by graphite-furnace atomic-absorption spectrometry [J]. Talanta, 1981, 28(1): 31-36.
[9] HAVEL J, MORENO C, HRDLICKA A, VALIENTE M. Spectrophotometric determination of rare earth elements by flow injection analysis based on their reaction with xylenol orange and cetylpyridinium bromide [J]. Talanta, 1994, 41(8): 1251-1254.
[10] BRAVERMAN D S. Determination of rare earth elements by liquid chromatographic separation using inductively coupled plasma mass spectrometric detection [J]. J Anal At Spectrom, 1992, 7(1): 43-46.
[11] WANG Xiao-yan, LI Yu-feng, LI Bai. Fast determination of heavy metals in human blood and urine samples by ICP-MS after simple dilution [J]. Chinese Journal of Analysis Laboratory, 2010, 29(6): 41-45.
[12] CROUDACE I W, MARSHALL S. Determination of rare earth elements and yttrium in nine geochemical reference samples using a novel group separation procedure involving mixed-acid elution ion-exchange chromatography [J]. Geostand Newsl, 1991, 15(1): 139-145.
[13] GUPTA J P. Analysis of the CCRMP Oka-2 rare-earth reference mineral of the britholite-apatite series by electrothermal atomic- absorption and inductively-coupled plasma atomicemission spectrometry [J]. Talanta, 1987, 34(12): 1043-1047.
[14] EID M A, BROEKAERT J A C, TSCH?PEL P. Application of ICPAES to the determination of rare earth elements in phosphate samples [J]. Fresenius J Anal Chem, 1992, 342(1/2): 107-112.
[15] WALSH J N, BUCKLEY F, BARKER J. The simultaneous determination of the rare-earth elements in rocks using inductively coupled plasma source spectrometry [J]. Chem Geol, 1981, 33(1/2/3/4): 141-153.
(Edited by HE Yun-bin)
Foundation item: Project(11JJ5053) supported by the Provincial Natural Science Foundation of Hunan Province, China
Received date: 2011-06-02; Accepted date: 2011-08-08
Corresponding author: GUI Ming, Associate Professor, PhD; Tel: +86-731-88618577; E-mail: zkbgm@126.com