
Application of cetylpyridinium chloride in dispersion of antibacterial ceramic glaze
CHAI Li-yuan(柴立元), CHENG Ming-ming(程明明),
PENG Bing(彭 兵), HUANG Yi(黄 毅), ZHANG Xiao-fei(张晓飞)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 21 April 2006; accepted 20 August 2006
Abstract: The adsorption of cetylpyridinium chloride(CPC) onto a ceramic glaze mixture composed of limestone, feldspar, quartz, kaolin, and antibacterial agent was studied. Both adsorption isothermals and the average particle zeta potential were investigated in order to understand the suspension stability as a function of pH, ionic strength, and surfactant concentration. The results show that under the optimal conditions such as pH value of 7 or 9, 0.01 mol/L of ionic strength and around critical micelle concentration(CMC) of surfactant, antibacterial ceramic glaze acquires fine dispersion stability. The adsorption of CPC on ceramic glaze particles is in accordance with Langmuir model in 0.01mol/L at pH=7 and pH=9. The adsorption of small amounts of cationic CPC onto the primarily negatively charged surfaces of the particles in the pH range of 7-9 produces strong attraction and flocculation due to hydrophobic interactions. High concentration of surfactant under 0.01 mol/L of salt provides high zeta potential, which produces a high stability.
Key words: surfactant; antibacterial glaze; adsorption isotheral; dispersion stability; zeta potential
1 Introduction
The adsorption of soluble surfactants to the solid/aqueous solution interface is a major factor in many processes including ceramics processing, waste water treatment, mineral flotation, dispersion stabilization and soil remediation[1,2]. Most of the solids involved in these processes have heterogeneous surfaces (including coexisting polar and nonpolar sites), and their interfacial properties depend on the history of their exposure to various species. The adsorption of surfactant onto the surface of these solids may be the result of several contributing forces, including covalent bonding, Coulombic interactions, ion exchange, hydrogen bonding, hydrophobic interactions, and van der Waals interactions [3].
The major components of the antibacterial ceramic glaze are limestone(CaCO3), feldspar(Al2O3?2SiO2?2H2O) quartz(SiO2), kaolin(KNaO·Al2O3·6SiO2) and antibacterial agent (nano-TiO2). The dispersion stability of the glaze mixture is important to control the various stages of the glazing process and maintain antibacterial activity[4]. The addition of a surfactant as a deflocculant can be used to stabilize a ceramic glaze suspension by keeping the particles well dispersed. In many industrial glazing processes the pH and ionic strength of the ceramic glaze suspensions are not controlled.
In previous studies, the effect of pH, ionic strength, and surfactant concentration on the adsorption of surfactants has only been studied on the single mineral ceramic glaze components, such as quart[5-7], feldspar [8], kaolin[9,10], and limestone[11]. In this study, the cationic surfactant cetylpyridinium chloride is adsorbed on the surface of the antibacterial ceramic glaze mixture. Adsorption isotherms, zeta potential measurements, and assessment of dispersion stability are used to illuminate the adsorption behaviour at various pH values, ionic strengths, and surfactant concentrations. The influence of the adsorbed surfactant on the stability of the ceramic glaze dispersion is then discussed. According to the interaction between surfactant and surface of particles, mechanism of the adsorption behaviour of CPC on the surface of the glaze is also analyzed.
2 Experimental
2.1 Materials
Ceramic glaze was supplied by Taixin Ceramics Co Ltd(Hunan). The average particle size of ceramic glaze was 10-30 mm. Antibacterial agent (nano-TiO2) was supplied by Degussa Co Ltd(Germany). The BET surface area was measured to be (50±15) m2/g by N2 adsorption. The average particle size of the antibacterial agent was 50 nm. The final composition of the mixture glaze and the isoelectric point (IEP) of each component are presented in Table 1. The cationic surfactant cetyl- pyridinium chloride (≥99%), NaCl, HCl, and NaOH were AR grade. Water was ultrapure water (R≥15).
Table 1 Composition and isoelectric point of antibacterial ceramic glaze

2.2 Methods
Adsorption isotherms were measured at 30 ℃ using the depletion method. To prepare the antibacterial ceramic glaze suspension, 0.20 g ceramic glaze and 0.01 g antibacterial agent were added to a 20 mL solution with desired ionic strength and pH inside a polycarbonate centrifuge tube. This suspension was sonicated for 5 min. The pH was adjusted if necessary using 0.01 or 0.1 mol/L HCl or NaOH. The mixtures were then vibrated for 24 h. The final pH was close to the initial pH, so no further adjustment was necessary after this equilibration. The solutions were centrifuged at 6 000 r/min for 20 min until a constant surfactant solution concentration was attained. The clear supernatant was removed for determination of the equilibrium surfactant concentration by UV-Vis spectrophotometry (U2010, Hitachi, Japan). The UV absorption of the cetylpyridinium ion in the supernatant was measured at 245 nm. A standard calibration series of known supernatant concentrations (at the necessary pH and NaCl concentration) were prepared for each experiment to permit the supernatant concentration to be determined. The calibration curve of CPC is shown in Fig.1. The amount of surfactant adsorbed was calculated with following equation:
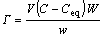 (1)
(1)
where G is the amount of surfactant adsorbed; Ceq and C are equilibrium concentration and initial concentration of surfactant respectively; V is volume of the glaze; w is mass of the glaze; and W is the relative molecular mass of the surfactant.
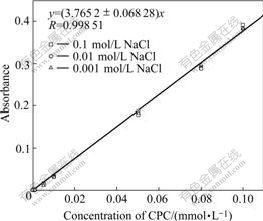
Fig.1 Calibration curve of CPC
An assessment of the stability of the antibacterial ceramic glaze dispersions was made by using the same adsorption isotherm samples and UV-Vis spectrophotometer. After equilibration, the dispersions were allowed to stand without disturbance for 2 h before measurement of the transmission of 500 nm light through the sample. The degree of dispersion of the ceramic particles was evaluated by the absorbance of the incident light by the top portion (clear region within 1 cm from the top of the sample in polycarbonate tube) of each aqueous suspension. A high absorbance indicated stability, whereas a low value indicated a flocculated or settled state.
Dispersion stability of antibacterial ceramic glaze depends on the zeta potential of the particles. The higher the potential with the same polarity, the more important the electrostatic repulsion between particles. On the other hand, when closing to the isoelectric point (z=0), the particles tend to flocculate, as illustrated in Fig.2. Zeta potential measurements were performed using Coulter Delsa Zeta (America).
3 Results and discussion
3.1 Adsorption isotherms
The adsorption isotherms for the cationic surfactant, CPC, on the antibacterial ceramic glaze particles as a function of ionic strength at pH values of 7 and 9 are shown in Fig.3. The adsorbed amount of CPC is low and increases slightly from 0.001 mmol/L to 0.1 mmol/L CPC. When the concentration of CPC is between 0.1 mmol/L and 1 mmol/L, the adsorbed amount of CPC increases dramatically and the isotherms reach a plateau with the adsorbed amount at a concentration that corresponds to the surfactant CMC[16] (the critical micelle concentration) in the presence of added salt. The arrows designate the CMC of CPC in 0.1, 0.01, 0.001 mol/L NaCl from left to right, respectively. The isotherms exhibit similar trends at values of pH 7 and 9. The maximum adsorbed amount of CPC increases slightly from pH=7-9. This attributes principally to the calcium carbonate (limestone) component, which is negatively charged at pH value of 9 but positively charged at pH value of 7 (see Table 1). As the pH is increased, the number of negative sites on the surface of kaolin particles also increases. Therefore there is an increase in the adsorption of the CPC on kaolin at high pH value. For quartz, feldspar and titanium oxide, the negative charge is similar at pH values of 7 and 9, so the adsorbed amount on these components will be similar in both cases.
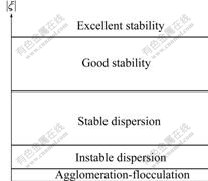
Fig.2 Stability of suspension versus zeta potential
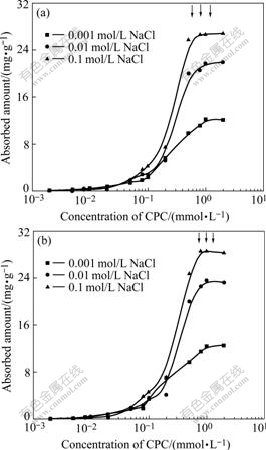
Fig.3 Adsorption isotherms of CPC on ceramic glaze: (a) pH=7; (b) pH=9
The adsorbed amount increases with added electrolyte at pH=7 and pH=9 under fixed surfactant concentration. Added electrolyte changes the initial adsorption to low equilibrium surfactant concentration.
3.2 Dispersion stability of antibacterial ceramic glaze with adsorbed surfactant
The dispersion stability of the antibacterial ceramic glaze in the presence of CPC as function of ionic strength at pH values of 7 and 9 are shown in Fig.4. Similar stability behaviour is observed at both pH values. The stability decreases with increasing electrolyte. In the presence of the low concentrations of CPC, flocculation occurs rapidly. The suspensions are completely stabilized and no flocculation occurs in the presence of the maximum adsorbed amount of CPC in 0.01 m/L NaCl. It is interesting that at high ionic strength (0.1 m/L NaCl) and low ionic strength (0.001 m/L NaCl) the dispersion stability slightly increases, yet is still poor at the maximum adsorption of CPC. Therefore the dispersion in 0.1 m/L NaCl could not be made stable by the addition of CPC, although the adsorbed amount of CPC is greater than that of lower salt concentrations.
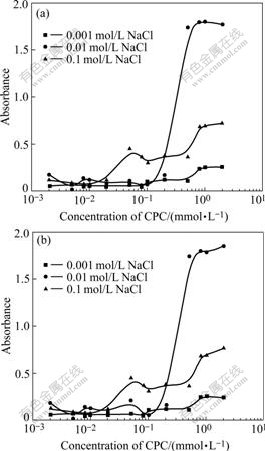
Fig.4 Stability of settled ceramic glaze suspensions adsorbed by CPC after 2 h: (a) pH=7; (b) pH=9
Fig.5 shows the absorbance of the antibacterial ceramic glaze suspension as a function of the time at different ionic strengths at pH=7. At pH=9 the results are similar. At low concentrations of initially added without CPC (0.1 mmol/L), the absorbance of the suspension decreases more rapidly than that without CPC. Compared with the absence of CPC, the suspensions are even less stable when a small amount of CPC is added. At high concentrations of initially added CPC (1 mmol/L), there is no change on the absorbance of the antibacterial ceramic glaze. Therefore the dispersions are more stable than the case of the absence of CPC.
Fig.6 gives the SEM images of surface morphology of ceramic glaze. In Fig.6(a) the size of the particles is much larger than that in Fig.6(b). It can be seen from Fig.6(b) that the bright spots are well dispersed in antibacterial ceramic glaze.
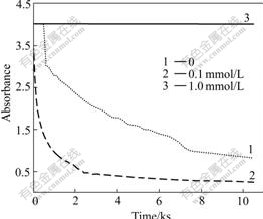
Fig.5 Time dependence of stability with different amounts of CPC under pH=7 and 0.01 mol/L NaCl
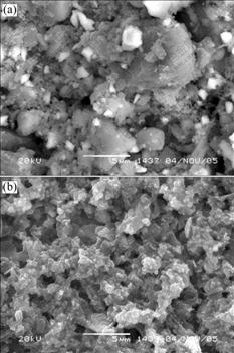
Fig.6 SEM images of ceramic glaze sediment: (a) Without CPC; (b) With CPC
3.3 Zeta potential of antibacterial ceramic glaze with adsorbed surfactant
Fig.7 shows that the zeta potential of aqueous suspensions of the antibacterial ceramic glaze is affected by adsorbed CPC with 0.01 mol/L NaCl. The zeta potential of the ceramic glaze particles under pH values of 7 and 9 without any surfactant is negative. The zeta potential shows a steep increase from negative to positive with the increase of CPC and reaches a plateau in the vicinity of the maximum adsorbed amount of CPC. The zeta potential respects for CPC absorption with the dispersion stability of the antibacterial ceramic glaze.
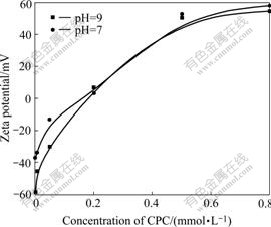
Fig.7 Zeta potential of ceramic glaze as function of added CPC concentration at pH=7 and pH=9
The ceramic glaze is more negatively charged under pH=9 than pH=7. It requires a greater adsorbed amount of CPC to reverse the sign of the charge from negative to positive. The positively charged cationic surfactant ions are more easily adsorbed to the negatively charged particles under pH=9. The calcium carbonate and kaolin in some edge sites are positively charged under pH=7, which makes CPC uneasy to be adsorbed. The main factor controlling the maximum zeta potential by CPC is the ionic strength.
4 Conclusions
1) Addition of low concentrations of CPC leads to charge neutralization. The suspension becomes flocculated due to hydrophobic attraction of adsorbed surfactant. At higher dosages, the ceramic particles become hydrophilic again once a bilayered surfactant coating forms.
2) For salt concentrations less than or equal to 0.01 mol/L, high zeta potential is achieved and stable suspensions result from the substantial electrical double-layer repulsion.
3) When the salt concentration is 0.1 mol/L, both adsorption amount and zeta potential value are large. But the stability of antibacterial ceramic glaze suspension is poor. This suggests that the steric repulsion due to adsorbed CPC is insufficient to overcome the van der Waals attraction.
4) The dispersion stability of the ceramic glaze in the presence of CPC has no evident changes at both pH values of 7 and 9. For CPC, the main factor controlling the maximum zeta potential is seen to be the ionic strength.
References
[1] HAN Bin-yong, ZhANG Xue-jun. Surfactants and Polymers in Aqueous Solution [M]. Beijing: Chemical Industry Press, 2005, 3. (in Chinese)
[2] ZHANG Zhi-hong, SHEN Zhong, SHAO Chang-sheng. Surface organization modifications of inorganic particles and their applications in rubber [J]. Journal of Petrochemical Universities, 1999, 12(2): 27-31. (in Chinese)
[3] WANG Shun, ZHAO Zheng-guo, LIU Ying-qing. The effect of adsorption of suifactants on stability of suspension of alumina powders [J]. Acta Scientiarum Naturalium Universitatis Pekinensis, 1998, 34(6): 735-740.
[4] FENG La-jun, LIU Bing. Dispersion of TiO2 nano-sized particles in liquor [J]. Powder Technology of China, 2004(3): 27-30. (in Chinese)
[5] KOOPAL L K, GOLOUB T P, KEIZER A, SIDOROVA M P. The effect of cationic surfactants on wetting colloid stability and flotation of silica [J]. Colloids Surf A, 1999(151): 15-25.
[6] MONTICONE V, TREINER C. Adsorption isotherms of cetylpyridinium chloride with iron Ⅲ salts at air/water and silica/water interfaces [J]. Colloids Surf A, 2000(230): 298-305.
[7] BREMMELL K E, JAMESON G J, BIGGS S. Forces between surfaces in the presence of a cationic polyelectrolyte and an anionic surfactant [J]. Colloids Surf A, 1999(146): 1-10.
[8] VIDYADHAR A, HANUMANTHA, RAO K, FORSSBERG K S E. Mechanisms of amine–feldspar interaction in the absence and presence of alcohols studied by spectroscopic methods [J]. Colloid Interface Sci, 2003(214): 127-142.
[9] SASTRY N V, SEQUARIS J M, SCHWUGER M J. Adsorption of polyacrylic acid and sodium dodecylbenzenesulfonate on kaolinite [J]. Colloid Interface Sci, 1995(171): 224-233.
[10] TORN L H, KEIZER A, KOOPAL L K, LYKLEMA J. Mixed adsorption of poly(vinylpyrrolidone) and sodium dodecylbenzenesul- fonate on kaolinite [J]. Colloid Interface Sci, 2003(260): 1-8.
[11] BACKFOLK K, LAGERGE S, ROSENHOLM J B. The influence of stabilizing agents on the interaction between styrene/butadiene latex and calcium carbonate: A calorimetric and a dynamic electrokinetic study [J]. Colloid Interface Sci, 2002(254): 8-16.
[12] LIU Xiao-wen, HU Yue-hua, QIU Guan-zhou. Chemical composition and surface property of kaolins [J]. Acta Mineralogical Sinica, 2001, 21(3): 443-447.
[13] NEDA V. Electrokinetic behaviour of calcite—the relationship with other calcite properties [J]. Chemical Geology, 2001, 177(3): 241-248.
[14] VIDYADHAR A, HANUMANTHA R K, FORSSBERG K S E. Mechanisms of amine–quartz interaction in the absence and presence of alcohols studied by spectroscopic methods [J]. Colloid Interface Sci, 2002(256): 59-72.
[15] ZHANG Zhi-hong, SHEN Zhong, SHAO Chang-sheng. Adsorption of anionic surfactant SDBS on TiO2/H2O and Al-TiO2/H2O interfaces [J]. Journal of Jiangsu Institute of Petrochemical Technology, 1996, 8(2): 1-4. (in Chinese)
[16] ATKIN R, CRAIG V S J, WANLESS E J, BIGGS S. Mechanism of cationic surfactant adsorption at the solid-aqueous interface [J]. Advances in Colloid and Interface Science, 2003(103): 219-304.
(Edited by YANG Bing)
Foundation item: Project(04GK2007) supported by Industrial Key Project of Science and Technology of Hunan Province, China
Corresponding author: CHAI Li-yuan; Tel: +86-731-8836921; E-mail: lychai@mail.csu.edu