固溶处理后的不同淬火过程对7050合金性能及析出行为的影响
来源期刊:中国有色金属学报(英文版)2018年第11期
论文作者:康雷 赵刚 王光东 刘坤 田妮
文章页码:2162 - 2172
关键词:7050铝合金;淬火方式;析出行为;电导率;形核位置
Key words:7050 aluminum alloy; quenching method; precipitation behavior; electrical conductivity; nucleation site
摘 要:采用扫描电镜(SEM)、透射电镜(TEM)、选区电子衍射(SAED)、硬度及电导率测试等手段,研究7050合金薄板固溶处理后通过不同方式淬火冷却至室温的性能及析出行为。结果表明:7050合金经不同方式淬火冷却后,在自然时效最初的48 h内样品的电导率迅速下降。合金自然时效状态的电导率由GP区的尺寸和密度共同决定,其中GP区的尺寸为主要影响因素。室温水淬火样品基体内GP区的尺寸最大,为1.8~2.6 nm;水雾和强风淬火样品基体内的GP区尺寸相近,为1.4~1.8 nm。水雾淬火为7050合金固溶处理后较理想的淬火冷却方式,由该方式淬火的7050合金先自然时效70 d再人工峰时效处理后的硬度为HV 193.6,电导率为30.5% (IACS),均高于相同状态下室温水淬火合金的。
Abstract: The effects of quenching in different ways following solid-solution treatment on properties and precipitation behaviors of 7050 alloy were investigated by transmission electron microscopy (TEM), scanning electron microscopy (SEM), selected area electron diffraction (SAED), hardness and electrical conductivity tests. Results show that after quenching in different ways, electrical conductivity of the alloy decreases rapidly in the first 48 h of natural aging. The electrical conductivity of 7050 alloy in natural aging state is determined by the size and density of GP zones, and the size of GP zones is the main factor. After natural aging for 70 d, the size of GP zones is 1.8-2.6 nm in matrix of the immersion quenched sample and it is 1.4-1.8 nm in matrix of both water mist and forced air quenched samples. After natural and artificial peak aging, the hardness of the water mist quenched sample is HV 193.6 and its electrical conductivity is 30.5% (IACS) which are both higher than those of the immersion quenched sample. Therefore, water mist quenching is an ideal quenching method for 7050 alloy sheets after solid-solution treatment.
Trans. Nonferrous Met. Soc. China 28(2018) 2162-2172
Lei KANG1, Gang ZHAO1, Guang-dong WANG1, Kun LIU2, Ni TIAN1
1. Key Laboratory for Anisotropy and Texture of Materials, Ministry of Education, School of Materials Science and Engineering, Northeastern University, Shenyang 110819, China;
2. Department of Applied Science, University of Quebec at Chicoutimi, Quebec, G7H 2B1, Canada
Received 2 August 2017; accepted 28 February 2018
Abstract: The effects of quenching in different ways following solid-solution treatment on properties and precipitation behaviors of 7050 alloy were investigated by transmission electron microscopy (TEM), scanning electron microscopy (SEM), selected area electron diffraction (SAED), hardness and electrical conductivity tests. Results show that after quenching in different ways, electrical conductivity of the alloy decreases rapidly in the first 48 h of natural aging. The electrical conductivity of 7050 alloy in natural aging state is determined by the size and density of GP zones, and the size of GP zones is the main factor. After natural aging for 70 d, the size of GP zones is 1.8-2.6 nm in matrix of the immersion quenched sample and it is 1.4-1.8 nm in matrix of both water mist and forced air quenched samples. After natural and artificial peak aging, the hardness of the water mist quenched sample is HV 193.6 and its electrical conductivity is 30.5% (IACS) which are both higher than those of the immersion quenched sample. Therefore, water mist quenching is an ideal quenching method for 7050 alloy sheets after solid-solution treatment.
Key words: 7050 aluminum alloy; quenching method; precipitation behavior; electrical conductivity; nucleation site
1 Introduction
As light structural materials, Al-Zn-Mg-Cu aluminum alloys have been widely used in aerospace industry due to their high specific strength, specific stiffness, fracture toughness, and damage tolerance [1-3]. The quenching process following the solid-solution treatment will greatly affect the comprehensive properties of Al-Zn-Mg-Cu alloys [4]. It is generally believed that cooling rates during the quenching process of heat-treatable aluminum alloys should be high enough to restrain the precipitation phenomenon to ensure that the alloys have a sufficient aging strengthening ability. However, excessive cooling rates will cause the large residual stress in quenching workpieces resulting in distortion, deformation and even cracking of the workpieces [5], and these quenching defects will be difficult to eliminate in subsequent processes. Therefore, it is particularly important to reveal the effect of different quenching processes on properties and precipitation behaviors of 7050 alloy. It is the key to develop a new quenching method which can obtain the sufficient aging strengthening ability and reduce the cooling intensity simultaneously, during the quenching process.
At present, the researches on the effect of different quenching processes on microstructures and properties of Al-Zn-Mg-Cu alloys are mainly concentrated in two aspects: one is the quench sensitivity and precipitation behaviors of these alloys during different quenching processes [6-9]; the other is the effect of different quenching methods on the formation and distribution of temperature field and stress field in quenching workpieces [10-13]. In the field of the effect of quenching methods on precipitation behaviors of Al-Zn-Mg-Cu alloys, GODARD et al [14] revealed the inhomogeneous precipitation sequences of 7010 alloy by interrupted quenching tests. ZHANG et al [1] investigated the influence of spraying parameters on microstructures and properties of 7050 alloy during water spraying quenching tests. DUMONT et al [15] studied the effect of the cooling rates during different quenching processes on the quenching-induced precipitation behaviors and mechanical properties of 7040 and 7050 alloy. ZHANG et al [16] reported the influence of different cooling rates on the inhomogeneous precipitation behaviors of 7021, 7085 and 7050 alloys by Jominy quenching tests. The effects of different quenching medium on the precipitation behaviors and corrosion resistance of 7085 alloy were discussed by CHEN et al [17]. In order to improve the corrosion resistance of Al-Zn-Mg-Cu alloys, HUANG [18] and LI et al [19] changed the precipitation behaviors on grain boundaries of Al-Zn-Mg alloys by the high temperature pre-precipitation treatment.
In summary, the existing researches are mainly focused on the effect of quenching processes on the quenching-induced precipitation behaviors and properties of Al-Zn-Mg-Cu alloys. However, the effects of different cooling intensities on the homogeneous precipitation behaviors in matrix of Al-Zn-Mg-Cu alloys, especially the formation and growth of GP zones, have been rarely studied. Therefore, in the present work, the solid-solution treated 7050 alloy sheets were quenched in different ways and the influence of cooling intensities on the properties and quenching-induced precipitation behaviors of 7050 alloy was systemically studied. The purpose is to provide some experimental basis for developing a new quenching method of 7050 alloy which can not only guarantee the sufficient aging strengthening ability, but also effectively reduce the quenching stress.
2 Experimental
The material used in the present work was a hot-rolled 7050 alloy plate with thickness of 30 mm and its chemical composition was measured by the Oxford- instruments FOUNDRY MASTER Pro direct-reading spectrometer. The surface of tested sample was mechanically ground with 2000# sand paper, and contaminants on the surface were removed by ultrasonic cleaning with ethanol. The content of alloying elements was determined by placing the sample on the electrospark holder of the spectrometer, and the result is given in Table 1. Samples with size of 20 mm × 20 mm × 2 mm were cut from the one-quarter of thickness of layers parallel to the rolling surface of the plate. After two-stage solid-solution treatment at 470 °C for 1 h followed by 484 °C for 2 h, the samples were cooled (transfer time was less than 2 s) to room temperature with five different quenching methods, including immersion quenching by room temperature water, 8 MPa water mist quenching, 8 MPa forced air quenching and isothermal treatment at 330 °C for 5 s and 30 s followed by water quenching (WQ). After quenching, one sample of each group was only naturally aged for 70 d, and the other was firstly naturally-aged for 70 d and then artificially peak-aged at 120 °C for 24 h. After different aging treatments, the electrical conductivity and Vickers hardness of the samples were measured.
Table 1 Chemical composition of 7050 aluminum alloy (mass fraction, %)

Vickers hardness of the samples was measured by a WILSON WOLPERT 450-SVD instrument with 5 kg load for 10 s and an average value was obtained from five measurements for each sample. Electrical conductivity of the samples was measured by a FISCHER SIGMASCOPE SMP10 conductivity tester. Microstructural features of the samples in natural aging, including characteristics of grain boundary precipitates, sub-grain boundary precipitates and inhomogeneous precipitates in matrix, were observed by OLYMPUS OLS 3100 laser scanning confocal microscopy (LSCM) and ZEISS Ultra Plus scanning electron microscopy (SEM) attached with the Energy Dispersive Spectrometer (EDS). Microstructural features of inhomogeneous and homogeneous precipitates in matrix of the natural aging samples were observed by JEOL JEM-2100 transmission electron microscopy (TEM) operated at 200 kV. The thickness of TEM foils was measured using an Electro Energy Loss Spectroscopy (EELS) attached in the TEM. Precipitates in matrix were identified and ascertained by the selected area electron diffraction (SAED) method. Foils for TEM observation were twin-jet electropolished in solution of 30% HNO3 + 70% CH3OH below -20 °C with the voltage of 12-15 V. The average diameter of GP zones was estimated by measuring at least 50 GP zones in the images taken along <100>Al direction on TEM.
3 Results
3.1 Effect of different quenching methods on electrical conductivity and hardness
Figure 1 shows the relationship between the electrical conductivity of the samples quenched in different ways and natural aging time. The as-quenched electrical conductivity, measured within 30 s after samples are cooled to room temperature, is the highest (34.1% (IACS)) in the sample isothermally treated at 330 °C for 30 s followed by water quenching, is the lowest (30.3% (IACS)) in the forced air quenching sample and is a medium-value (31.5% (IACS)) in the immersion quenching sample. The decreasing rates of the electrical conductivity of the immersion quenching sample and the samples isothermally treated at 330 °C for 5 and 30 s followed by water quenching are similar. However, the decreasing rates of the electrical conductivity of the water mist quenching and forced air quenching samples are much slower than those of the other three samples. Although at the beginning of natural aging, the electrical conductivity of the immersion quenching sample is higher than that of the forced air quenching sample, the decreasing rate of the electrical conductivity of the forced air quenching sample is the lowest, leading to the electrical conductivity of the forced air quenching sample higher than that of the immersion quenching sample after natural aging for 1 h. For instance, the decreasing amplitude of electrical conductivity of the immersion quenching sample is 4.4% (IACS), while the decreasing amplitude of the forced air quenching sample is only 2% (IACS) after natural aging for 48 h. When natural aging time is over 96 h, the changes of electrical conductivity of all the samples are very small compared with their decreasing amplitude of electrical conductivity in the first 48 h of natural aging.

Fig. 1 Effect of natural aging time on electrical conductivity of 7050 alloy samples quenched in different ways following solid-solution treatment
Figure 2 shows the distribution of electrical conductivity and hardness of the samples quenching in different ways of aging. It can be seen that the hardness of the water mist quenching sample is the highest (HV 158.4) after natural aging for 70 d, and the hardness gradually decreases following the sequence of the forced air quenching sample, immersion quenching sample and the samples isothermally-treated at 330 °C for 5 and 30 s followed by water quenching. When all samples are naturally aged for 70 d followed by artificial peak-aging treatment, the hardness of the water mist quenching sample is still the highest (HV 193.6) and its electrical conductivity is 30.5% (IACS) higher than that of the immersion quenching sample (29.7% (IACS)), indicating that the precipitation degree of the water mist quenching sample is more adequate. The hardness of the sample isothermally treated at 330 °C for 30 s followed by water quenching is the lowest (HV 167) but its electrical conductivity is the highest (33.4% (IACS)) after natural aging followed by artificial peak-aging, indicating that this sample has the highest precipitation degree after aging treatment.

Fig. 2 Distribution of electrical conductivity and hardness of 7050 alloy samples quenched in different ways following solid- solution treatment
3.2 Microstructure of 7050 alloy in natural aging
Figure 3 shows the optical microstructures in naturally aged samples quenched in different ways. When the sample is immersion quenched in room temperature water, the quenching-induced precipitates are invisible on grain boundaries, sub-grain boundaries and in matrix. Sub-grain boundaries are difficult to be distinguished and the grains with the fibrous shape distribute along the rolling direction, as shown in Fig. 3(a). However, some inhomogeneous precipitates appear on the partial grain boundaries of the water mist quenched sample (Fig. 3(b)), indicating that the cooling intensity of water mist quenching is lower than that of immersion quenching. By comparing Figs. 3(c), 3(d) with Fig. 3(b), it can be known that the inhomogeneous precipitates on grain boundaries and sub-grain boundaries gradually increase in the sequence of the water mist quenched sample, forced air quenched sample and the sample isothermally treated at 330 °C for 5 s followed by water quenching. Because of the boundaries of grains or sub-grains decorated with inhomogeneous precipitates, the grains and sub-grains of the samples are gradually revealed. When the sample is isothermally treated at 330 °C for 30 s followed by water quenching, the amount of inhomogeneous precipitates on grain boundaries and sub-grain boundaries further increases, and the sub-grain boundaries are the most obvious. Meanwhile, a large number of inhomogeneous precipitates appear in matrix of the sample (Fig. 3(e)).
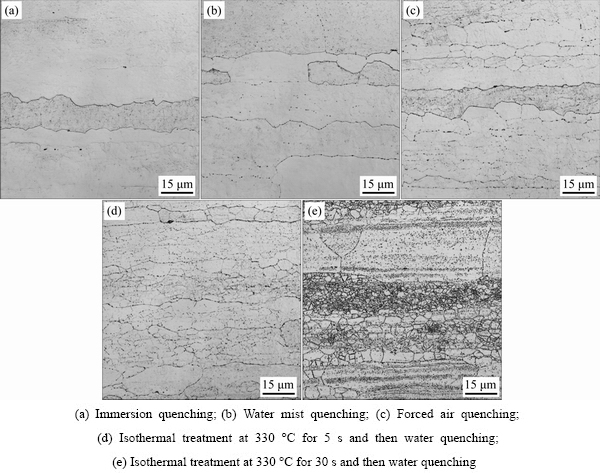
Fig. 3 Optical microstructures of naturally aged samples quenched in different ways (after etching)

Fig. 4 SEM microstructures in naturally aged samples quenched in different ways (without etching)
Figure 4 shows the SEM microstructures in naturally aged samples quenched in different ways. The microstructures in Fig. 4 further confirm metallographic results in Fig. 3. There is no precipitate on grain boundaries, sub-grain boundaries and in matrix of the immersion quenched sample. The second phases with the long rod shape distribute along the rolling direction, whose composition is similar to Al7Cu2Fe (Fig. 4(a)) and their EDS results are shown in Fig. 4(f). From Figs. 4(b) and 4(c), it can be seen that the density of precipitates on the grain boundaries and sub-grain boundaries of the forced air quenched sample is higher than that of the water mist quenched sample and the size range of these precipitates is 0.2-0.4 mm. When the sample is isothermally treated at 330 °C for 5 s followed by water quenching (Fig. 4(d)), not only the density of precipitates on the grain boundaries and sub-grain boundaries further increases, but also the size difference of the precipitates increases gradually. Meanwhile, some small inhomogeneous precipitates are also observed in matrix of this sample. When the sample is isothermally treated at 330 °C for 30 s followed by water quenching (Fig. 4(e)), the precipitates on the grain boundaries and sub-grain boundaries have grown to 0.5-2 mm and the number of the inhomogeneous precipitates in matrix increases significantly.
Figure 5 shows the TEM and SAED results recorded in the direction of <100>Al of the samples quenched in different ways followed by natural aging for 70 d. The thickness of the observation areas in TEM foils can be measured by the function of EELS. In order to exclude the influence of samples thickness on statistical results of GP zones, the bright field images are obtained at the positions with the similar thickness (50-60 nm) in TEM foils. From the bright field images with low magnification (Figs. 5(a) and 5(b)), it can be seen that there is no inhomogeneous precipitate in matrix of the immersion quenched and water mist quenched samples, except for some dispersed particles with the size of 20-40 nm. According to the SAED patterns corresponding to these dispersed particles, the diffraction spots are located at 1/2{200} and 1/2{220} (Figs. 5(g) and 5(h)), which proves that the particles are Al3Zr dispersoids [20,21]. However, from Fig. 5(c) in matrix of the forced air quenched sample, it is found that the inhomogeneous precipitates are precipitated on Al3Zr particles which act as the nucleation sites, and the size of the inhomogeneous precipitates is 30-50 nm.

Fig. 5 TEM images (a-f) and SAED analysis results (g-i) recorded near <100>Al of samples quenched in different ways followed by natural aging for 70 d
Figures 5(d), 5(e) and 5(f) show high magnification TEM bright field images corresponding to Figs. 5(a), 5(b) and 5(c), respectively. It can be seen that a large number of the fine precipitates with the dot-like shape are uniformly distributed in matrix of the samples quenched in three different ways. According to the SAED patterns corresponding to these dot-like shape precipitates (Figs. 5(g), 5(h) and 5(i)), the diffraction spots are located at {1, (2n+1)/4, 0} which are the most obvious at {1, 1/4, 0} and {1, 7/4, 0}, and there is no diffraction pattern at 1/3{220} and 2/3{220}, indicating that the dot-like shape precipitates in matrix are all GPI zones and not η’ phase [20,22-24]. In general, the shape of GPI zones in Al-Zn-Mg-Cu alloy is globular [25,26], which indicates that the two-dimensional morphology of GPI zones along the <100>Al direction is in the dot-like shape. The results of statistical analysis show that the size of GPI zones is the largest in matrix of the immersion quenched sample and the diameter of these GPI zones is 1.8-2.6 nm. Meanwhile, the size of GPI zones in matrix of the water mist quenched sample and the forced air quenched sample is similar, which is 1.4-1.8 nm. The density of GPI zones in the matrix of the samples gradually decreases in the order of the water mist quenched sample, the forced air quenched sample and the immersion quenched sample, which is consistent with the order of the brightness contrast in Figs. 5(a), 5(b) and 5(c). The statistical analysis results of the GPΙ zones are listed in Table 2.
Table 2 Statistical analysis results of GPΙ zones in matrix of 7050 alloy quenched in different ways


Fig. 6 TEM images (a, b) and SAED analysis results (c, d) recorded near <110>Al of samples isothermally treated at 330 °C for 5 (a, c) and 30 s (b, d) and then water quenching followed by natural aging for 70 d
Figure 6 shows the TEM and SAED results recorded in the direction of <110>Al of the samples isothermally treated at 330 °C for different time followed by natural aging for 70 d. The shape of the inhomogeneous precipitates is rod-like in matrix of the sample isothermally treated at 330 °C for 5 s and the size of these precipitates is 0.1-0.3 mm, as shown in Fig. 6(a). According to the SAED pattern (Fig. 6(c)) corresponding to the rod-like precipitates in Fig. 6(a), these rod-like precipitates are h phases (MgZn2) [23,27]. In addition, the h phases preferentially nucleate at Al3Zr particles and dislocations. The size of h phases in matrix of the sample isothermally treated at 330 °C for 5 s followed by water quenching is significantly larger than that in matrix of the forced air quenched sample (Fig. 5(c)). In the matrix of the sample isothermally treated at 330 °C for 30 s followed by water quenching (Fig. 6(b)), the shapes of inhomogeneous precipitates are rod-like or ellipsoidal, and the number of the inhomogeneous precipitates is significantly larger than that of the sample isothermally treated at 330 °C for 5 s. According to the SAED patterns (Fig. 6(d)) corresponding to the inhomogeneous precipitates in Fig. 6(b), the diffraction spots are located at 1/3{220} and 2/3{220}, indicating that these inhomogeneous precipitates are also h phases [20,28]. A comprehensive analysis of Fig. 6 shows that with prolonging isothermal time from 5 to 30 s, the morphology and size of inhomogeneous precipitates change a little, and the density of inhomogeneous precipitates increases remarkably.
4 Discussion
4.1 Relationship between electrical conductivity and precipitation behaviors
The inhomogeneous precipitation behaviors of the 7050 alloy samples quenched in different ways show that the cooling intensities gradually decrease following the sequence of immersion quenching by room temperature water, water mist quenching, forced air quenching, isothermal treatment at 330 °C for 5 s followed by water quenching, and isothermal treatment at 330 °C for 30 s followed by water quenching. According to the Matthiessen resistance law, the main factors influencing electrical resistivity include the density of solid solute atoms in matrix and the stress field of coherent or semi-coherent precipitates, which intensifies the scattering of free electrons [29,30]. When 2 mm sheets of 7050 alloy are immersion quenched by room temperature water following solid-solution treatment, the phenomenon of inhomogeneous precipitation is suppressed and the most of strengthening alloying elements dissolve in a(Al) matrix of the supersaturated solid solution, while a small amount of them maybe precipitate in the second phases with high melting point. Electrical conductivity of the as-quenched sample is mainly affected by solute atoms and vacancies in matrix, and it is not the lowest. As the cooling intensity of water mist quenching and forced air quenching is lower than that of immersion quenching, in a relatively long cooling process, the high density solute atom aggregation zones have been formed in matrix due to the Spinodal decomposition. The scattering effect of free electrons in the solute atom aggregation zones is stronger than that of the supersaturated solute atoms and vacancies in matrix, leading to the lower electrical conductivity of the water mist quenched and forced air quenched samples. When the samples are isothermally treated at 330 °C for a short time, which is the most sensitive temperature during the quenching process [31], large size of equilibrium precipitates have precipitated on grain boundaries, sub-grain boundaries and in matrix, resulting in the consumption of solute atoms and vacancies in matrix. Therefore, the scattering effect of free electrons in the samples isothermally treated at 330 °C is much lower than that of the samples quenched by the other three methods. In other words, the as-quenched electrical conductivity of the samples isothermally treated at 330 °C for a short time is higher.
When the samples are quenched to room temperature in different ways, in the subsequent natural aging process, GP zones will form in matrix due to the decomposition of supersaturated solid solution. The formation of GP zones leads to a strong effect of coherent strain and increases the lattice distortion in matrix, so that the electrical conductivity of the samples quenched in different ways gradually decreases with prolonging natural aging time [32]. Because the formation and growth of GP zones are related to the interaction between solute atoms and vacancies in matrix [33-35] and the concentration of vacancies in matrix decreases rapidly with prolonging aging time after quenching, the formation and growth of GP zones are obviously fast in the first 48 h of natural aging, leading to electrical conductivity of the samples decreasing rapidly. Prolonging natural aging time over 96 h, the number and size of GP zones in matrix are almost stable [36,37], so electrical conductivity of the samples is almost a constant in this stage.
According to the Hillel, Edwards and Wilkes (HEW) theory, the electrical resistivity of aluminum alloy has the maximum value [38,39], that is, the minimum value of the electrical conductivity during aging at low temperature, and the size range of GP zones corresponding to the minimum electrical conductivity is 2-2.4 nm [38,40], which is similar to the size of the GP zones in matrix of the immersion quenched sample after natural aging for 70 d (1.8-2.6 nm in Fig. 5(d)). Therefore, the electrical conductivity of the immersion quenched sample after natural aging for 70 d is the lowest. When the size of GP zones in matrix is similar, the electrical resistivity of the alloy is proportional to the volume fraction of GP zones [32]. When the samples are naturally aged for the same time, the electrical conductivity of the water mist quenched sample is lower than that of the forced air quenched sample. During the natural aging process, the decreasing rate of electrical conductivity is related to the formation and growth rates of GP zones in matrix. Because the cooling intensity of immersion quenching, water mist quenching and forced air quenching gradually decreases, the supersaturation of solute atoms and vacancies concentration of the immersion quenched sample are both higher than those of the forced air quenched sample. Therefore, the driving force of the formation and growth of GP zones is larger and the transformation rate is faster in the immersion quenched sample. Although the cooling intensity of the forced air quenched sample is lower compared with the immersion quenched sample, a large number of solute atom aggregation zones have been formed in matrix during the quenching process, which will act as the cores of GP zones. However, due to the low supersaturation of solute atoms and vacancies concentration in matrix of the forced air quenched sample, the nucleated GP zones grow slowly. Therefore, the decreasing rate of the electrical conductivity of the immersion quenched sample is much higher than that of the forced air quenched sample. In summary, the electrical conductivity of 7050 alloy samples in natural aging state is determined by the size and density of GP zones in matrix, in which the size of GP zones is the main factor.
4.2 Relationship between hardness and precipitation behaviors
The main strengthening phases of 7050 alloy are GP zones and η’ transition phases. It is generally believed that the strengthening phases in natural aging state are GP zones and in artificial peak-aging state they are η’ transition phases [41]. By analyzing the hardness of the samples quenched in different ways followed by natural aging for 70 d (Fig. 2), it is found that the hardness of the forced air quenched sample is obviously higher than that of the immersion quenched sample. By comparing Fig. 5(d) with Fig. 5(f), it is found that GP zones in matrix of the forced air quenched sample are slight smaller, but the density of GP zones is higher. The strengthening mechanism of 7050 alloy in natural aging state is the interaction of GP zones and their stress field with dislocations in matrix, which will hinder the motion of the dislocations. As the maximum size of GP zones in this work during natural aging is 2.6 nm which is less than 3 nm, the mechanism of dislocations passing through the GP zones is shearing [42] and the strengthening effect mainly comes from the strain strengthening and chemical strengthening [43]. Therefore, hardness should be proportional to the size of GP zones only when a single GP zone hinders dislocations motion [42]. However, when the interaction of the size, density and distribution of GP zones is synthetically considered, the hardness of the forced air quenched sample in natural aging state may be higher than that of the immersion quenched sample.
The precipitation sequence of 7050 alloy can be described as follows: supersaturated solid solution →GP zones→η’ phases→h phases, and the key factors for the formation and growth of η’ phases are the size, density and distribution of GP zones in matrix [29,44,45]. The samples quenched in different ways have been treated with natural aging for 70 d before the artificial peak-aging and the distribution of GP zones in matrix of the samples is shown in Fig. 5. The maximum-sized GP zones have formed in matrix of the immersion quenched sample, which are difficult to dissolve into matrix during the following artificial peak-aging. Because the supersaturation of solute atoms and vacancies concentration of the immersion quenching sample are both the largest, which is very favorable for the nucleation and growth of η’ strengthening phases, almost all GP zones gradually transform into η’ phases during the following artificial peak-aging. Therefore, the hardness of the immersion quenched sample after the artificial peak-aging has a great increase compared with its hardness in natural aging. During the water mist quenching process, the density and size of the inhomogeneous precipitates on grain boundaries and sub-grain boundaries are very small (Figs. 3(b) and 4(b)), which has little influence on the supersaturation of solute atoms. Due to the highest density and suitable size of GP zones in matrix during the quenching and following natural aging process, which promotes the formation of the high-density η’ strengthening phases, the water mist quenched sample will obtain the highest hardness after artificial peak-aging. Because of the formation of numerous large-sized inhomogeneous precipitates on grain boundaries, sub-grain boundaries and even on Al3Zr particles during the quenching process, which consumes a lot of solute atoms and vacancies resulting in inhibiting the formation and growth of η’ strengthening phases during the following artificial peak-aging process, the peak-aging hardness of the forced air quenched sample is slightly lower than that of the immersion quenched and water mist quenched samples.
4.3 Sequence of nucleation sites of inhomogeneous precipitation
According to microstructures of the samples quenched in different ways followed by natural aging treatment (Figs. 3-6), it is found that the most probable sequence of nucleation sites of the inhomogeneous precipitation can be described as follows: nucleation on grain boundaries, nucleation on sub-grain boundaries, and nucleation at Al3Zr particles or dislocations in matrix. The grain boundaries, sub-grain boundaries, dislocations and the surfaces of dispersed particles belong to the high-energy regions compared with the normal position in matrix and the nucleation energy barrier of these positions is much smaller than that of the homogeneous nucleation. Therefore, the inhomogeneous nucleation is always earlier than the homogeneous nucleation [46]. The sequence of inhomogeneous nucleation sites is closely related to the critical nucleation energy, so that the inhomogeneous precipitates preferentially nucleate at the positions which have the lowest critical nucleation energy. In addition, these preferential nucleation positions are beneficial to the diffusion of solute atoms; meanwhile, the inhomogeneous nucleation always happens at high temperature corresponding to the high diffusion coefficient of solute atoms. Therefore, the growth rate of inhomogeneous precipitates after nucleation is very fast, and the inhomogeneous precipitates are mostly coarse equilibrium phases.
After natural aging for 70 d and artificial peak- aging, the hardness of the water mist quenched sample is HV 193.6 and its electrical conductivity is 30.5% (IACS), which are both higher than those of the immersion quenched sample. It is considered that 8 MPa water mist quenching is the best in the five quenching methods of 7050 alloy sheets after solid-solution treatment. 7050 alloy sheets quenched by this method can obtain high hardness and electrical conductivity simultaneously, that is to say, the sheets will obtain a better corrosion resistance [47,48]. Moreover, during this quenching process, the suitable size, density and distribution of the precipitated nucleus happen on grain boundaries, sub-grain boundaries and in matrix of the alloy. Therefore, the water mist quenching process is equivalent to a pre-nucleation process. During the following artificial peak-aging process, these pre-nucleation precipitates will grow up gradually, inhomogeneous precipitates on grain boundaries will become more coarse and discrete, and the density and distribution of homogeneous strengthening precipitates in matrix will be more reasonable, which guarantees that the 7050 alloy sheets will get excellent properties after water mist quenching.
5 Conclusions
1) The electrical conductivity of the 7050 alloy samples quenched in different ways decreases rapidly in the first 48 h of natural aging. The decreasing amplitude of the immersion quenched sample is the maximum, which is 4.4% (IACS), while the decreasing amplitude of the forced air quenched sample is the minimum, which is only 2% (IACS).
2) The electrical conductivity of the 7050 alloy samples in natural aging state is determined by the size and density of GP zones, in which the size of GP zones is the main factor. After quenching and natural aging for 70 d, the size of GP zones of the immersion quenched sample is the largest, which is 1.8-2.6 nm. The size of GP zones of the water mist quenched and forced air quenched samples is similar, which is 1.4-1.8 nm.
3) During the quenching process of 7050 alloy, the most probable sequence of nucleation sites of the inhomogeneous precipitation can be described as follows: first nucleation on grain boundaries, next nucleation on sub-grain boundaries, and final nucleation at Al3Zr particles or dislocations in matrix.
4) After natural aging for 70 d followed by artificial peak-aging, the hardness of the water mist quenched sample is HV 193.6 and its electrical conductivity is 30.5% (IACS), which are both higher than those of the immersion quenched sample. Therefore, water mist quenching is the best in the five quenching methods of 7050 alloy sheets after solid-solution treatment.
References
[1] ZHANG J, DENG Y L, YANG W, HU S S, ZHANG X M. Design of the multi-stage quenching process for 7050 aluminum alloy [J]. Materials and Design, 2014, 56(4): 334-344.
[2] LI P Y, XIONG B Q, ZHANG Y A, LI Z H, ZHU B H, WANG F, LIU H W. Quench sensitivity and microstructure character of high strength AA7050 [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(2): 268-274.
[3] WILLIAMS J C, STARKE E A. Progress in structural materials for aerospace systems [J]. Acta Materialia, 2003, 51(19): 5775-5799.
[4] LANG Y J, ZHOU G X, HOU L G, ZHANG J S, ZHUANG L Z. Significantly enhanced the ductility of the fine-grained Al-Zn-Mg-Cu alloy by strain-induced precipitation [J]. Materials and Design, 2015, 88: 625-631.
[5] ROBINSON J S, TANNER D A, TRUMAN C E, PARADOWSKA A M, WIMPORY R C. The influence of quench sensitivity on residual stresses in the aluminium alloys 7010 and 7075 [J]. Materials Characterization, 2012, 65(3): 73-85.
[6] ROBINSON J S, CUDD R L, TANNER D A, DOLAN G P. Quench sensitivity and tensile property inhomogeneity in 7010 forgings [J]. Journal of Materials Processing Technology, 2001, 119(1-3): 261-267.
[7] LIU S D, LIU W J, ZHANG Y, ZHANG X M, DENG Y L. Effect of microstructure on the quench sensitivity of AlZnMgCu alloys [J]. Journal of Alloys and Compounds, 2010, 507(1): 53-61.
[8] DESCHAMPS A, BRECHET Y. Nature and distribution of quench-induced precipitation in an Al-Zn-Mg-Cu alloy [J]. Scripta Materialia, 1998, 39(11): 1517-1522.
[9] ZHANG X M, LIU W J, LIU S D, ZHOU M Z. Effect of processing parameters on quench sensitivity of an AA7050 sheet [J]. Materials Science and Engineering A, 2011, 528(3): 795-802.
[10] TANNER D A, ROBINSON J S. Residual stress prediction and determination in 7010 aluminum alloy forgings [J]. Experimental Mechanics, 2000, 40(1): 75-82.
[11] KOC M, CULP J, ALTAN T. Prediction of residual stresses in quenched aluminum blocks and their reduction through cold working processes [J]. Journal of Materials Processing Technology, 2006, 174(1-3): 342-354.
[12] LI Y N, ZHANG Y A, LI X W, LI Z H, WANG G J, YAN H W, JIN L B, XIONG B Q. Effects of heat transfer coefficients on quenching residual stresses in 7055 aluminum alloy [J]. Materials Science Forum, 2016, 877: 647-654.
[13] TANNER D A, ROBINSON J S. Effect of precipitation during quenching on the mechanical properties of the aluminium alloy 7010 in the W-temper [J]. Journal of Materials Processing Technology, 2004, 153-154(22): 998-1004.
[14] GODARD D, ARCHAMBAULT P, GAUTIER E A, LAPASSET G. Precipitation sequences during quenching of the AA 7010 alloy [J]. Acta Materialia, 2002, 50(9): 2319-2329.
[15] DUMONT D, DESCHAMPS A, BRECHET Y, SIGLI C, EHRSTROM J C. Characterisation of precipitation microstructures in aluminium alloys 7040 and 7050 and their relationship to mechanical behaviour [J]. Materials Science and Technology, 2004, 20(5): 567-576.
[16] ZHANG Z H, XIONG B Q, LIU S F, ZHU B H, ZUO Y T. Changes of microstructure of different quench sensitivity 7000 aluminum alloy after end quenching [J]. Rare Metals, 2014, 33(3): 270-275.
[17] CHEN S Y, CHEN K H, PENG G S, LIANG X, CHEN X H. Effect of quenching rate on microstructure and stress corrosion cracking of 7085 aluminum alloy [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(1): 47-52.
[18] HUANG L P, CHEN K H, LI S, SONG M. Influence of high-temperature pre-precipitation on local corrosion behaviors of Al-Zn-Mg alloy [J]. Scripta Materialia, 2007, 56(4): 305-308.
[19] LI H Z, YAO S C, LIANG X P, CHEN Y H, LIU C, HUANG L. Grain boundary pre-precipitation and its contribution to enhancement of corrosion resistance of Al-Zn-Mg alloy [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(10): 2523-2531.
[20] SHA G, CEREZO A. Early-stage precipitation in Al-Zn-Mg-Cu alloy (7050) [J]. Acta Materialia, 2004, 52(15): 4503-4516.
[21] SRIVATSAN T S, SRIRAM S, VEERARAGHAVAN D, VASUDEVAN V K. Microstructure, tensile deformation and fracture behaviour of aluminium alloy 7055 [J]. Journal of Materials Science, 1997, 32(11): 2883-2894.
[22] BERG L K, GJONNES J, HANSEN V, LI X Z, WEDEL M K, WATERLOO G, SCHRYVERS D, WALLENBERG L R. GP-zones in Al-Zn-Mg alloys and their role in artificial aging [J]. Acta Materialia, 2001, 49(17): 3443-3451.
[23] YANG W C, JI S X, WANG M P, LI Z. Precipitation behaviour of Al-Zn-Mg-Cu alloy and diffraction analysis from η' precipitates in four variants [J]. Journal of Alloys and Compounds, 2014, 610(15): 623-629.
[24] LIU Y, JIANG D M, LI B Q, YANG W S, HU J. Effect of cooling aging on microstructure and mechanical properties of an Al-Zn-Mg-Cu alloy [J]. Materials and Design, 2014, 57(5): 79-86.
[25] SCHMUCK C, AUGER P, DANOIX F, BLAVETTE D. Quantitative analysis of GP zones formed at room temperature in a 7150 Al-based alloy [J]. Applied Surface Science, 1995, 87-88: 228-233.
[26] ZHANG M, LIU T, HE C N, DING J, LIU E Z, SHI C S, LI J J, ZHAO N Q. Evolution of microstructure and properties of Al-Zn-Mg-Cu-Sc-Zr alloy during aging treatment [J]. Journal of Alloys and Compounds, 2016, 658: 946-951.
[27] KVERNELAND A, HANSEN V, THORKILDSEN G, LARSEN H B, PATTISON P, LI X Z, GJONNES J. Transformations and structures in the Al-Zn-Mg alloy system: A diffraction study using synchrotron radiation and electron precession [J]. Materials Science and Engineering A, 2011, 528(3): 880-887.
[28] BUHA J, LUMLEY R N, CROSKY A G. Secondary ageing in an aluminium alloy 7050 [J]. Materials Science and Engineering A, 2008, 492(1-2): 1-10.
[29] WANG T, YIN Z M, SHEN K, LI J, HUANG J W. Single-aging characteristics of 7055 aluminum alloy [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(3): 548-552.
[30] LI H Y, LIU J J, YU W C, ZHAO H, LI D W. Microstructure evolution of Al-Zn-Mg-Cu alloy during non-linear cooling process [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(5): 1191-1200.
[31] KANG L, ZHAO G, TIAN N, FU H. Measurement of TTP curves of 7050 aluminum alloy by conductivity [J]. Advanced Materials Research, 2015, 1095: 168-174.
[32] FERRAGUT R, SOMOZA A, TORRIANI I. Pre-precipitation study in the 7012 Al-Zn-Mg-Cu alloy by electrical resistivity [J]. Materials Science and Engineering A, 2002, 334(1-2): 1-5.
[33] SCHLOTH P, DESCHAMPS A, GANDIN C A, DREZET J M. Modeling of GP(I) zone formation during quench in an industrial AA7449 75 mm thick plate [J]. Materials and Design, 2016, 112: 46-57.
[34] LENDVAI J. Precipitation and strengthening in aluminium alloys [J]. Materials Science Forum, 1996, 217-222: 43-56.
[35] DUPASQUIER A, FERRAGUT R, IGLESIAS M M, MASSAZZA M, RIONTINO G, MENGUCCI P, BARUCCA G, MACCHI C E, SOMOZA A. Hardening nanostructures in an AlZnMg alloy [J]. Philosophical Magazine, 2007, 87(22): 3297-3323.
[36] ROSSITER P L, WELLS P. The electrical resistivity during pre-precipitation processes [J]. Philosophical Magazine, 1971, 24(188): 425-436.
[37] FERRAGUT R, SOMOZA A, TOLLEY A. Microstructural evolution of 7012 alloy during the early stages of artificial ageing [J]. Acta Materialia, 1999, 47(17): 4355-4364.
[38] OSAMURA K, OTSUKA N, MURAKAMI Y. Resistivity maximum during Guinier-Preston zone formation in an Al-4wt%Cu alloy [J]. Philosophical Magazine Part B, 1982, 45(6): 583-599.
[39] YONEMITSU K, MATSUDA T. Electrical resistivity of spherical Guinier-Preston zone [J]. Physica Status Solidi, 1976, 36(2): 791-798.
[40] OSAMURA K, HIRAOKA Y, MURAKAMI Y. The resistivity maximum during Guinier-Preston zone formation in Al-Zn alloys [J]. Philosophical Magazine, 1973, 28(4): 809-825.
[41] VIANA F, PINTO A M P, SANTOS H M C, LOPES A B. Retrogression and re-ageing of 7075 aluminium alloy: Microstructural characterization [J]. Journal of Materials Processing Technology, 1999, 92-93: 54-59.
[42] GUYOT P, COTTIGNIES L. Precipitation kinetics, mechanical strength and electrical conductivity of AlZnMgCu alloys [J]. Acta Materialia, 1996, 44(10): 4161-4167.
[43] GUO Z, SHA W. Quantification of precipitation hardening and evolution of precipitates [J]. Materials Transactions, 2002, 43(6): 1273-1282.
[44] LIU S D, LI C B, DENG Y L, ZHANG X M. Inflence of aging on the hardenability of 7055 aluminum alloy thick plate [J]. Acta Metallurgica Sinica, 2012, 48(3): 343-350. (in Chinese)
[45] WERENSKIOLD J C, DESCHAMPS A, BRECHET Y. Characterization and modeling of precipitation kinetics in an Al-Zn-Mg alloy [J]. Materials Science and Engineering A, 2000, 293(1-2): 267-274.
[46] YU Y N. Metallography principle [M]. 2nd ed. Beijing: Metallurgical Industry Press, 2013: 588-598. (in Chinese)
[47] LI Z H, XIONG B Q, ZHANG Y A, ZHU B H, WANG F, LIU H W. Microstructural evolution of aluminum alloy 7B04 thick plate by various thermal treatments [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(1): 40-45.
[48] FENG C, LIU Z Y, NING A L, LIU Y B, ZENG S M. Retrogression and re-aging treatment of Al-9.99%Zn-1.72%Cu-2.5%Mg- 0.13%Zr aluminum alloy [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(5): 1163-1170.
康 雷1,赵 刚1,王光东1,刘 坤2,田 妮1
1. 东北大学 材料科学与工程学院 材料各向异性与织构教育部重点实验室,沈阳 110819;
2. Department of Applied Science, University of Quebec at Chicoutimi, Quebec, G7H 2B1, Canada
摘 要:采用扫描电镜(SEM)、透射电镜(TEM)、选区电子衍射(SAED)、硬度及电导率测试等手段,研究7050合金薄板固溶处理后通过不同方式淬火冷却至室温的性能及析出行为。结果表明:7050合金经不同方式淬火冷却后,在自然时效最初的48 h内样品的电导率迅速下降。合金自然时效状态的电导率由GP区的尺寸和密度共同决定,其中GP区的尺寸为主要影响因素。室温水淬火样品基体内GP区的尺寸最大,为1.8~2.6 nm;水雾和强风淬火样品基体内的GP区尺寸相近,为1.4~1.8 nm。水雾淬火为7050合金固溶处理后较理想的淬火冷却方式,由该方式淬火的7050合金先自然时效70 d再人工峰时效处理后的硬度为HV 193.6,电导率为30.5% (IACS),均高于相同状态下室温水淬火合金的。
关键词:7050铝合金;淬火方式;析出行为;电导率;形核位置
(Edited by Bing YANG)
Foundation item: Project (2016YFB0300801) supported by the National Key Research and Development Program of China; Project (51371045) supported by the National Natural Science Foundation of China
Corresponding author: Ni TIAN; Tel: +86-24-83691571; E-mail: kangleiahut@126.com
DOI: 10.1016/S1003-6326(18)64861-7

