Synthesis of intracellular cobalt ferrite nanocrystals by extreme acidophilic archaea Ferroplasma thermophilum
来源期刊:中南大学学报(英文版)2020年第5期
论文作者:王军 邬柏强 何万里 杨宝军 廖蕤 周祎 刘玉玲 林墨 邱冠周
文章页码:1443 - 1452
Key words:Ferroplasma thermophilum; cobalt ferrite nanocrystals; biomineralization; bioleaching; extreme acidophilic microorganism
Abstract: Ferroplasma thermophilum, a sort of extreme acidophilic archaea, which can synthesize intracellular cobalt ferrite nanocrystals, is investigated in this study. The nanocrystals were analyzed with ultrathin sections and transmission electron microscope, with the size of 20-60 nm, the number of more than 30 in each cell at average, which indicated that F. thermophilum can synthesize intracellular nanocrystals and also belongs to high-yield nanocrystals-producing strain. Intriguingly, the nanocrystals contain ferrite and cobalt characterized by EDS X-ray analysis, suggesting that both cobalt and ferrite are potentially contributed to the formation of nanocrystals. Moreover, under the different energy source culture conditions of FeSO4 and CuFeS2, the size and the morphology of the nanocrystals are different. It was also found that the higher initial Fe availability leads to an induced synthesis of larger nanocrystals and the lower oxidation-reduction potential (ORP) leads to an induced effect on the synthesis of nanocrystals with abnormal unhomogeneous size, which suggested that the higher initial Fe availability and the lower oxidation-reduction potential lead to a higher uptake efficiency of iron ions of F. thermophilum by iron and ORP gradient culture.
Cite this article as: WU Bai-qiang, HE Wan-li, YANG Bao-jun, LIAO Rui, ZHOU Yi, LIU Yu-ling, LIN Mo, QIU Guan-zhou, WANG Jun. Synthesis of intracellular cobalt ferrite nanocrystals by extreme acidophilic archaea Ferroplasma thermophilum [J]. Journal of Central South University, 2020, 27(5): 1443-1452. DOI: https://doi.org/ 10.1007/s11771-020-4380-4.

J. Cent. South Univ. (2020) 27: 1443-1452
DOI: https://doi.org/10.1007/s11771-020-4380-4
WU Bai-qiang(邬柏强)1, 2, HE Wan-li(何万里)1, 2, YANG Bao-jun(杨宝军)1, 2,LIAO Rui(廖蕤)1, 2,
ZHOU Yi(周祎)1, 2, LIU Yu-ling(刘玉玲)1, 2, LIN Mo(林墨)1, 2, QIU Guan-zhou(邱冠周)1, 2, WANG Jun(王军)1, 2
1. Key Laboratory of Biohydrometallurgy of Ministry of Education, Central South University,Changsha 410083, China;
2. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract: Ferroplasma thermophilum, a sort of extreme acidophilic archaea, which can synthesize intracellular cobalt ferrite nanocrystals, is investigated in this study. The nanocrystals were analyzed with ultrathin sections and transmission electron microscope, with the size of 20-60 nm, the number of more than 30 in each cell at average, which indicated that F. thermophilum can synthesize intracellular nanocrystals and also belongs to high-yield nanocrystals-producing strain. Intriguingly, the nanocrystals contain ferrite and cobalt characterized by EDS X-ray analysis, suggesting that both cobalt and ferrite are potentially contributed to the formation of nanocrystals. Moreover, under the different energy source culture conditions of FeSO4 and CuFeS2, the size and the morphology of the nanocrystals are different. It was also found that the higher initial Fe availability leads to an induced synthesis of larger nanocrystals and the lower oxidation-reduction potential (ORP) leads to an induced effect on the synthesis of nanocrystals with abnormal unhomogeneous size, which suggested that the higher initial Fe availability and the lower oxidation-reduction potential lead to a higher uptake efficiency of iron ions of F. thermophilum by iron and ORP gradient culture.
Key words: Ferroplasma thermophilum; cobalt ferrite nanocrystals; biomineralization; bioleaching; extreme acidophilic microorganism
Cite this article as: WU Bai-qiang, HE Wan-li, YANG Bao-jun, LIAO Rui, ZHOU Yi, LIU Yu-ling, LIN Mo, QIU Guan-zhou, WANG Jun. Synthesis of intracellular cobalt ferrite nanocrystals by extreme acidophilic archaea Ferroplasma thermophilum [J]. Journal of Central South University, 2020, 27(5): 1443-1452. DOI: https://doi.org/ 10.1007/s11771-020-4380-4.
1 Introduction
Species of the genus Ferroplasma is cell wall-deficient archae which lives in extremely acid environment, and the species belongs to iron-oxidizing mixotrophic or organotrophic archae [1-3]. Ferroplasma spp. has several same metabolic features as the classical bioleaching bacteria Acidithiobacillus spp. and Leptospirillum spp [4, 5]. The most important feature of Ferroplasma spp. is its ability to oxidize ferrous iron and thus it has been widely reported in bioleaching [6-8]. Ferroplasma thermophilum can increase the copper dissolution in bioleaching process when it is working with Acidithiobacillus caldus and Leptospirillum ferriphilum [9]. Furthermore, some reports suggested that Ferroplasma thermophilum can remove the accumulating organic products in low pH leachates to catalyze metal sulphide oxidation [10, 11]. Therefore, the species currently plays a critical role in the biogeochemical process of sulphide metals in extremely acidic environments [12-17].
Magnetotactic bacteria (MTB) can synthesize ferrite nanocrystals that are called magnetosome, which is a kind of nano-sized crystal and is enveloped by a lipid bilayer membrane [18]. The diversity of magnetosomes includes cub octahedral, tooth-shaped, elongated prismatic and bullet-shaped morphologies [19-24]. Magnetosome is mineral crystal with magnetism, currently either magnetite (Fe3O4) or greigite (Fe3S4) [25-27]. It was also reported that some magnetosomes consist of cobalt [28-30]. There are a few reports that leaching bacteria can synthesize intracellular magnetic nanoparticle [31]. YAN et al [32] used transmission electron microscopy technology to characterize the typical mineral leaching bacteria Acidithiobacillus ferrooxidans intracellular magnetic nanoparticles, and found that the size was 20-30 nm, the distribution in the cell is relatively scattered, and one or more complete magnetic chains have not been formed. WU et al [33] investigated the magnetic nanoparticles of Acidithiobacillus ferrooxidans by synchrotron radiation soft X-ray fine near-edge absorption structure spectroscopy. The results show that the phase composition is close to Fe3O4. There are also a few reports on Ferroplasma thermophilum intracellular magnetic nanoparticles. XU et al [34] studied Ferroplasma thermophilum intracellular magnetic nanoparticles and found that Ferroplasma thermophilum intracellular magnetic nanoparticles are 47-100 nm in size and composed of iron and oxygen. However, previous studies did not establish a high-density culture system for Ferroplasma thermophilum, and failed to use different energy materials to cultivate Ferroplasma thermophilum. In this paper, strain F. thermophilum L1T, a nonmotile coccus lacking cell wall, isolated from a chalcopyrite-leaching bioreactor, was investigated about new function that can synthesize intracellular cobalt ferrite nanocrystals. Ultrathin sections, transmission electron microscopy (TEM) and EDS X-ray analysis were carried out to evaluate morphology and chemical elements composition of nanocrystals, especially. We investigated the nanocrystals under the different energy sources culture conditions of FeSO4 and CuFeS2.
2 Experimental
2.1 Pure culture of F. thermophilum L1T
The F. thermophilum L1T (EF062309) in the present work was provided by the School of Minerals Processing and Bioengineering, Central South University, China. The medium of the strain F. thermophilum L1T was modified in 9K medium (4.47 g FeSO4·7H2O, 4.47 g CuFeS2 and 0.2% (M/V) yeast extract) [35]. Modified 9K medium (per litre) contained 3.0 g (NH4)2SO4, 0.5 g H2PO4, 0.1 g KCl, 0.5 g MgSO4·7H2O and 0.01 g Ca(NO3)2. The pH of the medium was adjusted to 1.0 by adding 50% (V/V) H2SO4 which was autoclaved, and then FeSO4·7H2O was added. The rotation speed was 170 r/min and the temperature was 45 °C. The iron gradient culture of F. thermophilum L1T was as follows: The F. thermophilum L1T was purely cultured in different grads of iron concentration. The 100 mL of 9K medium was mixed into 1 mL of 0.2% leaven leaching solution and FeSO4·H2O in different mass (3 g and 7 g). And every experimental group was set up with three parallel tests. Then they were put into the incubator at 170 r/min and 45 °C. The potential gradient culture of F. thermophilum L1T was as follows: The F. thermophilum L1T was purely cultured in different grads of iron concentration. The 100 mL of 9K medium was mixed into 1 mL 0.2% leaven leaching solution and FeSO4·H2O in different mass. The proportion between FeSO4·7H2O and Fe2(SO4)3 was 1:1 and 1:5. And every experimental group was set up with three parallel tests. Then they were put into the incubator at 170 r/min and 45 °C.
2.2 Transmission electron microscopy (TEM) and energy spectrum analysis
The F. thermophilum L1T which has been magnetic isolated was put into centrifuge at 12000 r/min and 25 °C for 10 min. After 10 min, the supernatant fluid was washed, and it was added into 1 mL of 2.5% glutaric dialdehyde and phosphoric acid buffer to fix the concentration for 2 h or even more time. After that, the phosphoric acid was used to rinse it three times, every time for 10-15 min and the osmic acid was used to fix it for 1-2 h. Dehydration was as follows: The solution was dehydrated with acetone in four different concentrations, 50%, 70%, 90% and 100%, for 10-15 min, respectively, and changed at the middle time. Marinating and embedding were as follows: use pure acetone and embedding reagent at the proportion of 1:1 at 37 °C for 10-12 h and then use pure embedding reagent at 37 °C for 10-12 h. Solidifying was as follows: use oven at 37 °C over night and at 60 °C for 12-24 h. Use the section cutter to make every slice of 50-100 nm. The observations and recording of images were performed with a TEM (FEI Tecnai Spirit) at accelerating voltage of 80 kV.
2.3 Physical-chemical analysis
The oxidation–reduction potential (ORP) was measured directly in the pulp, respectively, with pH S–3C acid meter and a Pt electrode in reference to an Ag/AgCl electrode (INESA Scientific Instrument Co., Ltd., China). The concentrations of iron and cobalt in solution and inner the cell were measured by ICP-OES analysis.
3 Results and discussions
3.1 Results
The ultrathin sections and transmission electron microscopy observations (Figure 1) revealed that F. thermophilum L1T is a kind of coccus, and the ellipsoidal thalli of F. thermophilum L1T are 500-1000 nm (n=100). Moreover, some of the F. thermophilum L1T contained abundant nanocrystals, while others contain slight nanocrystals. The shape of the nanocrystals was inhomogenous and most of nanocrystals seem like hexagonal prisms. For the sizes and numbers of the nanocrystals of each thallus by the statistical analysis, 100 thalli of F. thermophilum L1T and 100 nanocrystals were randomly selected. The statistical analysis results showed that the number of the nanocrystals per F. thermophilum L1T approximately varies from 10 to 70 and the most of F. thermophilum L1T contains 20-30 nanocrystals and the average number (per cell) of nanocrystals was 30±5 (n=100). The size of the nanocrystals varies from 10 nm to 70; thereinto, most of nanocrystals is 20-30 nm and the average size is (25±5) nm (n=100). The arrangement of those nanocrystals in the cell of F. thermophilum L1T seems like stochastic and flocked together.

Figure 1 (a) Slice of F. thermophilum L1T, suggesting that nearly every cell had some nanocrystals without the same amount; (b) Statistics of the number of cellular nanocrystals for 100 cells and the size of every nanoparticle for 100 nanocrystals; (c) Arrangement of nanocrystals
The arrangement of those nanocrystals is random as shown in Figure 2(a). The nanocrystals seems like globular and tooth-shaped. The nanocrystals seems to be membrane-enveloped. EDX spectrum analysis showed that the nanocrystals contain high iron, cobalt and oxygen (Figure 2(b)). The peaks of iron element and cobalt are very near, and it also can illustrate that the contents of the two metal elements are abundant. Hence, it can infer that the nanocrystals may contain high amounts of iron, cobalt and oxygen. The stain is phosphotungstic acid and thus, it has a peak of tungsten shown in Figure 2(b).
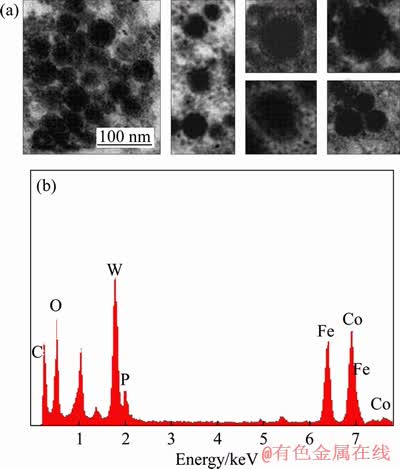
Figure 2 (a) Observation of nanocrystals; (b) Energy spectrum analysis of nanocrystals
F. thermophilum L1T grew under optimum condition in which as the energy source, one is 4.47 g CuFeS2 (Figure 3(a)) and another is 4.47 g FeSO4·7H2O (Figure 3(b)). The ultrathin sections and transmission electron microscopy (TEM) images revealed that the thalli can synthesize nanocrystals under the energy sources of CuFeS2 and FeSO4·7H2O, but it was obvious that the morphology and number of nanocrystals are different. For the previously mentioned thalli in which energy source is CuFeS2, the morphology of nanocrystals is globular, the number of nanocrystals per cell is approximately 30 at average, and the size of nanocrystals is 25 nm at average. With respect to the thalli in which the energy sources is FeSO4·7H2O, it was obviously different that the morphology is more inhomogeneous, the size is larger, approximately 50 nm, and the number is less, approximately 20 in each cell.
The size and morphology of nanocrystals are different under the different energy source cultures, so we thought that the initial Fe availability in the medium and the ORP of the media are the main reasons that caused the phenomenon. It showed that the concentration of iron ions in the medium in which energy source is CuFeS2 is 3500-3900 mg/L, and the FeSO4·7H2O is 9500-9700 mg/L by ICP-OES analysis after 2 h of inoculation, which means that the initial Fe availability of ferrous sulfate heptahydrate solution is far more than chalcopyrite solution [36-38]. Because the ferrous sulfate heptahydrate was immediately dissolved when it was added into the medium (Figure 3(a)), chalcopyrite was very hard to dissolve in the medium (Figure 3(b)).
3.2 Discussion
Magnetosomes, synthesized by magnetotactic bacteria, are intracellular membrane-enveloped iron minerals, individually less than 150 nm in size, and are normally organized as chain-like structures within the cell [19, 39, 40]. The obvious difference between the nanocrystals and magnetosome is the synthesis condition. The condition of synthesis of magnetosome is under microaerobic or anaerobic, alkaline or neutral conditions [1, 41, 42], and the synthesis condition of the nanocrystals synthesized by F. thermophilum L1T is in extreme acid [43] and aerobic environment [35, 44]. Magnetosomes, which are membrane-enveloped, can be proven by TEM images [45]. However, for the nanocrystals which are synthesized by F. thermophilum L1T, we did not find any directly evidence that the nanocrystals are membrane-enveloped (Figure 2(a)). Currently, magnetosomes are organized as chain (one or multiple) within the cell for optimizing the cellular magnetic dipole moment, and it will facilitate the navigation of MTB, like a compass needle, to make MTB orient along the lines of magnetic fields and find the anaerobic or microaerobic environment for living [18, 46-48]. However, the arrangement of those nanocrystals in F. thermophilum L1T is irregular, not forming a line, but it may get together as shown in Figure 1(c). In addition, the size of magnetosomes is less than 150 nm and generally is 20-30 nm. The morphology of magnetosome is multitudinous, including cub octahedral, elongated prismatic, tooth-shaped and bullet-shaped [19, 21, 39, 46]. The morphology of nanocrystals seems like globular (Figure 2(a)) and the size is 20-30 at average in common with magnetosome (Figure 1(b)). A similar nanocrystals production was previously reported for Acidithiobacillus ferrooxidans, whereas Acidithiobacillus ferrooxidans can produce nanoparticles which are magnetic, and the number of those nanoparticles is 1-3 per cell [49, 50]. Here, we found that the number of nanocrystals of F. thermophilum L1T is 30±5 (n=100) each cell. It suggested that F. thermophilum L1T belongs to a sort of high-yield nanocrystals-producing strain.
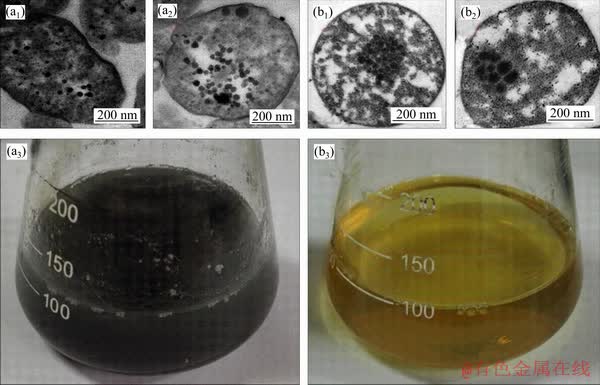
Figure 3 (a) CuFeS2 as energy source (the size of nanocrystals formed by F. thermophilum L1T was smaller, and the size was more well-distributed); (b) FeSO4·7H2O as energy source (the size of nanocrystals formed by F. thermophilum L1T was bigger, and the size was not well-distributed)
However, the chemical elements of the nanocrystals are iron, cobalt and oxygen, while sulphur is absent. It is different from magnetosomes that magnetosomes are iron minerals which currently are magnetite (Fe3O4) and/or greigite (Fe3S4) and contain high amounts of iron, oxygen and/or sulfur [18, 19, 39]. Synthesis of cobaltic nanocrystals was also reported by using the recombinant mms6 protein. Here, we found that the nanocrystals of F. thermophilum L1T contain iron as well as cobalt in nature conditions. It is notable that we did not add any cobaltic ions into the medium, but with respect to the source of cobalt, it may be from the 4.47 g/L FeSO4·7H2O that was added into the culture medium because the FeSO4·7H2O was not pure and contains cobalt ion. After ICP-OES analysis, it showed that the concentration of cobalt ions is 0.4-0.6 mg/L in the medium (Table 1). However, the concentration of iron is 9000-10000 mg/L in the medium, whereas the contents of iron and cobalt in the nanocrystals are similar, suggesting that both cobalt and ferrite potentially have contributed to the formation of nanocrystals, and the cobalt may be more important, and the cobalt ion uptake rate of F. thermophilum L1T is far more higher than the iron uptake rate.
The concentration of iron ions in chalcopyrite energy source is less than ferrous sulfate heptahydrate solution [2, 51]. Consistent with these findings, the initial Fe availability can influence the morphologies and sizes of mature magnetosomes synthesized by M. gryphiswaldense MSR-1 (DSM6361) [20, 52]. Although the size of the studied particles was similar to magnetosomes synthesized by MTB, the physical properties, like crystal-size distribution, morphology and aspect ratio, were significantly disparate. The formation of magnetite, a mixed-valence iron oxide, is only favored within a narrow redox range (n(Fe3+): n(Fe2+)=2:1). Thus, the formation of less and abnormal crystals of magnetite can be caused by strong perturbations of redox conditions, such as high oxygen levels, growth on highly oxidized carbon sources or inhibition of respiratory pathways. Here, we also found that the ORP of the chalcopyrite medium is higher than that of ferrous sulfate heptahydrate (Figure 4), indicating that the content of Fe3+ in the chalcopyrite medium is more than the content of Fe3+ in the ferrous sulfate heptahydrate medium. Thus Fe3+ led to an induced synthesis of nanoparticles with multiple shape CSDs and abnormal morphologies. Therefore, it is possible that the initial Fe availability and the ORP are the main reasons that caused the differences of size and morphology of nanocrystals.
Table 1 Concentrations of iron and cobalt in culture fluid of iron culture and mineral culture
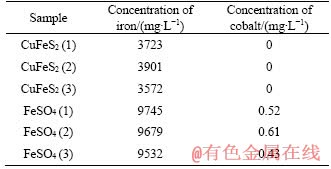
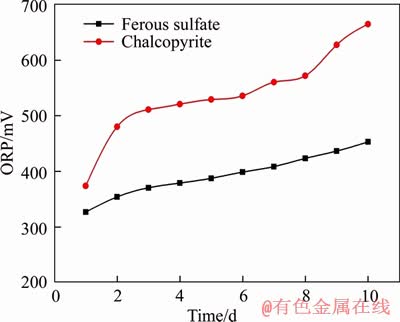
Figure 4 Change of potential in process of iron culture and mineral culture
The reason that the higher initial Fe availability leads to an induced synthesis of larger nanocrystals and the lower ORP leads to an induced synthesis of nanocrystals with positively abnormal inhomogeneous size, maybe that the higher initial Fe availability and the lower ORP induced a higher uptake efficiency of iron ions of F. thermophilum L1T. Under different culture conditions of F. thermophilum L1T shown in Figure 5, it suggested that when the initial Fe availability is 3000 mg/L in the medium, the concentration of iron of cystolic approximately is 1.21 mg/L in 10 mg F. thermophilum L1T, and the nanocrystals are small; However, when the initial Fe availability is 5000 mg/L in the medium, the concentration of iron of cystolic approximately is 3.26 mg/L in 10 mg F. thermophilum L1T, and the nanocrystals are large. It may infer that the higher initial Fe availability leads to a higher uptake efficiency of iron ions in F. thermophilum L1T, and then it has an induced synthesis of larger nanocrystals. When the concentration of Fe3+: Fe2+ is 1 : 1 in the medium, the concentration of iron of cystolic is approximately 3.71 mg/L in 10 mg F. thermophilum L1T, and the nanocrystals are inhomogeneous; When the concentration of Fe3+ : Fe2+ is 5 : 1 in the medium, the concentration of iron of cystolic is approximately 2.59 mg/L in 10 mg F. thermophilum L1T and the nanocrystals are homogeneous. It may infer that the different ORP leads to different uptake efficiency of ferrous and ferric of F. thermophilum L1T, and then different uptake efficiency of ferrous and ferric leads to the different size of nanocrystals. It suggested that the lower ORP (lower Fe3+) leads to an induced higher uptake efficiency of iron ions in F. thermophilum L1T, and it also leads to an induced synthesis of nanocrystals with abnormal unhomogeneous size.
This would also suggest that the formation of nanocrystals of F. thermophilum L1T is a strict gene regulation process, not an induced process by special energy source. With respect to the mechanisms of magnetosome biogenesis, the approximately 20 proteins which are magnetosome- specific have functions in the formation of vesicle, iron transport, the control of crystallization and intracellular magnetosomes arrangement [19, 53-55]. Although there are investigations of F. thermophilum in gene level, there is no paper to report the genes forming intracellular iron oxide and cobalt oxide mineral or any similar genes forming magnetosome in F. thermophilum [12, 50-52, 56-58]. But it doesn’t mean that F. thermophilum has no genes that regulate iron oxide and cobalt oxide [59, 60], and it may be a new approach of biomineralization and still needs more investigation.

Figure 5 Concentration of cellular iron, dry cell mass and TEM image of cell under different culture conditions
4 Conclusions
1) F. thermophilum can synthesize iron and cobalt-containing magnetic nanoparticles with a size of 25 nm in the cell and it is a magnetic nanoparticle high-yield strain.
2) Under different energy culture conditions, the spatial distribution of iron-cobalt magnetic nanoparticles in F. thermophilum is different.
3) The synthesis of iron-cobalt magnetic nanoparticles in F. thermophilum is affected by the initial iron concentration and ORP (potential), in which the initial iron concentration is positively correlated and the ORP value is negatively correlated.
References
[1] QIN Wen-qing, YANG Cong-ren, LAI Shao-shi, WANG Jun, LIU Kai, ZHANG Bo. Bioleaching of chalcopyrite by moderately thermophilic microorganisms [J]. Bioresource Technology, 2013, 129: 200-208. DOI: 10.1016/j.biortech. 2012.11.050.
[2] ZHAO Hong-bo, WANG Jun, YANG Cong-ren, HU Ming-hao, GAN Xiao-wen, TAO Lang, QIN Wen-qing, QIU Guan-zhou. Effect of redox potential on bioleaching of chalcopyrite by moderately thermophilic bacteria: An emphasis on solution compositions [J]. Hydrometallurgy, 2015, 151: 141-150. DOI: 10.1016/j.hydromet.2014.11.009.
[3] ZHAO Hong-bo, WANG Jun, HU Ming-hao, QIN Wen-qing, ZHANG Yan-sheng, QIU Guan-zhou. Synergistic bioleaching of chalcopyrite and bornite in the presence of Acidithiobacillus ferrooxidans [J]. Bioresource Technology, 2013, 149: 71-76. DOI: 10.1016/j.biortech.2013.09.035.
[4] ZHAO Hong-bo, WANG Jun, GAN Xiao-wen, HU Ming-hao, ZHANG Er-xing, QIN Wen-qing, QIU Guan- zhou. Cooperative bioleaching of chalcopyrite and silver- bearing tailing by mixed moderately thermophilic culture: An emphasis on the chalcopyrite dissolution with XPS and electrochemical analysis [J]. Minerals Engineering, 2015, 81: 29-39. DOI: 10.1016/j.mineng.2015.07.015.
[5] WU Shi-fa, YANG Cong-ren, QIN Wen-qing, JIAO Fen, WANG Jun, ZHANG Yan-sheng. Sulfur composition on surface of chalcopyrite during its bioleaching at 50 °C [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(12): 4110-4118. DOI: 10.1016/S1003-6326(15)64062-6.
[6] YANG Bao-jun, ZHAO Chun-xiao, LUO Wen, LIAO Rui, GAN Min, WANG Jun, LIU Xue-duan, QIU Guan-zhou. Catalytic effect of silver on copper release from chalcopyrite mediated by Acidithiobacillus ferrooxidans [J]. Journal of Hazardous Materials, 2020, 392: 122290. DOI: 10.1016/ j.jhazmat.2020.122290.
[7] CHANG Ke-xin, ZHANG Yan-sheng, ZHANG Jia-ming, LI Teng-fei, WANG Jun, QIN Wen-qing. Effect of temperature- induced phase transitions on bioleaching of chalcopyrite [J]. Transactions of Nonferrous Metals Society of China, 2019, 29(10): 2183-2191. DOI: 10.1016/S1003-6326(19)65124-1.
[8] WU Ai-xiang, HU Kai-jian, WANG Hong-jiang, ZHANG Ai-qing, YANG Ying. Effect of ultraviolet mutagenesis on heterotrophic strain mutation and bioleaching of low grade copper ore [J]. Journal of Central South University, 2017, 24(10): 2245-2252. DOI: 10.1007/s11771-017-3634-2.
[9] ZHAO Hong-bo, WANG Jun, QIN Wen-qing, HU Ming-hao, ZHU Shan, QIU Guan-zhou. Electrochemical dissolution process of chalcopyrite in the presence of mesophilic microorganisms [J]. Minerals Engineering, 2015, 71: 159-169. DOI: 10.1016/j.mineng.2014.10.025.
[10] ZHAO Hong-bo, WANG Jun, QIN Wen-qing, ZHENG Xi-hua, TAO Lang, GAN Xiao-wen, QIU Guan-zhou. Surface species of chalcopyrite during bioleaching by moderately thermophilic bacteria [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(8): 2725-2733. DOI: 10.1016/S1003-6326(15)63897-3.
[11] ZHAO Hong-bo, HU Ming-hao, LI Yi-ni, ZHU Shan, QIN Wen-qing, QIU Guan-zhou, WANG Jun. Comparison of electrochemical dissolution of chalcopyrite and bornite in acid culture medium [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(1): 303-313.
[12] NANCUCHEO I, JOHNSON D B. Production of glycolic acid by Chemolithotrophic iron- and sulfur-oxidizing bacteria and its role in delineating and sustaining acidophilic sulfide mineral-oxidizing consortia [J]. Applied and Environmental Microbiology, 2010, 76(2): 461-467. DOI: 10.1128/AEM.01832-09.
[13] WANG Xing-xing, LIAO Rui, ZHAO Hong-bo, HONG Mao-xing, HUANG Xiao-tao, PENG Hong, WEN Wen, QIN Wen-qing, QIU Guan-zhou, HUANG Cao-ming, WANG Jun. Synergetic effect of pyrite on strengthening bornite bioleaching by Leptospirillum ferriphilum [J]. Hydrometallurgy, 2018, 176: 9-16. DOI: 10.1016/ j.hydromet.2017.12.003.
[14] LIU Sheng, XIE Lei, LIU Jun, LIU Guang-yi, ZHONG Hong, WANG Yi-xiang, ZENG Hong-bo. Probing the interactions of hydroxamic acid and mineral surfaces: Molecular mechanism underlying the selective separation [J]. Chemical Engineering Journal, 2019, 374: 123-132. DOI: 10.1016/j.cej.2019.05.152.
[15] ZHAO Hong-bo, HUANG Xiao-tao, WANG Jun, LI Yi-ni, LIAO Rui, WANG Xing-xing, QIU Xiao, XIONG Yu-ming, QIN Wen-qing, QIU Guan-zhou. Comparison of bioleaching and dissolution process of p-type and n-type chalcopyrite [J]. Minerals Engineering, 2017, 109: 153-161. DOI: 10.1016/ j.mineng.2017.03.013.
[16] YANG Bao-jun, LIN Mo, FANG Jing-hua, ZHANG Rui-yong, LUO Wen, WANG Xing-xing, LIAO Rui, WU Bai-qiang, WANG Jun, GAN Min, LIU Bin, ZHANG Yi, LIU Xue-duan, QIN Wen-qing, QIU Guan-zhou. Combined effects of jarosite and visible light on chalcopyrite dissolution mediated by Acidithiobacillus ferrooxidans [J]. Science of the Total Environment, 2020, 698: 134175. DOI: 10.1016/j.scitotenv.2019.134175.
[17] QIN Wen-qing, LIU Kai, DIAO Meng-xue, WANG Jun, ZHANG Yan-sheng, YANG Cong-ren, JIAO Fen. Oxidation of arsenite (As(III)) by ferric iron in the presence of pyrite and a mixed moderately thermophilic culture [J]. Hydrometallurgy, 2013, 137: 53-59. DOI: 10.1016/ j.hydromet.2013.05.011.
[18] LEFEVRE C T, BAZYLINSKI D A. Ecology, diversity, and evolution of magnetotactic bacteria [J]. Microbiology and Molecular Biology Reviews, 2013, 77(3): 497-526. DOI: 10.1128/MMBR.00021-13.
[19] UEBE R, SCHUELER D. Magnetosome biogenesis in magnetotactic bacteria [J]. Nature Reviews Microbiology, 2016,14(10): 621-637. DOI: 10.1128/MMBR.00021-13.
[20] FAIVRE D, MENGUY N, POSFAI M, SCHUELER D. Environmental parameters affect the physical properties of fast-growing magnetosomes [J]. American Mineralogist, 2008, 93(2, 3): 463-469. DOI: 10.1038/nrmicro.2016.99.
[21] POSFAI M, LEFEVRE T., TRUBITSYN D, BAZYLINSKI A, FRANKEL B. Phylogenetic significance of composition and crystal morphology of magnetosome minerals [J]. Frontiers in Microbiology, 2013, 4: 344. DOI: 10.3389/ fmicb.2013.00344.
[22] LEAO P, TEIXEIRA S, CYPRIANO J, FARINA M, ABREU F, BAZYLINSKI A, LINS U. North-seeking magnetotactic gammaproteobacteria in the southern hemisphere [J]. Applied and Environmental Microbiology, 2016, 82(18): 5595-5602. DOI: 10.1128/AEM.01545-16.
[23] JI Bo-yang, ZHANG Sheng-da, ZHANG Wei-jia, ROUY Z, ALBERTO F, SANTINI C, MANGENOT S, GAGNOT S, PHILIPPE N, PRADEL N, ZHANG Li-chen, TEMPEL S, LI Ying, MEDIGUE C, HENRISSAT B, COUTINHO P M, BARBE V, TALLA E, WU Long-fei. The chimeric nature of the genomes of marine magnetotactic coccoid-ovoid bacteria defines a novel group of Proteobacteria [J]. Environmental Microbiology, 2017, 19(3): 1103-1119. DOI: 10.1111/1462-2920.13637.
[24] HUIZAR-FELIX A M, MUNOZ D, ORUE I, MAGEN C, IBARRA A, BARANDIARAN J M, MUELA A, FDEZ- GUBIEDA M L. Assemblies of magnetite nanoparticles extracted from magnetotactic bacteria: A magnetic study [J]. Applied Physics Letters, 2016, 108(6): 0631096. DOI: 10.1063/ 1.4941835.
[25] BAZYLINSKI D A, FRANKEL R B, HEYWOOD B R, MANN S, KING J W, DONAGHAY P L, HANSON A K. Controlled biomineralization of magnetite (Fe3O4) and greigite (Fe3S4) in a magnetotactic bacterium [J]. Applied and Environmental Microbiology, 1995, 61(9): 3232-3239. DOI: 10.1128/AEM.61.9.3232-3239.1995.
[26] LEFEVRE CHRISTOPHER T, MENGUY N, ABREU F, LINS U, POSFAI M, PROZOROV T, PIGNOL D, FRANKEL R B, BAZYLINSKI D A. A cultured greigite-producing magnetotactic bacterium in a novel group of sulfate-reducing bacteria [J]. Science, 2011, 334(6063): 1720-1723. DOI: 10.1126/science.1212596.
[27] BARBER-ZUCKER S, ZARIVACH R. A look into the biochemistry of magnetosome biosynthesis in magnetotactic bacteria [J]. ACS Chemical Biology, 2017, 12(1): 13-22. DOI: 10.1021/acschembio.6b01000.
[28] PROZOROV T, PALO P, WANG Li-jun, NILSEN- HAMILTON M, JONES D, ORR D, MALLAPRAGADA S K, NARASIMHAN B, CANFIELD P C, PROZOROV R. Cobalt ferrite nanocrystals: Out-performing magnetotactic bacteria [J]. ACS Nano, 2007, 1(3): 228-233. DOI: 10.1021/ nn700194h.
[29] STANILAND S, WILLIAMS W, TELLING N, van der LAAN G, HARRISON A, WARD B. Controlled cobalt doping of magnetosomes in vivo [J]. Nature Nanotechnology, 2008, 3(3): 158-162. DOI: 10.1038/nnano.2008.35.
[30] COKER V S, TELLING N D, van der LAAN G, PATTRICK R A D, PEARCE C I, ARENHOLZ El, TUNA F, WINPENNY RICHARD E P, LLOYD JONATHAN R. Harnessing the extracellular bacterial production of nanoscale cobalt ferrite with exploitable magnetic properties [J]. ACS Nano, 2009, 3(7): 1922-1928. DOI: 10.1021/ nn900293d.
[31] FANG Jing-hua, LIU Yong, HE Wan-li, QIN Wen-qing, QIU Guan-zhou, WANG Jun. Transformation of iron in pure culture process of extremely acidophilic microorganisms [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(5): 1150-1155. DOI: 10.1016/S1003-6326(17)60134-1.
[32] YAN Lei, LI Pei-ye, ZHAO Xiao-peng, JI Rong, ZHAO Li-juan. Physiological and metabolic responses of maize (Zea mays) plants to Fe3O4 nanoparticles [J]. Science of the Total Environment, 2020, 718: 137400. DOI: 10.1016/ j.scitotenv.2020.137400.
[33] WU Ling-bo, YANG Bao-jun, WANG Xing-xing, WU Bai-qiang, HE Wan-li, GAN Min, QIU Guan-zhou, WANG Jun. Effects of single and mixed energy sources on intracellular nanoparticles synthesized by Acidithiobacillus ferrooxidans [J]. Minerals, 2019, 9(3): 163. DOI: 10.3390/ min9030163.
[34] XU Hang, TAN Ling, CUI Hao, XU Mei-ying, XIAO Yong, WU Hai-yan, DONG Hai-gang, LIU Xin-xing, QIU Guan-zhou, XIE Jian-ping. Characterization of Pd(II) biosorption in aqueous solution by Shewanella oneidensis MR-1 [J]. Journal of Molecular Liquids, 2018, 255: 333-340. DOI: 10.1016/j.molliq.2018.01.168.
[35] ZHOU H, ZHANG R, HU P, ZENG W, XIE Y, WU C, QIU G. Isolation and characterization of Ferroplasma thermophilum sp nov., a novel extremely acidophilic, moderately thermophilic archaeon and its role in bioleaching of chalcopyrite [J]. Journal of Applied Microbiology, 2008, 105(2): 591-601. DOI: 10.1111/j.1365-2672.2008.03807.x.
[36] ZHAO Hong-bo, WANG Jun, GAN Xiao-wen, HU Ming-hao, TAO Lang, QIN Wen-qing, QIU Guan-zhou. Role of pyrite in sulfuric acid leaching of chalcopyrite: An elimination of polysulfide by controlling redox potential [J]. Hydrometallurgy, 2016, 164: 159-165. DOI: 10.1016/ j.hydromet.2016.04.013.
[37] WANG Jun, GAN Xiao-wen, ZHAO Hong-bo, HU Ming- hao, LI Kai-yun, QIN Wen-qing, QIU Guan-zhou. Dissolution and passivation mechanisms of chalcopyrite during bioleaching: DFT calculation, XPS and electrochemistry analysis [J]. Minerals Engineering, 2016, 98: 264-278. DOI: 10.1016/j.mineng.2016.06.006. DOI: 10.1016/j.mineng.2016.09.008.
[38] WANG Jun, TAO Lang, ZHAO Hong-bo, HU Ming-hao, ZHENG Xi-hua, PENG Hong, GAN Xiao-wen, XIAO Wei, CAO Pan, QIN Wen-qin, QIU Guan-zhou, WANG Dian-zuo. Cooperative effect of chalcopyrite and bornite interactions during bioleaching by mixed moderately thermophilic culture [J]. Minerals Engineering, 2016, 95: 116-123. DOI: 10.1016/j.mineng.2016.06.006.
[39] FAIVRE D, SCHUELER D. Magnetotactic Bacteria and Magnetosomes [J]. Chemical Reviews, 2008, 108(11): 4875-4898. DOI: 10.1021/cr078258w.
[40] WANG Jun, HU Ming-hao, ZHAO Hong-bo, GAN Min, bGAN Xiao-wen, QIN Wen-qing, QIU Guan-zhou. Well- controlled column bioleaching of a low-grade copper ore by a novel equipment [J]. Journal of Central South University, 2015, 22: 3318-3325. DOI: 10.1007/s11771-015-2872-4.
[41] BAZYLINSKI D A, LEFEVRE C T. Magnetotactic bacteria from extreme environments [J]. Life-Basel, 2013, 3(2, Sp. Iss. SI): 295-307. DOI: 10.3390/life3020295.
[42] ZHAO Hong-bo, GAN Xiao-wen, WANG Jun, TAO Lang, QIN Wen-qing, QIU Guan-zhou. Stepwise bioleaching of Cu-Zn mixed ores with comprehensive utilization of silver- bearing solid waste through a new technique process [J]. Hydrometallurgy, 2017, 171: 374-386. DOI: 10.1016/ j.hydromet.2017.06.002.
[43] FENG Ya-li, WANG Hong-jun, LI Hao-ran, CHEN Xi-pei, DU Zhu-wei, KANG Jin-xing. Effect of iron transformation on Acidithiobacillus ferrooxidans bio-leaching of clay vanadium residue [J]. Journal of Central South University, 2019, 26(4): 796-805. DOI: 10.1007/s11771-019-4049-z.
[44] HONG Mao-xin, WANG Xing-xing, WU Ling-bo, FANG Chao-jun, HUANG Xiao-tao, LIAO Rui, ZHAO Hong-bo, QIU Guan-zhou, WANG Jun. Intermediates transformation of bornite bioleaching by Leptospirillum ferriphilum and Acidithiobacillus caldus [J]. Minerals, 2019, 9(3): 159. DOI: 10.3390/min9030159.
[45] KOMEILI A. Molecular mechanisms of compartmentalization and biomineralization in magnetotactic bacteria [J]. Fems Microbiology Reviews, 2012, 36(1): 232-255. DOI: 10.1111/j.1574-6976.2011.00315.x.
[46] LIN Wei, BAZYLINSKI D A, XIAO Tian, WU Long-fei, PAN Yong-xin. Life with compass: Diversity and biogeography of magnetotactic bacteria [J]. Environmental Microbiology, 2014, 16(9SI): 2646-2658. DOI: 10.1111/ 1462-2920.12313.
[47] SCHEFFEL A, GRUSKA M, FAIVRE D, LINAROUDIS A, GRAUMANN P L, PLITZKO J M, SCHULER D. An acidic protein aligns magnetosomes along a filamentous structure in magnetotactic bacteria (vol 440, pg 110, 2006) [J]. Nature, 2006, 441(7090): 248. DOI: 10.1038/nature04382.
[48] WANG Jun, LIAO Rui, TAO Lang, ZHAO Hong-bo, ZHAI Rui, QIN Wen-qing, QIU Guan-zhou. A comprehensive utilization of silver-bearing solid wastes in chalcopyrite bioleaching [J]. Hydrometallurgy, 2017, 169: 152-157. DOI: 10.1016/j.hydromet.2017.01.006.
[49] YAN Lei, YUE Xiao-xuan, ZHANG Shuang, CHEN Peng, XU Zhi-liang, LI Yang, LI Hong-yu. Biocompatibility evaluation of magnetosomes formed by Acidithiobacillus ferrooxidans [J]. Materials Science & Engineering C-Materials for Biological Applications, 2012, 32(7): 1802-1807. DOI: 10.1016/j.msec.2012.04.062.
[50] YAN Lei, ZHANG Shuang, CHEN Peng, WANG Wei-dong, WANG Yan-jie, LI Hong-yu. Magnetic properties of Acidithiobacillus ferrooxidans [J]. Materials Science & Engineering C-Materials for Biological Applications, 2013, 33(7): 4026-4031. DOI: 10.1016/j.msec.2013.05.046.
[51] GAN Min, LI Jia-yu, SUN Sheng-jie, CAO Yuan-yan, ZHENG Zhi-he, ZHU Jian-yu, LIU Xin-xing, WANG Jun, QIU Guan-zhou. The enhanced effect of Acidithiobacillus ferrooxidans on pyrite based Cr(VI) reduction [J]. Chemical Engineering Journal, 2018, 341: 27-36. DOI: 10.1016/ j.cej.2018.02.014.
[52] MOISESCU C, ARDELEAN I I, BENNING L G. The effect and role of environmental conditions on magnetosome synthesis [J]. Frontiers in Microbiology, 2014, 5(49). DOI: 10.3389/fmicb.2014.00049.
[53] FUKUDA Y, OKAMURA Y, TAKEYAMA H, MATSUNAGA T. Dynamic analysis of a genomic island in Magnetospirillum sp strain AMB-1 reveals how magnetosome synthesis developed [J]. Febs Letters, 2006, 580(3): 801-812. DOI: 10.1016/j.febslet.2006.01.003.
[54] ULLRICH S, KUBE M, SCHUBBE S, REINHARDT R, SCHULER D. A hypervariable 130-kilobase genomic region of Magnetospirillum gryphiswaldense comprises a magnetosome island which undergoes frequent rearrangements during stationary growth [J]. Journal of Bacteriology, 2005, 187(21): 7176-7184. DOI: 10.1128/ JB.187.21.7176-7184.2005.
[55] JOGLER C, WANNER G, KOLINKO S, NIEBLER M, AMANN R, PETERSEN N, KUBE M, REINHARDT R, SCHUELER D. Conservation of proteobacterial magnetosome genes and structures in an uncultivated member of the deep-branching nitrospira phylum [J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(3): 1134-1139. DOI: 10.1073/pnas.1012694108.
[56] WANG Jun, ZHAO Hong-bo, QIN Wen-qing, QIU Guan- zhou. Bioleaching of complex polymetallic sulfide ores by mixed culture [J]. Journal of Central South University, 2014, 21(7): 2633-2637. DOI: 10.1007/s11771-014-2223-x.
[57] WANG Jun, ZHU Shan ZHANG Yan-sheng, ZHAO Hong-bo, HU Ming-hao, YANG Cong-ren, QIN Wen-qing, QIU Guan-zhou. Bioleaching of low-grade copper sulfide ores by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans [J]. Journal of Central South University, 2014, 21: 728-734. DOI:10.1007/s11771-014-1995-3.
[58] ZHAO Hong-bo, ZHANG Yi-sheng, ZHANG Xian, QIAN Lu, SUN Meng-lin, YAN Yu, ZHANG Yan-sheng, WANG Jun, KIM Hyunjung, QIU Guan-zhou. The dissolution and passivation mechanism of chalcopyrite in bioleaching: An overview [J]. Minerals Engineering, 2019, 136: 140-154. DOI: /10.1016/j.hydromet.2019.05.002.
[59] WANG Jun, ZHAO Hong-bo, ZHUANG Tian, QIN Wen-qing, ZHU Shan, QIU Guan-zhou. Bioleaching of Pb–Zn–Sn chalcopyrite concentrate in tank bioreactor and microbial community succession analysis [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(12): 3758-3762. DOI: 10.1016/S1003-6326(13)62926-X.
[60] ZHAO Hong-bo, HUANG Xiao-tao, HU Ming-hao, ZHANG Chen-yang, WANG Jun QIN Wen-qing, QIU Guan-zhou. Insights into the surface transformation and electrochemical dissolution process of bornite in bioleaching [J]. Minerals, 2018, 8: 173. DOI: 10.3390/ min8040173.
(Edited by YANG Hua)
中文导读
极端嗜酸古菌Ferroplasma thermophilum合成胞内含钴铁纳米颗粒
摘要:本文研究一种极端嗜酸古菌Ferroplasma thermophilum合成胞内含铁钴的纳米颗粒。通过冷冻切片和透射电镜发现纳米颗粒的大小在20~60 nm,其数目在每个细胞中平均超过30个,这表明Ferroplasma thermophilum不仅可以合成胞内纳米颗粒,还是一种高产菌。更有趣的是,通过EDS X-ray分析表明,纳米颗粒的元素组成包含铁与钴,这表明铁与钴都参与了纳米颗粒的合成。更重要的是,在不同能源物质由硫酸亚铁与黄铜矿的培养下,发现Ferroplasma thermophilum所合成的纳米颗粒大小与形态不同。进一步研究表明,这一现象与培养基中最初铁离子浓度和电位有关,在高初始铁离子浓度培养下,Ferroplasma thermophilum所合成的纳米颗粒较大,低电位导致纳米颗粒形态不均一, 原因在于高初始铁离子浓度与低电位促进了Ferroplasma thermophilum铁离子的吸收。我们的研究丰富了磁性细菌的趋磁种类,增加了微生物合成胞内纳米颗粒的多样性,尤其是钴的参与。
关键词:Ferroplasma thermophilum;钴铁纳米颗粒;生物矿化;生物浸出;极端嗜酸微生物
Foundation item: Project(2018JJ1041) supported by the Natural Science Foundation of Hunan, China; Projects(51774332, 51934009, U1932129) supported by the National Natural Science Foundation of China
Received date: 2018-03-16; Accepted date: 2020-05-13
Corresponding author: WANG Jun, PhD, Professor; Tel: +86-731-88876697; E-mail: wjwq2000@126.com; ORCID: 0000-0003-0931- 3946

