Order of sphalerite and galena precipitation: A case study from lead-zinc deposits in southwest China
来源期刊:中南大学学报(英文版)2020年第1期
论文作者:韩润生 张艳 魏平堂
文章页码:288 - 310
Key words:precipitation order; thermodynamic phase diagram; mineral zoning; lead and zinc deposit; southwest of China
Abstract: Most of the lead and zinc deposits in Southwest China, are characterized by mineral zoning, which is especially true for the Huize and Zhaotong deposits. The mineral assemblage zoning is consistent for both horizontal and vertical zoning, from the base (center) of the ore body to the top (outermost), the mineral zones are as follows. I-1: coarse-grained pyrite and a little puce sphalerite; I-2: brown sphalerite, galena, and ferro-dolomite; I-3: galena, sandy beige and pale yellow sphalerite, and calcite; and I-4: fine-grained pyrite, dolomite, and calcite. Among them, sphalerite is the landmark mineral of different zoning. From I-1 to I-3, the color of sphalerite changes from dark to light, its crystalline size changes from coarse to fine, and its structure changes from disseminated to veinlet. This mineral zoning is seen not only on a microscopic scale, but is also clear on a mesoscopic and macroscopic scale. It is caused by the order of the sphalerite and galena precipitation. We studied the metallic minerals and fluid inclusions using a thermodynamic phase diagram method, such as lgfO2–lgfS2, pH–lgfO2, pH–lg[Pb2+] and pH–lg[HS-], discussed the constraints on the order of the sphalerite and galena precipitation in the migration and precipitation process of lead and zinc under different pH values, oxygen fugacity, sulfur fugacity, and ionic activity. We also explain the formation mechanism and propose that the main controlling factor of the order of the sphalerite and galena precipitation is sulfur fugacity.
Cite this article as: ZHANG Yan, HAN Run-sheng, WEI Ping-tang. Order of sphalerite and galena precipitation: A case study from lead-zinc deposits in southwest China [J]. Journal of Central South University, 2020, 27(1): 288-310. DOI: https://doi.org/10.1007/s11771-020-4296-z.

J. Cent. South Univ. (2020) 27: 288-310
DOI: https://doi.org/10.1007/s11771-020-4296-z

ZHANG Yan(张艳)1, HAN Run-sheng(韩润生)1, WEI Ping-tang(魏平堂)2
1. Kunming University of Science and Technology, Southwest of Geological Survey, Geological Survey Center for Non-ferrous Mineral Resources, Kunming 650093, China;
2. Kunming Geological Prospecting Institute, China Metallurgical Geological Bureau,Kunming 650024, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract: Most of the lead and zinc deposits in Southwest China, are characterized by mineral zoning, which is especially true for the Huize and Zhaotong deposits. The mineral assemblage zoning is consistent for both horizontal and vertical zoning, from the base (center) of the ore body to the top (outermost), the mineral zones are as follows. I-1: coarse-grained pyrite and a little puce sphalerite; I-2: brown sphalerite, galena, and ferro-dolomite; I-3: galena, sandy beige and pale yellow sphalerite, and calcite; and I-4: fine-grained pyrite, dolomite, and calcite. Among them, sphalerite is the landmark mineral of different zoning. From I-1 to I-3, the color of sphalerite changes from dark to light, its crystalline size changes from coarse to fine, and its structure changes from disseminated to veinlet. This mineral zoning is seen not only on a microscopic scale, but is also clear on a mesoscopic and macroscopic scale. It is caused by the order of the sphalerite and galena precipitation. We studied the metallic minerals and fluid inclusions using a thermodynamic phase diagram method, such as lgfO2–lgfS2, pH–lgfO2, pH–lg[Pb2+] and pH–lg[HS-], discussed the constraints on the order of the sphalerite and galena precipitation in the migration and precipitation process of lead and zinc under different pH values, oxygen fugacity, sulfur fugacity, and ionic activity. We also explain the formation mechanism and propose that the main controlling factor of the order of the sphalerite and galena precipitation is sulfur fugacity.
Key words: precipitation order; thermodynamic phase diagram; mineral zoning; lead and zinc deposit; southwest of China
Cite this article as: ZHANG Yan, HAN Run-sheng, WEI Ping-tang. Order of sphalerite and galena precipitation: A case study from lead-zinc deposits in southwest China [J]. Journal of Central South University, 2020, 27(1): 288-310. DOI: https://doi.org/10.1007/s11771-020-4296-z.
1 Introduction
Although lead and zinc have a similar electronic configuration and similar geochemical behaviors, such as having a strong affinity to sulfur. However, researchers have observed a clear mineral zoning in lead and zinc sulfide deposits [1]. An increasing number of studies have confirmed the existence of this phenomenon [2-4]. The realization has grown that sphalerite and galena are not always closely associated each other, as traditionally believed. Instead, the order of sphalerite and galena precipitation is successive. The difference in the precipitation sequence has resulted in mineral zonation. Therefore, studies on the mechanism of the order of mineral deposits can considerably promote better understanding of the genesis of lead and zinc sulfide ore deposits.
Two factors, internal and external, control whether minerals can be precipitated from hydrothermal fluid. For lead and zinc, the internal cause is the solubility of galena and sphalerite, and the stability of the Pb and Zn complexes. The external factor includes a variety of environmental factors, such as temperature (T), pressure (P), Eh (Redox potential), pH, salinity, fugacity, and activity, which can change solubility or the complex instability. Many scholars have extensively studied the process of lead and zinc dissolution, mobilization, transportation, and precipitation in hydrothermal solutions, with a focus on chloric and sulfurous solutions [5-15]. Research has proven that the metallic elements in hydrothermal fluid mainly migrate in the form of complexes, such as chloride, sulfur, fluoride, and hydroxyl complexes [16-21]. The characteristics, activity, coordination field stability, and other endogenous factors of the central ion and ligand, and temperature, pressure, pH, oxygen fugacity, sulfur fugacity, and other environmental factors, all affect the stability of the complex [22-28]. Many achievements have been made in the field of mineral solubility [8, 9, 12, 29-39], T and pH are known as important factors affecting solubility.
Over 440 poly-metallic deposits and prospects occur in Sichuan, Yunnan, and Guizhou (SYG) provinces of Southwest China, among these, three super-large deposits are known, Huize, Hanyuan and Zhugongtang, as well as eight large deposits, Zhaotong, Tianbaoshan, Daliangzi, Chipu, Maozu, Fulechang, Lehong, and Xiaoshifang, and 21 small- to medium-sized deposits. Aside from Xiaoshifang, the remaining lead-zinc deposits are non-magmatic epigenetic-hydrothermal deposits hosted in carbonate deposits. LEACH et al [25, 40] defined that the lead-zinc deposits found on carbonate platforms are dominated by dolomite or limestone and are produced at the sides of basins, orogenic foreland, and foreland fold-thrust belts, like in the Mississippi Valley-type (MVT) deposits [41]. HAN et al [42-45] classified the non-magmatic epigenetic-hydrothermal lead-zinc deposits into MVT- and Huize-type (HZT) deposits, according to the mineralization type, temperature, and so on. The majority of the world’s non-magmatic epigenetic- hydrothermal lead-zinc deposits are a transition between these two deposit types.
Most of the lead-zinc deposits in this area are characterized by obvious mineral zoning. The Huize and Zhaotong Pb-Zn deposits are two of the richest large Pb-Zn deposits in the world [46]. They have a unique metallogenic system, characterized by large reserves of lead and zinc, with over eight and three megatons, respectively, high mean grades (Pb and Zn: 25%-35%, with some exceeding 60%), and obvious mineral zoning. These characteristics are found in the metallogenic zones in the SYG area. The deposits in this area have been exhaustively studied, and a series of important findings have been made [47-58]. However, little was known about the mechanism that the order of sphalerite and galena precipitation in the lead-zinc deposits in this area, which substantially affects our understanding of the temporal-spatial distribution law of ore-forming elements and deposit types to direct mineral resource exploration.
Therefore, we selected two representative deposits, Huize and Zhaotong, in the SYG area and used phase diagram (such as lgfO2-lgfS2, pH-lgfO2, pH-log[Pb2+], and pH-lg[HS-]) to elucidate the constraints on the zoning of lead and zinc with different pH, oxygen fugacity, sulfur fugacity, and ionic activity conditions. This study may introduce the connection between time and space of the sphalerite and galena, and the reason for the mineral zonation in the Huize and Zhaotong lead-zinc deposits. In addition, based on thermodynamic theory, this paper also discusses the mechanism of the zoning of lead and zinc minerals and reveals the main factors controlling zonation in the different types of lead-zinc sulfide deposits.
2 Ore deposits
The SYG Pb–Zn polymetallic metallogenic region is located within the Huili–Kunming rift, and is surrounded by deep and large faults that act as boundaries. This region is bordered by the N–S trending Anninghe–Luzhijiang Fault, Kangding– Yiliang–Shuicheng Fault, and Mile–Shizong– Shuicheng Fault to the west, north, and south, respectively. The Huize and Zhaotong deposits are two important deposits in this area. The regional stratigraphy is composed of Precambrian basement overlain by a sedimentary cover sequence deposited in the Late Sinian, with an angular unconformable contact between these two units. The metamorphic basement is mainly composed of the Mesoproterozoic Hekou and Huili groups, the Neoproterozoic Yanbian and Yanjing groups, and large Neoproterozoic magmatic complexes, as well as their equivalent strata. The sedimentary cover comprises marine sediments composed of strata ranging in age from Sinian to Permian, and terrestrial sediments that consist of Mesozoic and Cenozoic strata. The tectonic structure of this region comprises 15 faults, which may be categorized as near-NS-, NE-, and NW-trending faults. In particular, the N–S trending Xiaojiang Fault and Zhaotong–Qujing concealed fault play a significant role in controlling the magmatic activity in the region, as well as the distribution and development of lead, zinc, and silver deposits.
The Huize Pb-Zn deposit, composed of Qilinchang, Kuangshanchang, and Yinchangpo, is located on the southwestern margin of the Yangtze Plate (Figure 1), and is the world’s largest and richest lead-zinc deposit [46]. Its orebodies mainly occur within the medium-to-coarse-grained dolomites of the Lower Carboniferous Baizuo Formation (C1b). The northeast to southwest- trending reverse shear faults are important ore-controlling tectonic structures. The Qilinchang, Kuangshanchang, and Yinchangpo faults control the Qilinchang, Kuangshanchang, and Yinchangpo deposits (Figure 1), respectively.
The Zhaotong Pb-Zn deposit is located on the inverted limb of the Maomaoshan anticline and controlled by the northeast–southwest reversed anticline and the Maoping fault (Figure 2). In this mining area, the main emergence strata are the mid-upper Devonian, the Carboniferous, and the Permian.
The orebodies of these two deposits usually occur as sheets, lenticles, sacs, lentils, and irregular veins, and generally propagate along interlayer fractures. Lateral end-to-end alignment of structures and expansion and contraction are usually observed along the strike and dip of the orebodies. The boundaries between the orebodies and the host rocks are sharp.
The two deposits have a similar minmalogical composition and structure. The major ore minerals are massive and disseminated sphalerite, galena, and pyrite, and the main gangue minerals are calcite, dolomite, and quartz. The ore shows idiomorphic, hypidiomorphic, granular, metasomatic, mosaic, coedge, interstitial, embodying, internal cleavage, crumpled, and cataclastic textures.

Figure 1 Simplified geological map of Huize lead-zinc ore district (adapted from Refs. [43, 51])

Figure 2 Simplified geological map of Zhaotong lead-zinc ore district (modified from Refs. [51, 74])
Based on the macroscopic characteristics, ore structures and textures, and mineral assemblage characteristics of the deposit, the metallogenesis of the Huize and Zhaotong Pb-Zn deposit can be divided into two periods: hydrothermal metallogenesis, and supergene oxidation [59] (Table 1), the first of which can be further divided into four metallogenic stages: the pyrite (sphalerite) stage, sphalerite–galena stage, galena–sphalerite stage, and pyrite–carbonate stage (Figures 3(a)-(d)). Figures 3-5 depict the characteristics of mineral zoning for a lead and zinc sulfide deposit in Southwestern China. The figures show that the zoning of lead and zinc minerals manifests on both microscopic and macroscopic scale. As shown in Figures 3(a)-(d), three generations of sphalerite and two generations of galena were formed in the hydrothermal mineralization period. From the first to the third generation, the color of sphalerite changes from black to puce to brown, its crystalline size changes from coarse to medium-coarse grain to medium-fine grain, and its structure changes from disseminated to massive to veinlet. The first generation of galena is closely associated with sphalerite and the second generation of galena formed separately. Such obvious mineral zoning is also observed in hand specimens and the ore body (Figures 3(e)-(g)). From the base (center) to the top (outermost) of the orebodies, the minerals show a vertical and horizontal zoning change from a small amount of black sphalerite to puce sphalerite and galena, to galena and brown sphalerite to yellow sphalerite. The mineral zoning is obvious in the scale of the orebodies too (Figures 4 and 5). From base to top, the mineral assemblage changes as follows: coarse-grained pyrite and a small amount of dark brown sphalerite to brown sphalerite, galena, and ferro-dolomite to galena and sandy beige to pale yellow sphalerite, quartz, and calcite to fine-grained pyrite and calcite and dolomite (Figures 4 and 5).
Table 1 Mineral paragenesis and zoning of Huize and Zhaotong Pb–Zn deposits, SW(modified by Ref. [59])
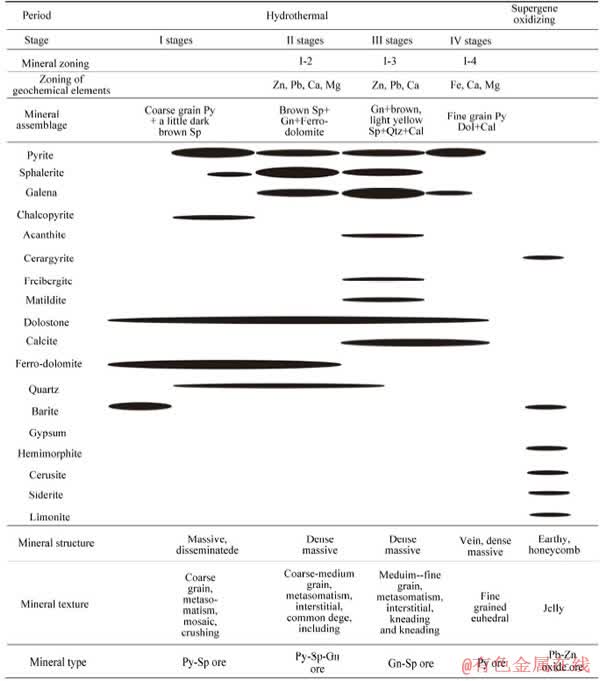

Figure 3 Mineral zoning at different scales:(Sp-sphalerite; Gn-galena, Dol-dolomite; Cal-Calcite. (-): Single polarization, (+): perpendicular polarization)

Figure 4 Alteration and mineralization zoning in Huize Pb-Zn deposit: Profile A-B: Alteration dolomite and mineral assemblage zonation in #86 transverse drift of 1261 m in the Qilinchang deposit. Profile C-D: Alteration of dolomite and mineral assemblage zonation in #90 transverse drift of 1261 m in the Qilinchang deposit. Profile E-F: Alteration of dolomite and mineral assemblage zonation in #15 stope of 1752 m in the Kuangshanchang deposit.(Gn-galena; Sp-sphalerite; Dol-dolomite; Cal-calcite; and Py- pyrite)

Figure 5 Alteration and mineralization zoning in Zhaotong Pb-Zn deposit:(Profile A-B: alteration of dolomite and mineral assemblage zonation in #98 transverse drift of 760 m; Profile C-D: alteration of dolomite and mineral assemblage zonation in #98 transverse drift of 670 m. Gn-galena; Sp-sphalerite; Qtz-quartz; and Cal-calcite)
Obvious mineral zoning is not only present in the Huize and Zhaotong deposits, but also in other Pb-Zn deposits in the SYG domain, such as Maozu, Jinshachang, and Fulechang. Based on detailed field work and petrological and mineralogical studies, we divided the mineral zoning into two types (Figure 6): horizontal zoning and vertical zoning. For a single orebody, the cross section AB mainly represents the horizontal zoning, and its longitudinal profile CD is represented as vertical zoning. This is the result of sulfide precipitation and the interaction between water and rock during the upward migration and diffusion around of hydrothermal fluid. The mineral zoning is consistent in both horizontal and vertical directions, that is, from the base (center) of the ore body to the top (outermost), the mineral assemblages are aligned. The sequence is as follows: I-1: coarse- grained pyrite and a little puce sphalerite; I-2: brown sphalerite, galena, and ferro-dolomite; I-3: galena and sandy beige and pale yellow sphalerite, and calcite; and I-4: fine-grained pyrite, dolomite, and calcite. This corresponds to the four metallogenic stages in the Huize and Zhaotong lead-zinc deposits [42, 44, 60], and corresponds to the zoning of geochemical elements(I-1: Fe and Zn; I-2: Zn, Pb, Ca, and Mg; I-3: Pb, Zn, and Ca; and I-4: Fe, Ca, and Mg).

Figure 6 Two zonation forms of metal minerals in lead and zinc deposits, Southwest China(AB profile-horizontal zoning; and CD profile-vertical zoning. Abbreviations: Gn-galena; Sp-sphalerite; Dol-dolomite)
3 Materials and methods
Based on studying petrographic data and details of the metallic minerals and relative fluid inclusions in the field, we achieved a series of plotting phase diagrams for pH-lgfO2, lgfO2-lgfS2, pH-lg[Pb2+], and pH-lg[HS-], which could reveal the physiochemical characteristics of lead and zinc sulfide deposits.
Previous studies [25, 61-63] and our recent studies [44, 64, 65] on the fluid inclusions demonstrated that the temperature in the main mineralizing phase ranges from 100 °C to 250 °C, being mostly around (200±20) °C. This means that the calculation method and thermodynamic data from the literature [66] could be used to plot the phase diagram under various temperatures. In order to draw these phase diagrams, the chemical equilibria involving the ore minerals should be established first (Tables 2-4).
The Nernst equation can be used to calculate the lgfO2 and lgfS2 of the reaction equations. The thermodynamic data were obtained from Ref. [66]:
Eh=Eh0+(RT/nF)×ln ([oxidation state]/[reduction state]) (1)
where R is the gas constant, which is 8.31 (volt–coulomb)/(mol·K), and n is the number of moles of electrons exchanged in the electrochemical reaction (mol).
Eh0=△GR,TΘ/nF (2)
Then the fO2 and fS2 are calculated by:
 (3)
(3)
where △GR,TΘ is under standard conditions.
Table 2 Chemical reactions for lgfO2 and lgfS2

For the reactions in which oxygen and sulfur participate simultaneously, the Gibbs-Duham equation is used to acquire the straight line slope:
 (4)
(4)
where C1 is the stoichiometric coefficient of oxygen and C2 is the stoichiometric coefficient of sulfur. Equation (4) can be rewritten as:
 (5)
(5)
Therefore, the straight line slope is:

The reactions (15)-(19) and (26)-(29) in Table 3 have a △G<0 and can occur spontaneously. Therefore, the stability ranges of sphalerite and galena are the same as those of H2S, HS-, and S2-. Among them, the reactions (15)-(19) divide the diagram into three stability regions: PbSO4, PbCO3, and PbS (Figure 8). For zinc, Zn2+, ZnS and ZnCO3 are stable in the diagram because ZnSO4 could easily dissolve in water. Because △G<0 in reaction (26), the equilibrium of Zn2+ and ZnCO3 is shown by the number 13 equilibrium line (Figure 8). Thus, reactions (26)-(29) divide the diagram into three stability areas: Zn2+, ZnS and ZnCO3 (Figure 8).
Table 3 Chemical reactions for pH-lgfO2

Table 4 Chemical reaction for pH-lga

According to reactions (20)-(25) and (30)-(35), the solubility isolines of the lead and zinc chloride complexes and the hydrogen sulfide complex were plotted.
To clearly express the related issues (Table 4), we made several assumptions when plotting the diagram of pH vs lga. When the relationship between the pH value and the metal ion concentration was studied, we assumed that [HS-]=0.1 mol/L and [S2-]=0.01 mol/L. When the relationship between pH value and [HS-] content was examined, the ratios between Pb and Zn must be established. According to previous studies [25, 42], the ratio of Pb: Zn can be set as 1:2. Based on HAN et al [44], the ore-forming pressure in lead and zinc sulfide deposits is between 100 and 1000×105 Pa, being mostly around 500×105 Pa. Thus, the pressure was set as 500×105 Pa. By substituting the above values into their relationship with pH, the lg[metal] and lg[HS-] were calculated. Then, according to the ratio of Pb and Zn, the log[metal] was transformed into lg[Pb2+]. Finally, the diagrams for pH-lg[Pb2+] and pH-lg[HS-] are shown in Figures 9 and 10, respectively.

Figure 7 lgfo2-lgfs2 phase diagram at different temperatures (Gn-galena; Sp-sphalerite; Py-pyrite; Po-pyrrhotite; Mt-magnetite; Hm-hematite; Spa-zincite; Li-masicotite; Pl-plattnerite; Mi-miniumite)

Figure 8 pH-lgfO2 phase diagram at different temperatures (The lead and zinc complex of solubility isoline from left to right is: –3, -4, …, –8)

Figure 9 pH-lg[Pb2+] phase diagram at main temperatures

Figure 10 pH-lg[HS-] phase diagram at main temperatures
4 Results
4.1 lgfO2-lgfS2 phase diagram
The lgfO2 and lgfS2 phase diagram (Figure 7) was drawn on the basis of our calculations. From Figure 7, various minerals and mineral assemblages have their own stable lgfO2 and lgfS2 ranges at a certain temperature. In addition, their redox increases with increasing lgfO2 and lgfS2, but the redox degree on the lgfS2 side is weaker than that on the lgfO2 side. Therefore, the redox trends reflect the geological correlation among minerals, mineral assemblages, and mineral zoning. All of these can be explained follows: 1) The fs2 is the critical factor in mineral zoning. The required lgfs2 value for sphalerite formation is lower compared to that of galena and pyrite formation, whereas the required lgfs2 value for pyrite formation is the highest. All three minerals share a common area in addition to their distinct areas in the phase diagram, leading to the mineral zoning from top to bottom along the ore bodies. 2) Lower temperatures are constructive to metal sulfide precipitation under the same oxygen and sulfur fugacity. Comparing the four phase diagrams in Figure 7 shows that as temperature drops, the required lgfO2 and lgfS2values for mineral precipitation decrease.
4.2 pH-lgfO2 phase diagram
The pH-lgfo2 diagrams and the solubility isoline (Figure 8) of lead and zinc complex were then plotted. Comparing the phase diagrams of pH-lgfO2 (Figure 8) at different temperatures indicates that: 1) The stability and solubility of galena and sphalerite control the precipitation range of sulfide. In general, the pH-lgfO2 stability ranges largely overlap in different forms of lead and zinc. In particular, the stability ranges of sphalerite and galena are almost the same. In other words, in the range where sphalerite is stable, so is galena. In addition, both sphalerite and galena are stable in a relatively large range. Therefore, the geochemical similarity of lead and zinc is one of the important reasons for the paragenesis of sphalerite and galena. However, the solubility isoline shows that sphalerite has a greater solubility than galena. If both are at the same concentration in the hydrothermal solution, lead may reach saturation earlier than zinc, thus the zinc precipitation range may be slightly wider than that of lead. Once lead has precipitated, zinc continues migrating in the hydrothermal fluids, causing the differentiation of lead and zinc. 2) Oxygen fugacity will affect the solubility of lead and zinc. When the oxygen fugacity increases, the zinc solubility increases, and zinc is likely to move with hydrothermal fluids unless the fluids are strongly alkaline, where zinc becomes smithsonite, which is only slightly soluble. On the contrary, lead tends to form lead sulfate and lead carbonate when oxygen fugacity increases. Both minerals are slightly soluble in water. When S2- concentration reaches a certain value, the slightly soluble lead sulfate and lead carbonate will transform to insoluble galena, which has a lower Ksp which is the solubility product constant. 3) pH is another vital factor affecting the solubility of lead and zinc. At a certain temperature, when pH value increases by one unit, the solubility will decrease by about two orders of magnitude. In other words, the number of metal ions dissolved under acidic conditions is 10 to 1000000 times of that in alkaline conditions. 4) Precipitation of sulfides is related not only to the form of Zn and Pb present, but also to the temperature of the fluid during migration. In general, lead and zinc mainly migrate as chloride in a fluid system containing sulfur and chlorine when pH is less than 5.3, but migrate as a hydrogen sulfide compound when pH is greater than 8. Thus, the stable range for the Pb-Zn sulfides should be near neutral (5 O2 value to form sphalerite and galena decreases. If the temperature reduces by 50 K, the required lgfO2 value for sulfides will decrease by five to eight orders of magnitude.
4.3 pH-lga phase diagram
From Figures 9 and 10, we determined that:1) The assemblage of pyrite, sphalerite, and galena can occur in a wide pH range and is mainly controlled by the activity of metal ions at a certain temperature. Only when lgaHS-> -6.6 and pH>6.5, or lgaHS->-4.3 and pH>9.7 (T=373 K), the assemblage is not stable and mainly exists with H2S and S2-. 2) When temperature, metal ion activity, and HS- activity are constant, the metal sulfides will easily precipitate in alkaline conditions. 3) In the pH-lga phase diagram, the precipitation order of sulfides is Py to Gn to Sp, and in the pH-lg[HS-] phase diagram, the order is Gn to Py and Sp. That means the HS- activity required for the formation of pyrite and sphalerite is quite similar, and could be one cause of the formation of christophite. 4) Lower temperatures are helpful for the formation of sulfides. When temperature decreases, the required metal ion and HS- activities to form sulfides will decrease.
5 Discussion
5.1 Order of sphalrite and galena precipitation
The deposit formation is a complex process and comprehensively constrained by various permeability features, mineralogy of the host rocks as the carbonates can solubilize and thus change the pH and the same is true for the solubilization of silica along the faults, physical and chemical conditions. Once one or several conditions are met, a leading factor may only depend on one condition.
The order of sphalerite and galena precipitation is a result of the multiple factors. When the ore-bearing hydrothermal fluids migrates to the ore-bearing zone, for example, the interlayer fracture zone, the solubility of the lead-zinc complexes will decrease with the change in the physical and chemical conditions, including temperature, pH, lgfO2, lgfS2 and lga, leading to complex decomposition and sulfide precipitation. The solubility isolines (Figure 8) show that when the lead concentration is roughly equal to that of zinc, galena should precipitate earlier than sphalerite. On the other hand, the required lgfS2 values for the formation of sphalerite, galena, and pyrite, in turn are increasingly higher (Figure7). This results in the precipitation of sphalerite earlier than galena. In a hydrothermal solution, the sulfur fugacity and the lead-to-zinc ratio are the key factors for the precipitation sequence of sphalerite and galena.
In general, the Zn2+ concentration in the ore-forming fluid is much higher than that of Pb2+. The ratio of Zn: Pb ranges from 2:1 to 4:1 in Sediment-Hosted Pb-Zn deposits in China [48]. All major MVT districts are Zn rich relative to Pb and have Zn/(Zn+Pb) ratios greater than 0.5, with the Southeast Missouri district being the exception. In contrast, this ratio in the Southeast Missouri district is less than 0.1. Some deposits are entirely lead, such as those in the Viburnum Trend, East Tennessee, USA, and Newfoundland Zinc districts [25]. Most SEDEX (CD) deposits are Zn rich relative to Pb and have Zn/(Zn+Pb) ratios that average about 0.7, with the Mount Isa and Sullivan deposits being the exceptions, containing near-equal proportions of Pb and Zn [67]. Because of these disparities in the concentration between Zn2+ and Pb2+, the sulfur fugacity is considered as the leading factor in controlling mineral precipitation rather than the solubility. Hence, when the temperature drops, the pH value and S2- concentration increase and the required sulfur fugacity for sphalerite formation is reached, then sphalerite will precipitate before galena. Therefore, in most of lead-zinc deposits rich in Zn, sphalerite precipitated earlier than galena (Table 5).
The mineral zoning of Pb and Zn is mainly controlled by the geochemical environment. Among the various physical and chemical conditions, temperature and pH are the vital factors controlling the solubility of Pb and Zn, and the stability of the complexes, which determine the precipitation interval of sulfides. The lga values are more related to the scale and grade of deposits, and the lgfS2 values are the key factors controlling the mineral zoning of the lead-zinc.
Table 5 Main mineral assemblage and features of zoning in different Pb-Zn sulfide deposits

5.2 Relationship between mineral zoning and metallogenic process in Huize and Zhaotong deposits
Combining the study of the phase diagrams and the deposit geologic features, we inferred that the formation of the Pb-Zn deposit in the SYG metallogenic provinces experienced the following processes during hydrothermal mineralization period.
5.2.1 Mineralization stage 1
When the metal-bearing fluid migrated upward from the bottom, the lead and zinc ions in the fluid migrated mainly in the form of chlorides. In the beginning, the hydrothermal fluid had a relatively high temperature and low pH value, thus pyrite formed during the diagenetic period began to recrystallize and coarse during this stage.
With increasing of the temperature and dropping of the pH, the enrichment of lead and zinc by dolomite become stronger. When the acidic, medium and high temperature fluid enter the compression torsion fracture, the longer residence time can be obtained. Due to the low pH of fluid [65], the fine-grained dolomite and dolomitic limestone formed during diagenesis are dissolved. It is generally known that calcite is easier to dissolve than dolomite. Therefore, the dense and smooth rock surface becomes loose and porous due to the dissolution of large amounts of calcite and a small amount of dolomite. After the water rock reaction, the fluid pH increases to about 6. According to the experiment by CALUGARU et al [99], when the initial pH is greater than 6, charring of dolomite led to a rigid and porous material showed a sorption capacity increased sevenfold for Zn treatment in contaminated neutral drainage, relative to the raw material. The charring dolomite after water rock reaction is similar in structure to that of charring dolomite. Therefore, when the metal-containing fluid does not mix the fluid rich in reduced sulfur, dolomite can preserve the heavy metals (lead and zinc) on the surface of the particles by adsorption, precipitation, ion exchange and other ways. As lead and zinc sulfides have smaller Ksp, the addition of reduced sulfur will lead to the precipitation of sphalerite and galena by the process as follow.
In the process of the hydrothermal fluid migration, the solubility of H2S increased as the temperature decreased. As H2S dissociated, the S2- and HS- concentrations increased (Figure 8). On the other hand, as the pressure was released, volatile components, such as HF and HCl, were separated out from the fluid, leading to the increase in pH value of the fluid. In addition, the increasing OH- concentration also accelerated the H2S dissociation.
The ratio Zn/Pb was set as 2:1 in the Huize and Zhaotong deposits. As mentioned above, sphalertie will precipitate first and simultaneously replace pyrite, forming the poikilitic texture that includes pyrite in the sphalerite crystals, and providing partial S sources with the released H2S for sulfide precipitation, as illustrated in the following formulae:
2PbCln2–n+2FeS2+2H2O=2PbS+2(n-1)Cl-1+ 2FeCl++2H2S+O2
2ZnCln2-n+2FeS2+2H2O=2ZnS+2(n-1)Cl-1+ 2FeCl++2H2S+O2
Although the metal ion and HS- activities required for pyrite precipitation at the weak acidic condition are similar to that of sphalerite, the lgfO2 and lgfS2 values had not yet reached the levels required for pyrite precipitation. In addition, the radius of Fe2+ is similar to that of Zn2+, so isomorphous substitution may have occurred between Fe2+ and Zn2+. Therefore, a large number of Fe2+ may have integrated into the sphalerite lattices. Thus, sphalerite formed in this stage is darker in color.
Through this process, a small amount of dark brown sphalerite was formed and overlapped the coarse-grained pyrite in the mineralizing phase. The mineral assemblage is that of I-1, according to metallogenic stage I.
5.2.2 Mineralization stage 2
Why are lead and zinc sulfides associated with hydrothermal dolomite (HTD) in this stage? High carbonate alkalinity and temperature are crucial to the dolomitization processes in natural hydrothermal systems [99-103]. The experiment [103] shows that a heat-ageing step from 200 to 300 °C promotes the fast formation of dolomite from magnesium calcite-rich system under high- carbonate alkalinity. It can be proposed for the formation of hydrothermal dolomite [104] that associate with sphalerite and galena of the main metallogenic stage [42, 44, 59] in Huize and Zhaotong Pb-Zn deposits.
When sphalerite precipitate, acid produced:
ZnCln2-n+H2S=ZnS+nCl-+2H+
At the same time, limestone and dolomite dissolved by reacting with acid as an acid-consuming process:
Ca(CO3)2+4H+=Ca2++2CO2+2H2O
CaMg(CO3)2+4H+=Ca2++Mg2++2CO2+2H2O
Thus, the PCO2 and fluid contents of Fe2+ and Mg2+ increased, causing dolomite to transform into ferro-dolomite through the following reaction:
2Ca2++Mg2++Fe2++3CO32-=CaMg(CO3)2-CaFe(CO3)2 (ferro-dolomite)
The ferro-dolomitization occurred near the ore bodies, showing the balance of the production and consumption of acid so that the fluid pH value was maintained at a weak alkaline condition, causing sulfide to be constantly precipitated, while the surrounding dolomite altered significantly. After that, as the Fe2+ concentration in the hydrothermal fluid decreased, it became increasingly difficult for Fe2+ to enter into sphalerite lattices, so the sphalerite color gradually lightened.
When the temperature continued to decrease and the lgfO2 value increased, although the lgfS2 value slightly decreased, the required lgfS2 and lgfO2 values for mineral precipitation were much lower than before. For example, at 473 K, the conditions required for sphalerite formation were lgfO2≥-69.9 and lgfS2≥-49.8, while at 423 K, the values were lgfO2≥-79.1 and lgfS2≥-54.8. The required lgfO2 and lgfS2 values met the galena precipitation conditions, and galena precipitated accordingly.
The mineral assemblages of brown sphalerite, galena, and ferro-dolomite were formed in this mineralizing phase. The mineral assemblage is of I-2, according to the metallogenic stage II.
5.2.3 Mineralization stage 3
With the mineral precipitation and evolution of the fluid, the lgfO2 value increased and the zinc concentration decreased, causing the zinc ion migration upward and outward with the fluid. At this time, the major mineral being precipitated was galena. The ferro-dolomite formation consumed massive amounts of magnesium in the fluid, resulting in a high ratio of Ca2+: Mg2+. With the precipitation of calcite being an acid production process, the following reaction occurred:
Ca2++2CO2+2H2O=Ca(CO3)2+4H+
Galena precipitation is also an acid production process:
PbCln2-n +H2S=PbS+nCl-+2H+
The weak acidic environment was conducive to SiO2 precipitation. Therefore, the mineral assemblages of galena, sandy beige and pale yellow sphalerite, quartz, and calcite were formed in this mineralizing phase. The mineral assemblage is that of I-3, according to metallogenic stage III.
5.2.4 Mineralization stage 4
When the lgfS2 value finally satisfied the conditions required to precipitate pyrite, a large amount of pyrite was precipitated as fine particles from the hydrothermal fluid. With the ratio of Ca2+ to Mg2+ in the fluid becoming lower due to calcite precipitation, calcite and dolomite were co-precipitated.
The mineral assemblages of fine-grained pyrite, calcite, and dolomite were formed in this mineralizing phase. The mineral assemblage is that of I-4, according to metallogenic stage IV.
The horizontal and vertical zonation are basically the same. As the lgfS2 value is lower in the center and becomes higher moving outward in the deposit, zinc tends to be found near to the center and lead is found closer to the edges. The ideal zonation from center to the edges of orebodies is: sphalerite, more sphalerite than galena, more galena than sphalerite, then galena, according to the zoning of elements zinc, more zinc than lead, more lead than zinc, and lead.
5.3 Mineral zoning in other deposit types
This mineral zoning is common in lead and zinc sulfide deposits, regardless of the type and district of the deposit (Table 5). Because of the difference in deposit types, the surrounding rocks, and the associated minerals or elements, this paper only focused on the zonation of sphalerite and galena. The zonation between the two can be summarized as follows: the center (base) of the orebodies is rich in sphalerite, whereas the outermost edge of the orebodies (top) is rich in galena, regardless of deposit type, or the relationship to granite, volcanic rocks, carbonatite, mudstones, or fine clastic rock (Figure 11). This zoning is by no means fortuitous; it is essentially due to the difference in the precipitation order of sphalerite and galena. Sphalerite precipitated earlier than galena in the deposits, as listed in Table 5.

Figure 11 Two zonation forms of metal minerals in lead and zinc sulfide deposits
5.4 Significance for mineral exploration
The galena and sphalerite zonation indicate different ore-forming conditions, which is not only significant for the creation of theory but also for mining exploration. Using some of the Pb-Zn deposits in the northeast of the Yunnan province as examples, if the ore-bodies on the surface mainly consist of black sphalerite, it means that the upper layer of these ore-bodies has been eroded and the prospects for the underlying layers appear unfavorable. On the contrary, if a large amount of galena is found on the surface of the ore body, the prospecting value at a depth appears favorable. The Huize super-large Pb-Zn deposit in this area is characteristic of the mineral zonation of sphalerite, galena, pyrite, and barite due to the high lgfO2 values of fluids as the fluids migrated near to the surface, if [SO4]2- was the main form of S in the fluids. When the Ba2+ concentration is high enough, a large amount of barite exists above the galena. If barite is discovered at a certain level, the lead and zinc ore bodies must be below. In the porphyry deposits, if the black sphalerite ore can be seen in the drill hole, the lead and zinc ore bodies may occur in a horizontal direction in the upper part, and copper and molybdenum may occur in the lower or base part.
In addition to the general rules mentioned above, comprehensive analyses in combination with other geological and geochemical factors are important. For example, when the zinc content is quite low in a mining area, sometimes being slightly higher than the Clarke value, the normal zoning of lead and zinc did not develop completely. On the other hand, the late structures might also destroy the zoning to a certain degree and cause discontinuity or reverse distribution. The zoning rules should be carefully applied through comprehensive geological analysis.
6 Conclusions
We determined that the mineral zoning was caused by different precipitation sequences of sphalerite and galena, and was mainly controlled by the properties of Pb and Zn and the geochemical environment. The mineral zoning corresponds to the four metallogenic stages of hydrothermal ore-forming periods in two deposits. Among various physical and chemical conditions such as temperature, pH, lgfO2, lgfS2 and lga, temperature and pH determined the precipitation interval of sulfides. The ion concentrations were more strongly related to the scale and grade of deposits. The temperature, pH, and the fS2 were the key factors controlling the mineral zoning.
References
[1] GARRELS R M. The Mississippi valley type lead-zinc deposits and the problem of mineral zoning [J]. Econ Geol, 1941, 36: 729-744. DOI:10.2113/gsecongeo.36.7.729.
[2] MIYAZAWA T. Regional lateral zoning of the Mesozoic to early Tertiary endogenic lead-zinc and copper deposits in East Asia and its geological background, with some comments on the drifting of the Japanese Islands [J]. Shigen-Chishitsu, 1985, 35: 31-39. DOI: 10.11456@ shigenchishitsu1951.35.31.
[3] WEI Ai-ying, XUE Chuan-dong, HONG Tuo, LUO Da-feng, LI Lian-ran, WANG Feng, ZHOU Gao-ming, LIU Xing. The alteration-mineralization zoning model for the Maoping lead-zinc deposit, northeastern Yunnan Province: Evidence from alternation-lithofacies mapping [J]. Acta Petrologica Et Mineralogica, 2012, 31(5): 723-735. DOI: 10.3969/j.issn. 1000-6524.2012.05.010. (in Chinese)
[4] WU Guang-feng, LI Jian, WANG Bo, LI Xiao-ya. Geochemical characteristics of primary halo of low grade lead-zinc at Jiazitaigou [J]. Geology of Chemical Minerals, 2016, 38(1): 7-15. DOI: 10.3969/j.issn.1006-5296.2016. 01.002. (in Chinese)
[5] BEALES F W, JACKSON S A. Precipitation of lead-zinc ores in carbonate reservoirs as illustrated by Pine Point ore field, Canada [J]. T I Min Metall B, 1966, 75: 278-285.
[6] SKINNER B J. Precipitation of Mississippi Valley-type ores: A possible mechanism. In: J.S. Brown, Genesis of Stratiform Lead-Zinc-Barite-Fluorite Deposits (Mississippi Valley-type Deposits) [J]. Economic Geology, 1967, 3: 363-370.
[7] HELGESON H C. A chemical and thermodynamic model of ore deposition in hydrothermal systems [C]// MORGAN B A. Symposium: Mineralog. Soc America SPEC, 1970: 155-186.
[8] ANDERSON G M. The hydrothermal transport and deposition of galena and Sphalerite near 100 °C [J]. Economic Geology, 1973, 68(4): 480-492. DOI: 10.2113/ gsecongeo.68.4.480.
[9] ANDERSON G M. Precipitation of Mississippi Valley-Type ores [J]. Economic Geology, 1975, 70(5): 937-942. DOI: 10.2113/gsecongeo.70.5.937.
[10] BEALES F W. Precipitation mechanisms for Mississippi Valley-type ore deposits [J]. Economic Geology, 1975, 70(5): 943-948. DOI:10.2113/gsecongeo.70.5.943.
[11] HAGNI R D, TRANCYNGER T C. Sequence of deposition of the ore minerals at the Magmont Mine, Viburnum Trend, Southeast Missouri [J]. Economic Geology, 1977, 72(3): 451-464. DOI: 10.2113/gsecongeo.72.3.451.
[12] BARNES H L. Geochemistry of hydrothermal ore deposits [M]. John Wiley and Son, 1997.
[13] GIORDANO T H, BARNES H L. Lead transport in Mississippi valley-type ore solutions [J]. Economic Geology, 1981, 76(8): 2200-2211. DOI:10.2113/gsecongeo.76.8.2200.
[14] APPOLD M S, WENZ Z J. Composition of ore fluid inclusions from the viburnum trend, southeast Missouri District, United States: Implications for transport and precipitation mechanisms [J]. Economic Geology, 2011, 106(1): 55-78. DOI:10.2113/econgeo.106.1.55.
[15] WILKINSON J J. Sediment-hosted zinc-lead mineralization: Processes and perspectives [J]. Treatise on Geochemistry, 2013: 219-249. DOI: 10.1016/B978-0-08- 095975-7. 01109-8.
[16] BAYOT D, DEVILLERS M. Peroxo complexes of niobium(v) and tantalum(v) [J]. Coordination Chemistry Reviews, 2006, 250: 2610-2626. DOI: 10.1016/j.ccr.2006. 04.011.
[17] LIU W, ETSCHMANN B, FORAN G, SHELLEY M, BRUGGER J. Deriving formation constants for aqueous metal complexes from XANES spectra: Zn2+ and Fe2+ chloride complexes in hypersaline solutions [J]. American Mineralogist, 2007, 92(5, 6): 761-770. DOI: 10.2138/am. 2007.2225.
[18] ANDRI S. Iron(III) hydrolysis and solubility at 25 °C [J]. Environmental Science & Technology, 2007, 41(17): 6117-23. DOI: 10.1021/es070174h.
[19] ANTIGNANO A, MANNING C E. Rutile solubility in H2O, H2O-SiO2, and H2O-NaAlSi3O8 fluids at 0.7-2.0 GPa and 700-1000 °C: Implications for mobility of nominally insoluble elements [J]. Chemical Geology, 2008, 255(s1, 2): 283-293. DOI: 10.1016/j.chemgeo.2008.07.001.
[20] WILLIAMS-JONES A E, BOWELL R J, MIGDISOV A A. Gold in solution [J]. Elements, 2009, 5(5): 281-287. DOI: 10.2113/gselements.5.5.281.
[21] YARDLEY B W D, BODNAR R J. Fluids in the Continental Crust [J]. Geochemical Perspectives, 2014, 3(1): 1-127. https://pubs.geoscienceworld.org/perspectives/article-abstract/3/1/1/217791/fluids-in-the-continental-crust?redirectedFrom=fulltext.
[22] ANDERSON A J, MAYANOVIC R A, CHOU Yi-ming, BASSETT W A. XAFS investigations of zinc halide complexes up to supercritical conditions [M]. Ottawa: ON: NRC Research Press, 2000.
[23] BASUKI N I. A review of fluid inclusion temperatures and salinities in Mississippi Valley-type Zn-Pb deposits: Identifying thresholds for metal transport [J]. Exploration & Mining Geology, 2002, 11(1-4): 1-17. DOI: 10.2113/11.1- 4.1.
[24] HARRIS D J, BRODHOLT J P, SHERMAN D M. Zinc complexation in hydrothermal chloride brines:Results from ab initio molecular dynamics calculations [J]. Journal of Physical Chemistry A, 2003, 107(7): 614-619. DOI: 10.1021/jp026098g.
[25] LEACH D L, SANGSTER D F, KELLEY K D, LARGE R R, GARVEN G, ALLEN C R, GUTZMER J, WALTERS S. Sediment-hosted lead-zinc deposits: A global perspective [J]. Econ Geol, 2005, 100: 561-607.
[26] TAGIROV B R, SULEIMENOV O M, SEWARD T M. Zinc complexation in aqueous sulfide solutions: Determination of the stoichiometry and stability of complexes via ZnS (cr) solubility measurements at 100 °C and 150 bars [J]. Geochimica Et Cosmochimica Acta, 2007, 71(20): 4942-4953. DOI: 10.1016/j.gca.2007.08.012.
[27] TAGIROV B R, SEWARD T M. Hydrosulfide/sulfide complexes of zinc to 250 °C and the thermodynamic properties of sphalerite [J]. Chemical Geology, 2010, 269(3, 4): 301-311. DOI: 10.1016/j.chemgeo.2009.10.005.
[28] TAGIROV B, ZOTOV A, SCHOTT J, SULEIMENOV O, KOROLEVA L. A potentiometric study of the stability of aqueous yttrium–acetate complexes from 25 to 175°C and 1–1000bar [J]. Geochimica Et Cosmochimica Acta, 2007, 71(7): 1689-1708. DOI:10.1016/j.gca.2007.01.003.
[29] GIORDANO T H, BARNES H L. Ore solution chemistry VI; PbS solubility in bisulfide solutions to 300 °C [J]. Economic Geology, 1979, 74(7): 1637-1646. DOI: 10.2113/gsecongeo. 74.7.1637.
[30] HAMANN R J, ANDERSON G M. Solubility of galena in sulfur-rich nacl solutions [J]. Economic Geology, 1978, 73(1): 96-100. DOI: 10.2113/gsecongeo.73.1.96.
[31] BARRETT T J, ANDERSON G M. The solubility of sphalerite and galena in NaCl brines [J]. Economic Geology, 1982, 77(8): 1923-1933. DOI: 10.2113/gsecongeo.77.8. 1923.
[32] SEWARD T M. The formation of lead(II) chloride complexes to 300 °C: A spectrophotometric study [J]. Geochimica Et Cosmochimica Acta, 1984, 48(1): 121-134. DOI: 10.1016/0016-7037(84)90354-5.
[33] RUAYA J R, SEWARD T M. The stability of chlorozinc(II) complexes in hydrothermal solutions up to 350°C [J]. Geochimica Et Cosmochimica Acta, 1986, 50(5): 651-661. DOI: 10.1016/0016-7037(86)90343-1.
[34] BOURCIER W L, BARNES H L. Ore solution chemistry: VII. Stabilities of chloride and bisulphide complexes of zinc to 350 °C [J]. Economic Geology, 1987, 82(7): 1839-1863. DOI: 10.2113/gsecongeo.82.7.1839.
[35] BARRETT T J, ANDERSON G M. The solubility of sphalerite and galena in 1–5 m NaCl solutions to 300 °C [J]. Geochimica Et Cosmochimica Acta, 1988, 52(4): 813-820. DOI: 10.1016/0016-7037(88)90353-5.
[36] SHANG Lin-bo, FAN Wen-ling, HU Rui-zhong, DENG Hai-lin. A thermodynamic study on paragensis and separation of silver, lead and zinc in hydrothermal solutions [J]. Acta Mineralogica Sinica, 2004, 24(1): 81-86. DOI: 10.3321/j.issn:1000-4734.2004.01.013. (in Chinese)
[37] SHANG Lin-bo, HU Rui-zhong, FAN Wen-ling. The mechanisms of paragenesis and separation of silver, lead and zinc in hydrothermal solutions [J]. Chinese Journal of Geochemistry, 2005, 24(1): 82-89. DOI: 10.1007/ bf02869692. (in Chinese)
[38] NRIAGU J O. Studies in the system pbs-nacl-h2s-h2o: Stability of lead(II) thiocomplexes at 90 °C [J]. Chemical Geology 1971, 8: 299-310. DOI: 10.1016/0009-2541(71) 90023-4.
[39] NRIAGU J O, ANDERSON G M. Stability of the lead (II) chloride complexes at elevated temperatures [J]. Chemical Geology, 1971, 7: 171-184. DOI: 10.1016/0009-2541(71) 90007-6.
[40] LEACH D L, TAYLOR R D. A deposit model for mississippi valley-type lead-zinc ores [R]. Chapter A of mineral deposit models for resource assessment: Scientific Investigations Report. U.S.geological Survey, 2010. http://minerals.cr. usgs.gov/.
[41] LEACH D L, SANGSTER D F. Mississippi Valley-Type lead-zinc deposits [J]. Geological Association of Canada Special Paper, 1993, 40: 289-314.
[42] HAN Run-sheng, CHEN Jin, HUANG Zhi-long. Dynamics of tectonic ore-forming process and localization-prognosis of concealed orebodies-As exemplified by the huize super-large Zn-Pb-(Ag-Ge) District, Yunnan [M]. Beijing, China: Beijing Science Press, 2006. (in Chinese)
[43] HAN Run-sheng, HU Yu-zhao, WANG Xue-kun, HOU Bao-hong, HUANG Zhi-long, CHEN Jin, WANG Feng, WU Peng, LI Bo, WANG Hong-jiang, DONG Ying, LEI Li. Mineralization model of rich Ge-Ag-bearing Zn-Pb polymetallic deposit concentrated district in Northeastern Yunnan, China [J]. Acta Geologica Sinica, 2012a, 86(2): 280-293. DOI: 10.3969/j.issn. 0001-5717.2012.02.007. (in Chinese)
[44] HAN Run-sheng, LIU Cong-qiang, HUANG Zhi-long, CHEN Jin, MA De-yun, LEI Li, MA Geng-sheng. Geological features and origin of the Huize carbonate-hosted Zn-Pb-(Ag) district, Yunnan [J]. Ore Geology Reviews, 2007, 31: 360-383. DOI: 10.1016/ j.oregeorev. 2006.03.003.
[45] HAN Run-sheng, WANG Feng, HU Yu-zhao, WANG Xue-kun, REN Tao, QIU Wen-long, ZHONG Kang-hui. Metallogenic tectonic dynamics and chronology constrains on the Huize-Typ (HZT) germanium-rich silver-zinc-lead deposits [J]. Geotectonic et Metallogenia, 2014, 38(4): 758-771. DOI: 10.3969/j.issn.1001-1552.2014.04.003. (in Chinese)
[46] DAI Zi-xi. The distributions, types and rules of exploration of lead and zinc all over the world [J]. World Nonferrous Metals, 2005(3): 15-23. (in Chinese)
[47] XIE Jia-rong. A discussion on the deposits classify [M]. Beijing, China: Science Press, 1963. (in Chinese)
[48] TU Guang-zhi. Geochemical of strata bound ore deposits in China [M]. Beijing, China: Science Press, 1984. (in Chinese)
[49] HAN Run-sheng, LIU Cong-qiang, HUANG Zhi-long, MA De-yun, LI Yuan, HU Bin. Sources of ore-forming fluid in huize Zn-Pb-(Ag-Ge) district, Yunnan, China [J]. Acta Geologica Sinica, 2004, 78(2): 583-591. DOI: 10.1111/j.1755-6724.2004.tb00170.x.
[50] HUANG Zhi-long, CHEN Jin, HAN Run-sheng. Geochemistry and ore genesis of Huize super-large lead-zinc deposit, Yunnan Province, concurrently discuss the relationship between Emeishan basalt and lead-zinc deposits [M]. Beijing, China: Geological Publishing House, 2004. (in Chinese)
[51] LIU He-chang, LIN Wen-da. Metallogenic rules of Zn-Pb-(Ag) deposits in Northeastern Yunnan [M]. Kunming, China: Yunnan University Publishing House, 1999. (in Chinese)
[52] ZHANG Chang-qing. The genetic model of mississippi valley-type deposits in the boundary area of Sichuan, Yunnan and Guizhou Provinces, China [D]. Beijing, China: Chinese Academy of Geological Sciences, 2008: 67-98. (in Chinese)
[53] ZHOU Jia-xi, LUO Kai, WANG Xuan-ce, WILDES A, WU TAO, HUAN Zhi-long, CUI Yin-liang, ZHAO Jian-xin. Ore genesis of the fule Pb-Zn deposit and its relationship with the Emeishan Large Igneous Province: Evidence from mineralogy, bulk C-O-S and in situ S-Pb isotopes [J]. Gondwana Research, 2018, 54: 161-179. DOI: 10.1016/ j.gr.2017.11.004.
[54] ZHOU Jia-xi, XIANG Zhen-zhong, ZHOU Mei-fu, FENG Yue-xing, LUO Kai, HUANG Zhi-long, WU Tao. The giant Upper Yangtze Pb–Zn province in SW China: Reviews, new advances and a new genetic model [J]. Journal of Asian Earth Sciences, 2018, 154: 280-315. DOI: 10.1016/ j.jseaes.2017.12.032.
[55] ZHOU Jia-xi, HUANG Zhi-long, ZHOU Mei-fu, ZHU Xiang-kun, PHILIPPE M. Zinc, sulfur and lead isotopic variations in carbonate-hosted Pb–Zn sulfide deposits, southwest China [J]. Ore Geology Reviews, 2014, 58(3): 41-54. DOI:10.1016/j. oregeorev.2013.10.009.
[56] ZHOU Jia-Xi, HUANG Zhi-long, LV Zhi-cheng, ZHU Xiang-Kun, JIN Zhong-guo, HASSAN M. Geology, isotope geochemistry and ore genesis of the Shanshulin carbonate-hosted Pb–Zn deposit, southwest China [J]. Ore Geology Reviews, 2014, 63(1): 209-225. DOI: 10.1016/ j.oregeorev.2014.05.012.
[57] ZHOU Jia-xi, HUANG Zhi-long, ZHOU Mei-fu, Li Xiao-biao, JIN Zhong-guo. Constraints of C–O–S–Pb isotope compositions and Rb–Sr isotopic age on the origin of the Tianqiao carbonate-hosted Pb–Zn deposit, SW China [J]. Ore Geology Reviews, 2013, 53: 77-92. DOI: 10.1016/j.oregeorev.2013.01.001.
[58] ZHOU Jia-xi, HUANG Zhi-long, BAO Guang-ping, GAO Jian-guo. Sources and thermo-chemical sulfate reduction for reduced sulfur in the hydrothermal fluids, southeastern SYG Pb-Zn metallogenic province, SW China [J]. Journal of Earth Science, 2013, 24(5): 759-771. DOI: 10.1007/ s12583-013-0372-8.
[59] ZHANG Yan, HAN Run-sheng, WEI Ping-tang, WANG Lei. Identification of two types of metallogenic fluids in the ultra-large Huize Pb–Zn Deposit, SW China [J]. Geofluids, 2017: 6345810. DOI: 10.1155/2017/6345810.
[60] ZOU Hai-jun, HAN Run-sheng, HU Bin. New evidences of origin of metallogenic materials in the Maoping Pb-Zn ore deposit, Zhaotong, Yunnan, R-factor analysis results of trace elements in NE-extending fractural tectonited [J]. Geology and Prospecting, 2004, 40(5): 43-48. (in Chinese)
[61] MARIE J S, KESLER S E. Iron-rich and iron-poor Mississippi Valley-Type mineralization, metaline district, Washington [J]. Econ Geol, 2000, 95(5): 1091-1106. DOI: 10.2113/gsecongeo.95.5.1091.
[62] SAVARD M M, CHI G, SAMI T, WILLIAMS-JONES A E, LEIGH K. Fluid inclusion and carbon, oxygen, and strontium isotope study of the Polaris Mississippi Valley-type Zn–Pb deposit, Canadian Arctic Archipelago: Implications for ore genesis [J]. Mineralium Deposita, 2000, 35(6): 495-510. DOI: 10.1007/s001260050257.
[63] GRANDIA F, CANALS A, CARDELLACH E, BANKS D A, PERONA J. Origin of ore-forming brines in sediment- hosted Zn-Pb deposits of the Basque-Cantabrian Basin, Northern Spain [J]. Econ Geol, 2003, 98(7): 1397-1411. DOI: 10.2113/gsecongeo.98.7.1397.
[64] HAN Run-sheng, LI Bo, NI Pei, QIU Wen-long, WANG Xu-dong, WANG Tian-gang. Infrared micro-thermometry of fluid inclusions in sphalerite and geological significance of the Huize super-large Zn-Pb-(Ge-Ag) deposit, Yunnan Province [J]. Journal of Jilin University: Earth Science Edition, 2016, 46(1): 91-104. DOI: 10.13278/j.cnki.jjuese. 201601109. (in Chinese)
[65] ZHANG Yan, HAN Run-sheng, WEI Ping-tang, QIU Wen-long. Fluid Inclusion Features and physical and chemical conditions of the ore-forming fluid in Kuangshanchang Pb-Zn Deposit, Huize, Yunnan [J]. Journal of Jilin University: Earth Science Edition, 2017, 2017, 47(3): 719-733. DOI: 10.13278/j.cnki.jjuese.201703107. (in Chinese)
[66] LIN Zhuan-xian, BAI Zheng-hai, ZHANG Zhe-ru. The thermodynamic manual book of minerals and related compounds [M]. Beijing, China: Science Press, 1985. (in Chinese)
[67] LARGE R R, BULL S W, MCGOLDRICK P J, WALTERS S G. Stratiform and strata-bound Zn-Pb-Ag deposits in Proterozoic Sedimentary Basins, Northern Australia [C]//Economic Geology, 2005, 100th Anniversary Volume: 931-963. http://www.segweb.org/journal.htm.
[68] ZHANG Cheng-shuai, WANG En-de, SONG Jian-chao, QI Hong-yan, LI Peng-fei. Zonation of the Skarn-type of polymetal deposit in Huanren, Liaoning province [J]. Geology & Resources, 2009, 18(1): 23-26. DOI: 10.13686/ j.cnki.dzyzy.2009.01.008. (in Chinese)
[69] LU Wen-ju, KONG Xiang-chao, LAN Xin-jie, ZHANG Li-jun, LIAN Shu-ting, XIN Wei. Geochemical metallogenic mechanism of the deep Xiangkuang Deposit in the Qixia Area of Shandong Province [J]. Geology & Exploration, 2016, 31(2): 89-96. DOI: 10.13712/j.cnki.dzykt.2016.06.010. (in Chinese)
[70] ZHAO Guo-bin, YANG He-qun, REN Hua-ning, XIE Xie, JIA Jian. Discussion on some problems about baiyinchang copper-polymetallic orefield in north Qilian [J]. Acta Geologica Sinica, 2016, 90(10): 2863-2873. (in Chinese)
[71] SONG Zhi-gao. The environments of formation of the Baiyinchang massive sulfide deposit and the implication of its origin [J]. Geological Review, 1982, 28(4): 335-343. DOI: 10.16509/j.georeview.1982.04.006. (in Chinese)
[72] ZHENG Yi, ZHANG Li, CHEN Yan-jing, HOLLINGS P, CHEN Hua-yong. Metamorphosed Pb-Zn-(Ag) ores of the Keketale VMS deposit, Xinjiang: Evidence from ore textures, fluid inclusions, geochronology and pyrite compositions [J]. Ore Geology Reviews, 2013, 54: 167-180. DOI: 10.1016/ j.oregeorev.2013.03.009
[73] ZHENG Yi, ZHANG Li, GUO Zhen-lin. The zircon LA-ICP-MS U–Pb and biotite 40Ar/39Ar geochronology, and implications for genesis of the Tiemuert Pb-Zn-Cu deposit, Xinjiang [J]. Acta Petrologica Sinica, 2013, 29(1): 191-204.
[74] ZHENG Yi, ZHANG Li, LI Deng-feng, ARGYRIOS K, CHEN Yan-jing. Genesis of the Dadonggou Pb-Zn deposit in Kelan basin, Altay, NW China: Constraints from zircon U–Pb and biotite 40Ar/39Ar geochronological data. Ore Geology Reviews, 2015, 64: 128-139. DOI: 10.1016/ j.oregeorev.2014.07.002.
[75] SUBIAS P L, LOPEZ C A, FANLO G I , FERNANDEZ N C. La mineralización de Pb-An-Cu-Ag de Valdeplata (Calcena, Zaragoza) [J]. Boletín De La Sociedad Espanola De Mineralogía, 1994, 17: 95-102.
[76] BARRIE C T, HANNINGTON M D. Volcanic-associated massive sulfide deposits: Pocesses and examples in modern and ancient settings [J]. Society of Economic Geologists, 1999, 8: 325-356.
[77] SUBIAS I, FANLO I, MATEO E, BILLSTR M K, RECIO C. Isotopic studies of Pb–Zn–(Ag) and barite Alpine vein deposits in the Iberian Range (NE Spain) [J]. Chemie der Erde-Geochemistry, 2010, 70(2): 149-158. DOI: 10.1016/ j.chemer.2009.12.004.
[78] YE Qing-tong. A preliminary study on hypogene zoning of the Yinshan copper-lead-zinc deposit [J]. Geological Review, 1981, 3(2): 199-206. (in Chinese)
[79] WANG Guo-guang, NI Pei, ZHAO Kui-dong, LIU Jia-run, XIE Guo-ai, XU Ji-hun, ZHANG Zhi-hui. Comparison of fluid inclusions in coexisting sphalerite and quartz from Yinshan deposit, Dexing, Northeast Jiangxi Province [J]. Acta Petrologica Sinica, 2011, 27(5): 1387-1396. (in Chinese)
[80] ROEDDER E. Temperature, salinity, and origin of the ore-forming fluids at Pine Point, Northwest Territories, Canada, from fluid inclusion studies [J]. Economic Geology, 1968, 63(5): 439-450.
[81] RHODES D, LANTOS E A, LANTOS J A, WEBB R J, OWENS D C. Pine Point orebodies and their relationship to the stratigraphy, structure, dolomitization, and karstification of the Middle Devonian barrier complex [J]. Economic Geology, 1984, 79(5): 991-1055. DOI: 10.2113/gsecongeo. 79.5.991.
[82] HANNIGAN P, GOODFELLOW W. Metallogeny of the Pine Point Mississippi Valley-type zinc-lead district, southern Northwest territories. Mineral Deposits of Canada: A synthesis of major deposit-types, district metallogeny, the evolution of geological provinces, and exploration methods [M]// W D GOODFELLOW Edited. Geological Association of Canada, Mineral Deposits Division, 2007, 5: 609-632.
[83] ASHTON J H, DOWNING D T, FINLAY S. The geology of the Navan Zn-Pb orebody [M]// ANDREW C J, CROWE R W A, FINLAY S, PENNEL W M, PYNE J F. Geology And Genesis Of Mineral Deposits in Ireland, Dublin. Irish Association for Economic Geology, 1986: 243-280.
[84] ASHTON J. The Navan carbonate-hosted Zn-Pb deposit, Ireland [J]. Ore Geology Reviews, 2005, 27(1-4): 270.
[85] LI Deng-feng, CHEN Hua-yong, ZHANG Li, HOLLINGS P, CHEN Yan-jing, LU Wan-jian, ZHENG Yi, WANG Cheng-ming, FANG Jing, CHEN Gang, ZHOU Gang. Ore geology and fluid evolution of the giant Caixiashan carbonate-hosted Zn–Pb deposit in the Eastern Tianshan, NW China [J]. Ore Geology Reviews, 2015, 72: 355-372. DOI: 10.1016/j.oregeorev.2015.08.007.
[86] LU Wan-jian, ZHANG Li,CHEN Hua-yong, HAN Jin-sheng, JIANG Hong-jun, LI Deng-feng, FANG Jing, WANG Cheng-ming, ZHENG Yi, TAN Zhi-xiong. Geology, fluid inclusion and isotope geochemistry of the Hongyuan reworked sediment-hosted Zn–Pb deposit: Metallogenic implications for Zn–Pb deposits in the Eastern Tianshan, NW China [J]. Ore Geology Reviews, 2018, 100: 504-533. DOI: 10.1016/j.oregeorev.2017.01.004.
[87] LIU Wen-jun, ZHENG Rong-cai, LI Yuan-lin, CHANG Si-he. Research of the daughter minerals in fluid inclusions of the Huayuan lead and zinc deposit [J]. Journal of Chengdu University of Technology, 1997(4): 65-69. (in Chinese)
[88] HAN Run-sheng, ZOU Hai-jun, HU Bin, HU Yu-zhao, XUE Chuan-dong. Features of fluid inclusions and sources of Ore-forming fluid in the Maoping Carbonate-hosted Zn-Pb-(Ag-Ge) Deposit, Yunnan, China [J]. Acta Petrological Sinica, 2007, 23(9): 2109-2118. (in Chinese)
[89] QIU Wen-long. Fluid geochemistry of the Zhaotong Pb-Zn deposit in Yunnan [D]. Kunming: Kunming University of Science and Technology, 2013. (in Chinese)
[90] SI Rong-jun , GU Xue-xiang, XIIE Liang-xian, ZHANG Na. Geological characteristics of the Fule polymetallic deposit in Yunnan Province: A Pb-Zn deposit with dispersed elements and unusual enrichment [J]. Geology & Exploration, 2013, 49(2): 313-322. (in Chinese)
[91] LV Yu-hui, HAN Run-sheng, REN Tao, QIU Wen-long, HAO Rang, GAO Yuan. Ore-controlling characteristics of fault structures and their relations to mineralization at Fulechang Zn-Pb Mining District in deposit concentration district of Northeastern Yunnan, China [J]. Geoscience, 2015, 29(3): 563-575. (in Chinese)
[92] ZHOU Jia-xi, HUANG Zhi-long, YAN Zai-fei. The origin of the Maozu carbonate-hosted Pb–Zn deposit, southwest China: Constrained by C–O–S–Pb isotopic compositions and Sm–Nd isotopic age [J]. Journal of Asian Earth Sciences, 2013, 73(5): 39-47. DOI: 10.1016/j.jseaes.2013.04.031.
[93] PLUMB K A, AHMAD M, WYGRALAK A S. Mid- Proterozoic basins of the North Australian Craton- regional geology and mineralisation [J]. Australasian Institute of Mining and Metallurgy Monograph, 1998, 22: 881-902.
[94] LOGAN R G, MURRAY W J, WILLIAMS N. HYC silver- lead-zinc deposit, McArthur river [J]. Australasian Institute of Mining and Metallurgy Monograph, 1990, 14: 907-911.
[95] KELLEY K D, LEACH D L, JOHNSON C A, CLARK J L, FAYEK M, AYUSO R A. Textural, compositional, and sulfur isotope variations of sulfide minerals in the red dog Zn-Pb-Ag deposits, brooks range, alaska: Implications for ore formation [J]. Economic Geology, 2004, 99(7): 1509-1532. DOI: 10.2113/gsecongeo.99.7.1509.
[96] LEACH D L, MARSH E, EMSBO P, ROMBACH C S, KELLEY K D, ANTHONY M. Nature of Hydrothermal Fluids at the Shale-Hosted Red Dog Zn-Pb-Ag deposits, Brooks Range, Alaska [J]. Economic Geology, 2004, 99(7): 1449-1480. DOI: 10.2113/gsecongeo.99.7.1449.
[97] MA Guo-liang, BEAUDOIN Georges, QI Si-jing, LI Ying. Geology and geochemistry of the Changba SEDEX Pb-Zn deposit, Qinling orogenic belt, China [J]. Mineralium Deposita, 2004, 39(3): 380-395. DOI: 10.1007/s00126- 004-0416-1. (in Chinese)
[98] MA Guo-liang, BEAUDOIN Georges, ZHONG Shao-jun, LI Ying, ZENG Zhang-ren. Geology and geochemistry of the Dengjiashan Zn-Pb SEDEX deposit, Qinling Belt, China [J]. Canadian Journal of Earth Sciences, 2007, 44(4): 479-492. DOI: 10.1139/e06-093.
[99] CALUGARU I L, NECULITA C M, GENTY T, BUSSIERE B, POTVIN R. Performance of thermally activated dolomite for the treatment of Ni and Zn in contaminated neutral drainage [J]. Journal of Hazardous Materials, 2016, 310: 48. DOI: 10.1016/j.jhazmat.2016.01.069.
[100] MACHEL H G. Bacterial and thermochemical sulfate reduction in diagenetic settings —Old and new insights [J]. Sedimentary Geology, 2001, 140(1, 2): 143-175. DOI: 10.1016/S0037-0738(00)00176-7.
[101] JACQUEMYN C, DESOUKY H E, HUNT D, CASINI G, SWENNEN R. Dolomitization of the Latemar platform: Fluid flow and dolomite evolution [J]. Marine & Petroleum Geology, 2014, 55: 43-67. DOI: 10.1016/j.marpetgeo. 2014.01.017.
[102] MONTESHERNANDEZ G, FINDLING N, RENARD F, AUZENDE A L. Precipitation of ordered dolomite via simultaneous dissolution of calcite and Magnesite: New experimental insights into an old precipitation enigma [J]. Crystal Growth & Design, 2014, 14(14): 671-677. DOI: 10.1021/cg401548a.
[103] MONTES-HERNANDEZ G, FINDLING N, RENARD F. Dissolution-precipitation reactions controlling fast formation of dolomite under hydrothermal conditions [J]. Applied Geochemistry, 2016, 73: 169-177. DOI: 10.1016/ j.apgeochem.2016.08.011.
[104] WEN De-xiao, HAN Run-sheng, WANG Feng, HE Jiao-jiao, QIU Wen-long, XIA Yan-le, CHEN Sui-hai, NI Er-jian. Features and formation mechanism of HTD dolomites in the Huize lead-zinc deposit, Yunnan Province [J]. Acta Petrologica et Mineralogica, 2014, 33(6): 1086-1098. DOI: 10.3969/j.issn.1000-6524.2014.06.007. (in Chinese)
(Edited by HE Yun-bin)
中文导读
闪锌矿和方铅矿的沉淀顺序:以中国西南铅锌矿床为例
摘要:中国西南的大部分铅锌矿床具有明显的矿物组合分带特征,尤其是会泽和昭通铅锌矿床。矿物组合分带具有垂向分带和水平分带,即从矿体底部(中心)到顶部(外围)具有如下矿物分带:I-1:粗晶黄铁矿+少量深色闪锌矿;I-2:棕色闪锌矿+方铅矿+铁白云石;I-3:方铅矿+浅褐色和黄色闪锌矿+方解石;I-4:细晶黄铁矿+白云石+方解石。其中,闪锌矿是不同分带的标志性矿物。从I-1到I-3,闪锌矿颜色从深变浅,晶粒从粗晶变为细晶,结构从浸染状变为细脉状。这种矿物分带现象不仅在微观尺度可以观察到,在宏观尺度上也同样存在。它是由闪锌矿和方铅矿的沉淀顺序引起的。本文在研究金属矿物和流体包裹体的基础上绘制了几类热力学相图,如lgfO2–lgfS2, pH–lgfO2, pH–lg[Pb2+] 和 pH–lg[HS-],讨论了不同pH,氧逸度,硫逸度和离子活度条件下铅锌运移沉淀过程中闪锌矿和方铅矿沉淀顺序的制约条件,解释了矿物组合分带的形成机制,认为闪锌矿和方铅矿沉淀顺序的核心控制条件是硫逸度。
关键词:沉淀顺序;热力学相图;矿物分带;铅锌矿床;中国西南
Foundation item: Projects(41572060, 41802089, U1133602) supported by the National Natural Science Foundation of China; Project(2017M610614) supported by the Postdoctoral Science Foundation, China; Projects(2008, 2012) supported by the YM Lab [2011] and Innovation Team of Yunnan Province and KMUST, China
Received date: 2019-05-26; Accepted date: 2019-08-02
Corresponding author: HAN Run-sheng, PhD, Professor; Tel: +86-13687166018; E-mail: 554670042@qq.com; ORCID: 0000-0002- 8088-0321

