Fabrication, property characterization and thermal performance of composite phase change material plates based on tetradecanol-myristic acid binary eutectic mixture/expanded perlite and expanded vermiculite for building application
来源期刊:中南大学学报(英文版)2019年第9期
论文作者:孔祥飞 杨华 陈万河 戎贤
文章页码:2578 - 2595
Key words:thermal storage; phase change material; expanded perlite; expanded vermiculite; binary eutectic mixture
Abstract: A binary eutectic mixture composed of tetradecanol (TD) and myristic acid (MA) was maximally absorbed into the microstructures of expanded perlite (EP) and expanded vermiculite (EVMT), respectively, through a self-made vacuum adsorption roller to prepare phase change material (PCM) particle (PCP). Then EP and EVMT-based composite PCM plates were respectively fabricated through a mold pressing method. The thermal property, chemical stability, microstructure and durability were characterized by differential scanning calorimeter (DSC), Fourier transform infrared spectroscope (FT-IR), scanning electron microscope (SEM) and thermal cycling tests, respectively. The results show that both PCPs have high latent heats with 110 J/g for EP-based PCP and more than 130 J/g for EVMT-based PCP, compact microstructure without PCM leakage, stable chemical property and good durability. The research results have proved the feasibility for the vacuum adsorption roller used in the composite PCM fabrication. Results of thermal storage performance experiment indicate that the fabricated PCM plates have better thermal inertia than common building materials, and the thermal storage performance of PCM plates has nonlinearly changed with outside air velocity and temperature increase. Therefore, PCM plates show a significant potential for the practical application of building thermal storage.
Cite this article as: YANG Hua, CHEN Wan-he, KONG Xiang-fei, RONG Xian. Fabrication, property characterization and thermal performance of composite phase change material plates based on tetradecanol-myristic acid binary eutectic mixture/expanded perlite and expanded vermiculite for building application [J]. Journal of Central South University, 2019, 26(9): 2578-2595. DOI: https://doi.org/10.1007/s11771-019-4196-2.

J. Cent. South Univ. (2019) 26: 2578-2595
DOI: https://doi.org/10.1007/s11771-019-4196-2

YANG Hua(杨华)1, CHEN Wan-he(陈万河)1, KONG Xiang-fei(孔祥飞)1, RONG Xian(戎贤)2
1. School of Energy and Environmental Engineering, Hebei University of Technology,Tianjin 300401, China;
2. School of Civil and Transportation Engineering, Hebei University of Technology, Tianjin 300401, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: A binary eutectic mixture composed of tetradecanol (TD) and myristic acid (MA) was maximally absorbed into the microstructures of expanded perlite (EP) and expanded vermiculite (EVMT), respectively, through a self-made vacuum adsorption roller to prepare phase change material (PCM) particle (PCP). Then EP and EVMT-based composite PCM plates were respectively fabricated through a mold pressing method. The thermal property, chemical stability, microstructure and durability were characterized by differential scanning calorimeter (DSC), Fourier transform infrared spectroscope (FT-IR), scanning electron microscope (SEM) and thermal cycling tests, respectively. The results show that both PCPs have high latent heats with 110 J/g for EP-based PCP and more than 130 J/g for EVMT-based PCP, compact microstructure without PCM leakage, stable chemical property and good durability. The research results have proved the feasibility for the vacuum adsorption roller used in the composite PCM fabrication. Results of thermal storage performance experiment indicate that the fabricated PCM plates have better thermal inertia than common building materials, and the thermal storage performance of PCM plates has nonlinearly changed with outside air velocity and temperature increase. Therefore, PCM plates show a significant potential for the practical application of building thermal storage.
Key words: thermal storage; phase change material; expanded perlite; expanded vermiculite; binary eutectic mixture
Cite this article as: YANG Hua, CHEN Wan-he, KONG Xiang-fei, RONG Xian. Fabrication, property characterization and thermal performance of composite phase change material plates based on tetradecanol-myristic acid binary eutectic mixture/expanded perlite and expanded vermiculite for building application [J]. Journal of Central South University, 2019, 26(9): 2578-2595. DOI: https://doi.org/10.1007/s11771-019-4196-2.
1 Introduction
As people spending more time indoors, the demands on the thermal comfort have been gradually improved. With the rapid development of the living standard, people are increasingly relying on space cooling/heating system to control the building indoor environment. The extensive use of heating systems and air-conditioning increases the energy consumption of buildings. It is believed that building accounted for almost 40% of the global energy consumption and should be responsible for over 30% of the CO2 emissions in the world [1].With the increasingly focuses on the energy shortage and environmental pollution, it is imminent to reduce the building energy consumption through reasonable and effective means [2]. A high-performance building envelope can reduce the energy use of the space cooling/ heating system, which is generally considered to be the largest part of building energy consumption.
Phase change material (PCM), a kind of function material that can absorb or release thermal energy, when incorporated with building envelope, it can enhance the thermal mass of buildings [3]. With the thermal mass increasing, the influence of outside surrounding variation on the inside thermal environment is decreased, the indoor temperature fluctuation can be passively reduced, and the thermal comfort is also enhanced [4]. More importantly, because the thermal storage capacity of PCM is larger than that of common building materials, the thermal inertia of building envelope can be improved, which is deemed to be beneficial to reduce the energy consumption of space cooling/heating system [5].
The combination method of PCM with building envelope has drawn significant attentions in the PCM application. Considering the leakage problem of PCM in the liquid phase, PCM is usually made into the composite material to resolve this leaking problem before the combination with building envelope. The shape-stabilized composite PCM has the advantage of stable solid appearance during the phase change process. It refers to that the liquid PCM is absorbed into three-dimensional micropores of supporting material, and PCM is maintained and encapsulated by the supporting material [6-8]. The shape-stabilized composite PCM is mainly composed of two important components [9, 10], PCM and supporting material. PCM in the liquid state should have good fluidity, and it is available to be absorbed by the supporting material. Fatty acids (capric acid, lauric acid, myristic acid, palmitic acid and stearic acid) [11, 12], as an organic PCM, have been widely applied due to their excellent properties such as little supercooling, good chemical stability, no corrosion and no toxicity, and they have good compatibility with the supporting materials [13]. Another organic PCM-fatty alcohols have the advantages of low price and high latent heat, but single component of the fatty alcohol has multiple phase change peaks in the phase change process [14, 15]. The binary low eutectic mixture of fatty acid and fatty alcohol has a phase change point that is lower than both the two components [16]. YUAN et al [13] have introduced 15 fatty acid binary eutectic mixtures, including capric-lauric acid (66.3: 33.7, mass ratio), lauric- stearic acid (85.7:14.3, mass ratio), etc, and the melting points and latent heats of the eutectic mixtures were calculated by a theoretically formula, which can be used for engineering application predictions. Therefore, a PCM low eutectic mixture with the appropriate phase change point can be obtained through this binary mixing method, making it suitable for practical application. Besides, fatty alcohol has no pungent odor, which can weaken pungent smell from the pure fatty acid [17], increasing the availability of fatty acids for building application.
The other component for the shape-stabilized composite PCM is the supporting material, which should have large amount of internal micropores to contain and encapsulate the liquid PCM [17-20]. Thus, the large specific surface area is a significant characteristic for the supporting material. Expanded perlite (EP) and expanded vermiculite (EVMT) are two kinds of lightweight building materials, which are produced through perlite and vermiculite to be heated with a high temperature, respectively. They not only have the large specific surface areas but also are uninflammable. Therefore, EP and EVMT are the perfect supporting materials for the shape-stabilized composite PCM. Some studies have reported the results for EP and EVMT in the fabrication of shape-stabilized composite PCM. ZHANG et al [21] have used lauric–palmitic– stearic acid ternary eutectic mixture as the PCM to form shape-stabilized composite PCM with expanded perlite. LIU et al [22] prepared the shape- stabilized composite PCM by absorbing lauric acid into porous networks of EP, with a melting point of 43.2 °C and latent heat of 105.58 J/g, and the microstructure analysis showed that lauric acid can be quite evenly dispersed in the micropores of EP. LI et al [23] utilized EP as the supporting material to absorb paraffin, and studied a new surface modification method by using hydrophobic silane to prevent the liquid PCM leakage in mixing with cement.
In summary, the organic materials such as fatty acids and paraffin have been proven to be useful as a phase change material of the shape-stabilized composite PCM; meanwhile, EP and EVMT have been verified that they are appropriate to be the supporting materials of shape-stabilized composite PCM. Besides, the eutectic mixture PCM is also a commonly used PCM, the main advantage of which is that their phase change temperature can be adjusted by combining different mass ratios of the components [16]. For the supporting materials, EP and EVMT have the pressure bearing capacity, which indicated that they can be processed into PCM plates. The application form of thin plate attached to the building wall has the advantages of simple operation and without decreasing the mechanical property of buildings. Due to no need premixing with building cement components, these PCM plates not only can be used in the new buildings, but also can be utilized for the existing building retrofit.
A binary eutectic mixture of tetradecanol (TD) and myristic acid (MA) was chosen to be PCM for the shape-stabilized composite PCM. The optimum ratio of binary components and the maximum adsorption rates of PCM mixture into supporting materials (EP and EVMT, respectively) were determined in this study. Based on the shape-stabilized composite PCM, a PCM plate that can be incorporated with the building envelope of both new and existing buildings was also developed by a mold pressing method. In order to enhance the production efficiency, the shape-stabilized composite PCM was fabricated through a self-made vacuum adsorption roller. The thermal properties, chemical stability, morphology and thermal stability of shape- stabilized composite PCM prepared by the new method were characterized by DSC, thermal conductivity measuring instrument, FT-IR, SEM and long thermal cycling tests, respectively. Besides, a novel test installation was applied to study their thermal storage performance.
2 Materials and preparation
2.1 Materials
TD and MA, 99% pure, with respectively melting points of 38.34 °C and 54.74 °C, were supplied by Tianjin Institute of Fine Chemical Rehabilitation, China and Shandong Youso Chemical Technology Co, Ltd, China, respectively. EP and EVMT, the supporting materials, were respectively purchased from Licheng perlite company and Xinlei mineral powder processing plant, China.
Equation (1) [24] was utilized to forecast the optimal mass ratio of the two components, the calculation results of which are shown in Figure 1. The result shows that the binary eutectic mixture of TD and MA is most stable under the condition of approximate 70% TD mass fraction.
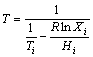 (1)
(1)
where T is the phase change temperature; H is the thermal enthalpy; X is the molar fraction in the mixture; and R is the gas constant. Subscript i indicates the organic species (A or B).
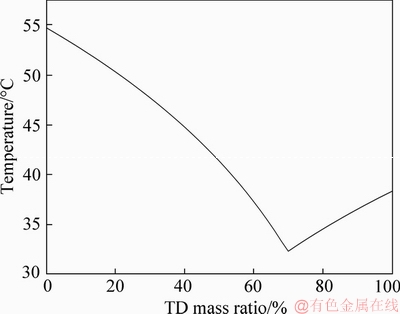
Figure 1 Phase change points of TD-MA eutectic with different TD mass ratios
The precise mass ratios of TD and MA were confirmed through an experimental method. TD and MA were mixed with different mass ratios (66.65%, 67.65%, 68.65%, 69.65%, 70.65% and 71.65%), and the mixtures were heated by a water bath at 80 °C for 30 min. The prepared TD-MA eutectics with the above mass ratios were measured by DSC to obtain the phase change points and latent heats. According to binary eutectic mechanism, the test sample with the lowest phase change point is the most stable binary eutectic mixture, and the mass ratios of the two raw materials is the optimum component proportion of the binary eutectic mixture. The DSC results are shown in Table 1. Figure 2 shows the DSC curves with different TD mass ratios, in which when the mass ratio of TD is 69.65%, TD-MA mixture has the lowest phase change point that is generally named the eutectic temperature [25], and the latent heats of melting and freezing are 205.32 and 204.26 J/g, respectively.
Table 1 DSC results of TD-MA mixture with different mass fractions
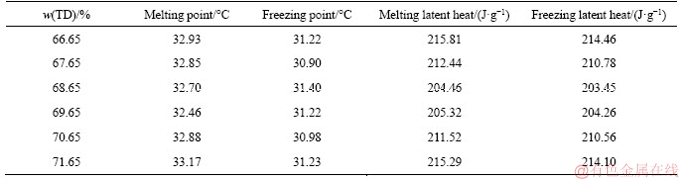
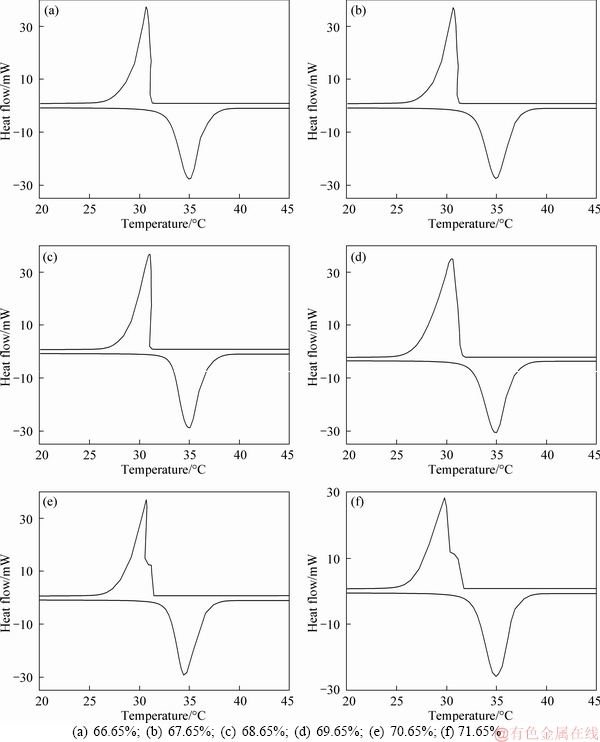
Figure 2 DSC curves with different TD mass ratio:
2.2 Preparation of shape-stabilized composite PCM
TD-MA mixture with mass ratios of 69.65% TD and 30.35% MA was absorbed into the supporting materials of EP and EVMT, respectively, so as to obtain the required phase change material particle (PCP) for the fabrication of composite phase change material plate (CPCP). As shown in Figure 3, a self-made vacuum adsorption roller that can improve production efficiency was used to prepare PCPs, the specific steps of which are stated as follows.
1) The solid-phase TD-MA mixture and the supporting materials were put into the vacuum box. After the upper cover of the roller was closed, the vacuum pump was started to extract the air inside the supporting materials.
2) The electric heater inside the water on the bottom of roller was opened, so as to uniformly heat the vacuum box by water vapor. When the TD-MA mixture fully melted in the vacuum box, the three-phase asynchronous motor began to work.
3) With adjusting the frequency of motor, a rotational speed of 60 rad/min was obtained. Under the effect of centrifugal force, TD-MA mixture has uniformly mixed with supporting materials. After 2 h, the electric heater and motor were closed, and the preparation of PCP was finally completed.
2.3 Fabrication of composite phase change material plate
Figure 4 has shown the fabrication process of CPCP. PCP1 (EP as the supporting material), styrene acrylic emulsion and environmental protection paint with the mass ratio of 85:9:6 were added to the mixer, and the motor began to stir.
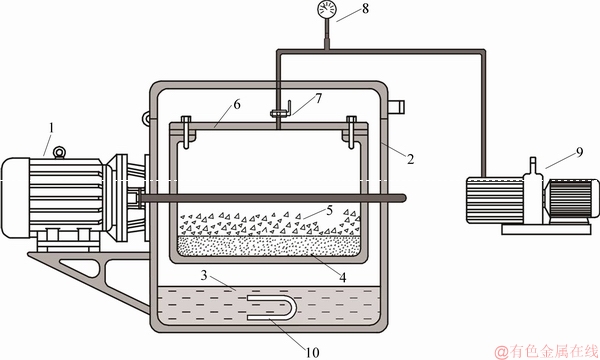
Figure 3 Schematic diagram of vacuum adsorption roller: (1—Three-phase asynchronous motor; 2—Gaine; 3—Water; 4—Porous materials; 5—Phase change materials; 6—Vacuum roller box; 7—Vacuum valve; 8—Vacuum meter; 9—Vacuum pump; 10—Electrical heating)
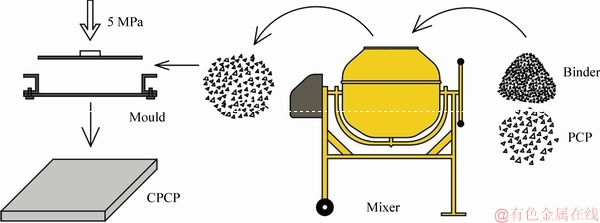
Figure 4 Preparation process of CPCP
After sufficient stirring, the mixture was evenly laid in a pre-prepared mold and given a constant pressure of 5 MPa, at room temperature. After PCP1 pressed well, the mold was disassembled and PCP1 dried naturally at room temperature, and then CPCP1 with a size of 400 mm×400 mm×30 mm was completed. Following the above steps, CPCP2 (EVMT as the supporting material) with the same size of 400 mm×400 mm×30 mm was also prepared. The appearances of the fabricated CPCP1 and CPCP2 samples are shown in Figure 5, in which EP plate (EPP) and EVMT plate (EVMTP) without PCM were also demonstrated.
3 Experimental methodology
3.1 Optimum adsorption ratio of phase change material particles
PCPs with different PCM mass fractions were prepared by the above vacuum absorption process. The diffusion-oozing circle method [7, 26] was applied to determine the optimum adsorption ratio of PCM. In this experiment, a standard circle with a diameter of 30 mm was drawn on the filter paper, and PCPs were evenly spread in this circle. Then, the filter paper was placed on a hot plate at 50 °C for 1 h to make PCM completely melt. After the completion of heating process, PCPs were removed from the filter paper. Finally, the diameter of the leaking PCM circle was measured.
3.2 Property characterization
The phase change points and the latent heats of PCM mixture and the PCPs were measured by DSC, under the conditions of heating rate of 5 °C/min, with the temperature range of 0-70 °C and constant nitrogenous atmosphere. Another important thermal parameter, the thermal conductivity, was measured by a thermal conductance meter. The chemical reactions between PCM and supporting materials were analyzed by FT-IR, with a wave number range of 400-4000 cm-1. SEM was used to observe the microstructure of supporting materials and PCPs.
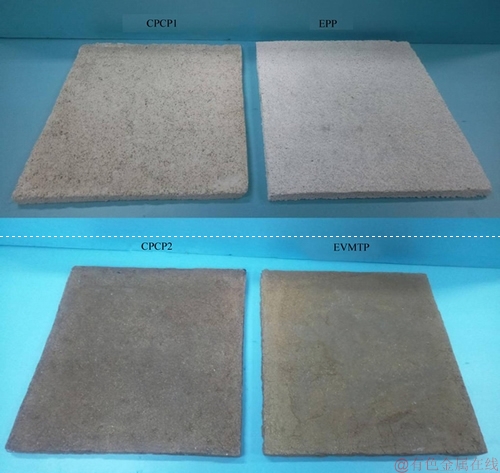
Figure 5 Appearances of prepared CPCPs, EPP and EVMTP
The durability of PCP was determined by a large number of accelerated thermal cycles. The test sample was placed inside a copper sleeve, which was heated by a hot water bath or cooled by a cold water bath. The temperature sensor inside the sample was used to confirm whether the PCM completely melting or freezing. And the temperature sensor was also connected with the temperature controller and the electric three-way valve, so as to switch heating and cooling process. After 200, 500 and 1000 times of thermal cycling, the sample was tested by DSC respectively, for comparison with the result before the cycles.
3.3 Thermal storage performance experiment
A novel device for the thermal storage performance test, as shown in Figure 6, has been used in the thermal storage performance experiment. It is made of steel plates of 2 mm thickness, with sizes of 125 cm×30 cm×30 cm. A temperature- adjustable water bath and an electric heater were installed in this experiment to generate the required heat that was transferred into the internal space at a variable speed. Besides, nine thermocouples were used to monitor the temperature variations of air temperature and surface and internal temperatures of test sample. The schematic diagram of thermal storage performance test device and the thermocouple layout are exhibited in Figure 7. This experiment is to obtain three targets:
1) After finishing the fabrication of PCM plate, the total thermal storage ability of PCM plate was determined by this thermal storage performance test device.
2) The air temperature is an important factor that influences the thermal storage performance of PCM plate; the air temperature was changed in the experiment process, so as to obtain the effect of temperature variation on the thermal storage of PCM plate.

Figure 6 Appearance of thermal storage performance test device

Figure 7 Schematic diagram of thermal storage performance test device
3) Another key factor of thermal storage performance for the plate-shaped composite PCM is the heat convection; the study of different convective heat transfer coefficients caused by different fan speeds influencing on the thermal storage performance was also conducted in this experiment.
The detailed experiment process is explained as follows:
1) With the heating system and fan system started, the required air temperature and wind speed in the left space of test sample were obtained. The temperature of other space was maintained to be equal to the room temperature.
2) The heated air was circulated through the connect pipe. Until that the middle temperature of the test sample reached to 45°C, the heating system was closed.
3) The valve between connect pipe and external environment was opened, and then, the surrounding air was introduced to cool the test sample. After that the middle temperature of the test sample dropped to 25°C, the whole system was turned off.
3.4 Main equipment
Table 2 lists the detailed information of the equipments used, which includes the equipments for the composite PCM preparation, instruments for characterization and data measuring and collecting devices in the experiments.
4 Results and discussion
4.1 Optimum adsorption ratio result
The results of the diffusion-oozing circle test can be reflected by α [7, 26], which was calculated by the formulation (2):
α=Dl/Ds×100% (2)
where D is the diameter of circle; subscripts s and l represent the standard circle and the leaking PCM circle, respectively.
The results are shown in Table 3 and Figure 8, in which it can be found that when the mass ratios of EP and EVMT are 47.5% and 40%, respectively, the liquid TD-MA mixture can be firmly encapsulated by EP and EVMT, and the leakage of PCP is acceptable. Due to the difference, three dimensional microstructures between the EP and EVMT, they have different adsorption ratios of PCM. Therefore, the mass ratios of EP: PCM= 47.5:52.5 and EVMT:PCM=40:60 were the optimum adsorption ratios.
Table 2 Instrument details
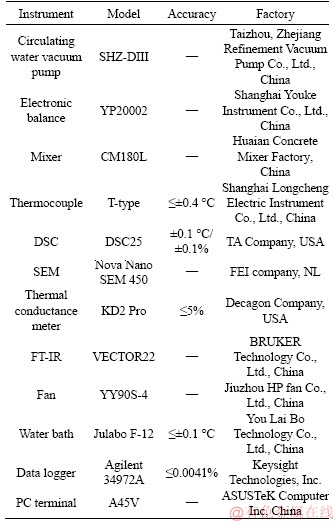
Table 3 Result of diffusion-oozing circle test
4.2 Morphology and microstructure
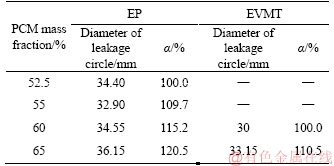
As can be seen from Figure 9(a), the expanded perlite is loose and porous with a random plate-like structure. Irregular micropores with different sizes formed the three-dimensional microstructure of EP. The numerous of micropores provides sufficient space for absorbing PCM. The walls of micropores (the light-colored region in the figures) provide good mechanical strength for PCP1. In Figure 9(b), the black-colored part is the absorbed PCM, which fills into the micropores, indicating that the PCM is uniformly distributed in EP internal structure and the three-dimensional microstructure of EP can prevent PCM leakage through the inter capillarity and surface tension.

Figure 8 Figures of composite PCM with different adsorption ratios:
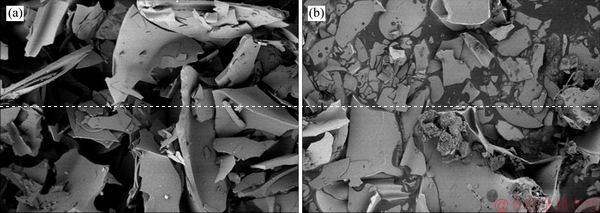
Figure 9 SEM images of EP before (a) and after (b) adsorption of TD-MA
As shown in Figure 10(a), EVMT presents a compact and irregular layered structure, which is different from the pore structure of EP. The internal structure of EVMT consists of many layers stacked at widely spaced intervals that provide sufficient spaces to contain PCM. After PCM absorption, as shown in Figure 10(b), PCM with black color is hold in the laminar internal space of EVMT. In other words, the laminar three-dimensional microstructure encapsulates PCM with a multilayer form, resulting in the adsorption mechanism difference with EP. These microstructure analyses clearly show that the TD-MA mixture was successfully absorbed and encapsulated by the supporting materials.
4.3 Thermal property
Figure 11 shows the DSC curves of PCP1 and PCP2. It can be seen that the melting and freezing points are respectively 32.32 °C and 31.02 °C for PCP1, and 32.37 °C and 31.14 °C for PCP2, which was close to the phase change points of 32.46°C and 31.22 °C for TD-MA eutectic mixture. Compared with pure TD-MA mixture, PCPs have a little reduction in phase change temperature, which is due to the weak interaction between the PCM and the interstitial surface of the micropores of supporting materials, which is corresponded with the published research reports of KARAIPEKLI et al [10], FENG et al [27], LIAO et al [28], and ZHANG et al [29].
The latent heats of melting and freezing were 205.32 J/g and 204.26 J/g for the TD-MA eutectic mixture, 113.45 J/g and 112.27 J/g for the PCP1, and 133.32 J/g and 131.43 J/g for the PCP2, respectively. Table 4 lists the latent heat ratios of PCP1 and PCP2 to TD-MA eutectic mixture. Both ratios of latent heats of melting and freezing were nearly equal to the optimum adsorption mass ratios of PCMs to PCPs, but the former was slightly larger than the latter, which was probably caused by the interactions between PCM and the inner surface of supporting materials [20]. Table 5 shows the thermal property comparison of prepared PCPs with other composite PCMs from the references. It can be found that PCPs have the high-level latent heats in the low phase change point range. This conclusion indicates that the obtained PCPs have a promising application prospect in building energy efficiency.
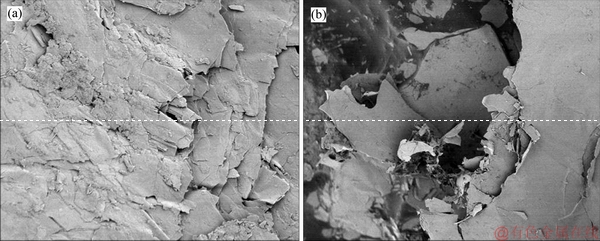
Figure 10 SEM images of EVMT before (a) and after (b) adsorption of TD-MA
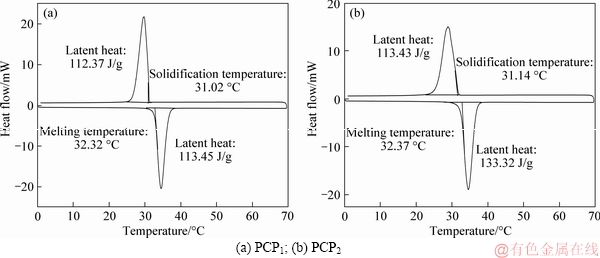
Figure 11 DSC curves:
Table 4 Latent heat ratio of PCP to TD-MA eutectic mixture
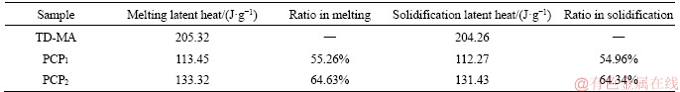
Table 5 Comparison of thermal properties of prepared PCPs with that of some composite PCMs in literatures

In the areas with large temperature differences between winter and summer (hot summer and cold winter), PCM with a single melting point could not be active all over the year through the whole year. Aiming to solve this problem, the form of PCM wall with double layers [30, 31] has been proposed by researchers, which was composed by PCM with high melting point in outside layer, basic wall materials and PCM with low melting point in inside layer. The outside layer is active during the cooling season and its function is to attenuate the amplitude of temperature fluctuations on the wall side exposed to the ambient; meanwhile, the inside layer is active during the heating season and contributes to increasing the indoor thermal comfort and attenuating the indoor temperature fluctuations. Based on this concept, PCP1 and PCP2 in this study were prepared to apply in the outside layer of building envelop (walls and roofs). The heat from the ambient can be absorbed by PCPs in the daytime and then release back to the ambient in the nighttime, reducing the heat gain of building envelop in summer. Therefore, the developed PCP1 and PCP2 have higher phase change points than that of PCMs used in the interior of buildings.
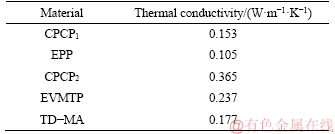
The KD2 Pro thermal conductivity meter was used to measure the thermal conductivity of the prepared PCM plate (400 mm×400 mm×30 mm) in room temperature. An average value from five measurements was taken as the final result. The results are shown in Table 6, in which both the thermal conductivities of PCM plates (CPCP1 and CPCP2) are higher than that of the plates (EPP and EVMTP) without PCM. In detail, the thermal conductivities of CPCP1 and CPCP2 increased by 45.7% and 54.0%, respectively, compared with EPP and EVMTP. It can be found that the thermal conduction has been enhanced by the absorption of PCM, which is mainly caused by that the original air in the internal structure of supporting materials is extruded by PCM, and PCM has a higher thermal conductivity than air. Besides, CPCP2 shows higher thermal conductivity than CPCP1, which is probably due to that the microstructure of EVMT- based CPCP2 is more compact than that of EP- based CPCP2.
Table 6 Thermal conductivity comparison
4.4 Chemical compatibility between TD-MA eutectic mixture and supporting material
The interaction between PCM and the support material was analyzed by FT-IR. The results are shown in Figure 12. For the supporting materials of EP and EVMT, the absorption peak at 3428 cm-1 was attributed to the stretching vibration of —OH, and the absorption peak at 1636 cm-1 was due to the bending vibration of —OH. Moreover, the absorption peaks at 1040 and 626 cm-1 were caused by the stretching vibration and bending vibration of Si—O—Si, respectively. Because the same chemical composition of EP and EVMT, they have nearly identical characteristic absorption peaks in their infrared spectrum. For the TD-MA mixtures, the peaks at 2925 and 2855 cm-1 correspond to the C-H stretching vibration, and the absorption peaks at 3428 and 1712 cm-1 represent the stretching vibration of H—OH and C=O, respectively. In addition, due to the anti-symmetric bending vibration of —CH3, the absorption peak at 1465 cm-1 can be observed.
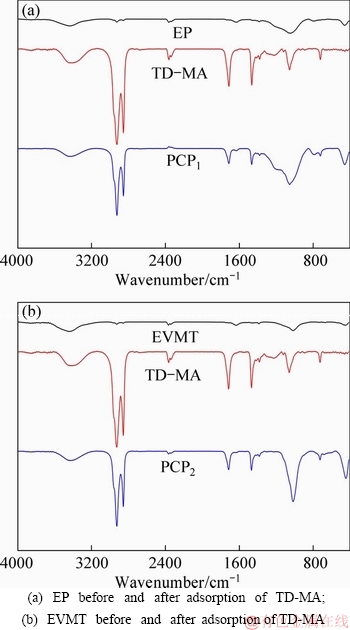
Figure 12 FT-IR spectra:
As seen in the infrared spectrum of PCP, no new absorption peak was observed in the curve. The FT-IR curve of PCP was only obtained by the superposition of the identical characteristic absorption peaks of supporting materials and TD-MA, which means that these functional groups were not destroyed during the absorption process. However, compared with pure TD-MA mixtures and porous materials, the absorption peak of PCP deviates slightly. These frequency shifts were attributed to the interactions between the —COOH group of TD-MA mixtures and alkaline region (K2O, Na2O, CaO) of EP (K2O, MgO, CaO) of EVMT. Therefore, it can be considered that there was only physical adsorption between the porous materials and PCM, and no chemical reaction occurs.
4.5 Durability
The composite PCM to be used in buildings should have good durability, which is commonly characterized by little or no change on the phase change parameters after the long term using. In this study, the rapidly melting and freezing cycling tests with 200, 500 and 1000 times repetition were utilized to evaluate the durability of the prepared PCPs. Figure 13 shows the DSC results of tested samples before and after thermal cycling, and the comparison on the key thermal parameters are presented in Table 7.
For the phase change point, after 200, 500, 1000 times of thermal cycling, the melting and freezing points of PCP1 decreased by 0.37, 0.43, 0.25 °C and 0.39, 0.22, 0.1 °C, respectively, while the corresponding shifts of the two points for PCP2 were respectively -0.76, -0.83, -0.17 °C and -0.37, -0.49, -0.13 °C. According to the research conclusions of study [32], the slight shifts on the phase change points have limited influence on the thermal storage capacity for building applications, and the attenuation of the phase change temperature is independent with the number of accelerated thermal cycles [39]. Thus, it can be said that both the prepared EP-based PCP1 and EVMT-based PCP2 have good reliability for the phase change point. The latent heat of composite PCM is a direct reflection of its thermal storage capacity, which is an important indicator for the PCM application performance in buildings. After the thermal cycling, the latent heats of melting and freezing of PCP1 changed by -6.9%, -2.6%, -5.0%, and -4.3%, -4.0%, -5.3%, respectively; meanwhile, the corresponding latent heats of PCP2 respectively decreased by 6.7%, 9.5%, 3.8% and 2.8%, 6.1%, 3.7%. In view of the reports of Ref. [25], the little decrease in latent heat value of shape-stabilized PCM is negligible for their applications in buildings. Therefore, it can be concluded that, the prepared EP-based PCP1 and EVMT-based PCP2 have good durability after a large number of repeated thermal cycles, indicating the significant potential for their applications in buildings.

Figure 13 DSC results before and after thermal cycling:
Table 7 Thermal performance parameters before and after thermal cycling

4.6 Thermal storage performance
The thermal storage performance of prepared plate with the same size (400 mm×400 mm×30 mm) and different mass (CPCP1:3.22 kg; EPP:1.64 kg; CPCP2:5.11 kg; EVMTP:2.38 kg) have been tested. Figure 14 shows the thermal storage performance test results of the prepared plates under the condition of air speed 0.5 m/s and air temperature 60 °C. In the heating period, the temperature of CPCPs increased more slowly than that of the plates without PCM, which proved that the prepared CPCPs have a larger thermal inertia. PCMs in the CPCPs absorbed a part of heat that transferred from outside hot air, while they had a small temperature changes in this heat absorption, resulting in the fact of better thermal inertia. Furthermore, although the thermal conductivity of EPP was small, its thermal diffusivity was larger than that of EVMTP, which led to that EPP had a faster temperature-rise rate than EVMTP, and Table 8 lists the thermal diffusivity of prepared plates. In the cooling period, when the outside temperature was decreased to the phase change points, PCMs began to release the absorbed heat in a narrow temperature range. Thus, a smooth curve section that represents this heat releasing process of PCM appeared in Figure 14(b), which indicated PCM functioning well in CPCPs. Besides, the latent heat of CPCP2 was larger than that of CPCP1, resulting in the fact that the heat storing and releasing time of CPCP2 is longer than that of CPCP1. According to the results, EP-based CPCP1 and EVMT-based CPCP2 had different thermal storage characteristics, which can be the basis for their practical applications. The good potential for the novel CPCPs has been determined through this thermal storage performance experiment, and based on this result, their usage effect will be further studied in the next experiment of real full-size buildings.
Table 8 Thermal diffusivity of prepared plate

Figures 15 and 16 show the test results of the CPCPs with air velocities of 0.3, 0.5 and 0.7 m/s under the air temperature of 60 °C. It can be found that when the air velocity was 0.5 m/s, both CPCPs absorbed most quickly, which were followed by air velocities of 0.3 and 0.7 m/s. Because of the phase transition in the heat absorption, the rate of temperature changes in the prepared CPCP does not increase with air velocity linearly, which caused the fact that under the condition of air velocity of 0.5m/s rather than that of 0.7 m/s, the internal temperature of CPCPs increased most rapidly. While it had a different result in the cooling period, in which the condition of 0.7 m/s air velocity led to the shortest heat-releasing time interval, and the second and third time intervals were under the conditions of 0.3 and 0.5 m/s air velocity, respectively. Besides, the conditions of 0.3 m/s and 0.5 m/s air velocities had very close results. This fact indicates that CPCPs have a larger sensitivity to the high air velocity than low air velocity.
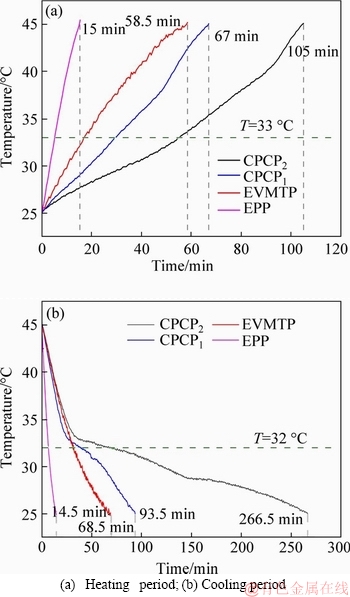
Figure 14 Thermal storage experiment results at air speed of 0.5 m/s and 60 °C:
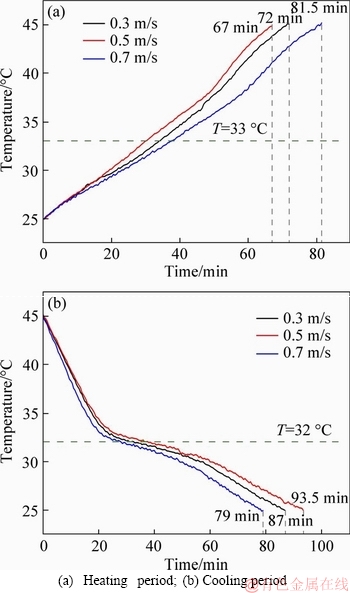
Figure 15 Thermal storage experiment results for CPCP1 with different air velocity:
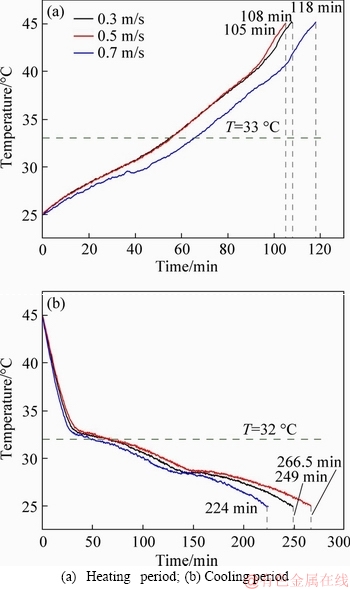
Figure 16 Thermal storage experiment results for CPCP2 with different air velocity:
Figure 17 shows the test results of the CPCPs with different heating temperatures of 55, 60 and 65 °C under the air velocity of 0.5 m/s. The time intervals for heat absorption were reduced with the increase of heating temperature. When the heating temperature was 65 °C, the heat absorption period was the shortest: 65 and 104 min for CPCP1 and CPCP2, respectively; and the lowest heating temperature of 55 °C obtained the longest heat absorption period. This result illustrates that the high outside temperature has a positive driving force on the phase change process. In other words, a large heat gain can accelerate the thermal storage of CPCPs. However, the close results between the heating temperatures of 65 and 60 °C also indicated that the high heating temperature can always reduce the heat absorption of CPCPs, and there should be an optimum heating temperature that can lead to a shortest heat absorption period of CPCPs. A more systematic research to quantitatively reveal the influences of the outside air velocity and temperature on the CPCP will be conducted in the next research with the numerical model.
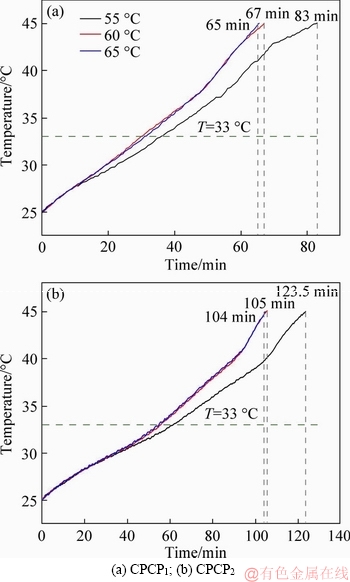
Figure 17 Thermal storage experiment results with different air temperatures:
5 Conclusions
Two new kinds of composite PCM plates which can be applied to buildings were studied. A binary eutectic mixture TD and MA was used as PCM, with supported materials of EP and EVMT, respectively. A self-made vacuum adsorption roller was used to prepare composite PCMs, and the composite PCM plate was fabricated through a mould pressing method. The main conclusions can be summarized as follows.
1) The melting and freezing points are 32.32 and 31.02 °C for PCP1, and 32.37 and 31.14 °C for PCP2, respectively. The latent heats of melting and freezing of are 113.45 and 112.37 J/g for PCP1, and 133.32 and 131.43 J/g for PCP2. After the comparison with similar composite PCMs in other studies, it is concluded that the two composite PCMs with high latent heat have the promising potential for building application.
2) TD-MA mixture is evenly distributed in the microstructures of EP and EVMT, and PCM is encapsulated well by the two supporting materials, without PCM leakage and chemical reaction. After the rapidly melting and freezing cycling tests with 200, 500 and 1000 times repetition, composite PCMs have no obvious changes on phase change points and latent heats before and after thermal cycles, which shows their good durability. The above results on the property characterization indicate that the self-made vacuum adsorption roller is a feasible method for the composite PCM fabrication, and the prepared PCM has high-quality property.
3) Compared with the traditional insulation materials, both the novel CPCPs have better thermal inertia, but because of the higher per-unit latent heat for PCP2 than PCP1, PCP2 has a longer thermal storing/releasing time period than that of PCP1. Besides, the thermal storage performances of CPCPs have nonlinearly changed with the outside air velocity and temperature increase. The above analyses have provided theoretical basis for CPCPs practical application.
References
[1] ELNAJJAR E. Using PCM embedded in building material for thermal management: Performance assessment study [J]. Energy and Buildings, 2017, 151: 28-34.
[2] YAO Cheng-qiang, KONG Xiang-fei, LI Yan-tong, DU Ya-xing, QI Cheng-ying. Numerical and experimental research of cold storage for a novel expanded perlite-based shape-stabilized phase change material wallboard used in building [J]. Energy Conversion and Management, 2018, 155: 20-31.
[3] CABEZA L F, CASTELL A, BARRENECHE C, GRACIA A D, FERNANDEZ A I. Materials used as PCM in thermal energy storage in buildings: A review [J]. Renewable and Sustainable Energy Reviews, 2011, 15(3): 1675-1695.
[4] SOARES N, COSTA J J, GASPAR A R, SANTOS P. Review of passive PCM latent heat thermal energy storage systems towards buildings’ energy efficiency [J]. Energy and Buildings, 2013, 59: 82-103.
[5] KONG Xiang-fei, LU Shi-lei, HUANG Jing-yu, CAI Zhe, WEI Sha-sha. Experimental research on the use of phase change materials in perforated brick rooms for cooling storage [J]. Energy and Buildings, 2013, 62: 597-604.
[6] XIAO Min, FENG Bo, GONG Ke-cheng. Preparation and performance of shape stabilized phase change thermal storage materials with high thermal conductivity [J]. Energy Conversion and Management, 2002, 43(1): 103-108.
[7] KONG Xiang-fei, ZHONG Yu-liang, RONG Xian, MIN Chun-hua, QI Cheng-ying. Building energy storage panel based on paraffin/expanded perlite: Preparation and thermal performance study [J]. Materials, 2016, 9(2): 70.
[8] MEHRALI M, LATIBARI S T, MEHRALI M, METSELAAR H S C, SILAKHORI M. Shape-stabilized phase change materials with high thermal conductivity based on paraffin/graphene oxide composite [J]. Energy Conversion and Management, 2013, 67: 275-282.
[9] WANG En-yu, KONG Xiang-fei, RONG Xian, YAO Cheng-qiang, YANG Hua, QI Cheng-ying. A study on a novel phase change material panel based on tetradecanol/ lauric acid/expanded perlite/aluminium powder for building heat storage [J]. Materials, 2016, 9(11): No. 896.
[10] KARAIPEKLI A, SARI A. Preparation, thermal properties and thermal reliability of eutectic mixtures of fatty acids/expanded vermiculite as novel form-stable composites for energy storage [J]. Journal of Industrial and Engineering Chemistry, 2010, 16(5): 767-773.
[11] ZHANG Zhao-li, YUAN Yan-ping, ZHANG Nan, CAO Xiao-ling. Experimental investigation on thermophysical properties of capric acid–lauric acid phase change slurries for thermal storage system [J]. Energy, 2015, 90(1): 359-368.
[12] YUAN Yan-ping, ZHANG Nan, LI Tian-yu, CAO Xiao-ling, LONG Wei-yue. Thermal performance enhancement of palmitic-stearic acid by adding graphene nanoplatelets and expanded graphite for thermal energy storage: A comparative study [J]. Energy, 2016, 97: 488-497.
[13] YUAN Yan-ping, TAO Wen-quan, CAO Xiao-ling, BAI Li. Theoretic prediction of melting temperature and latent heat for a fatty acid eutectic mixture [J]. Journal of Chemical & Engineering Data, 2011, 56(6): 2889-2891.
[14] ZUO Jian-guo, LI Wei-zhong, WENG Lin-dong. Thermal properties of lauric acid/1-tetradecanol binary system for energy storage [J]. Applied Thermal Engineering, 2011, 31(6): 1352-1355.
[15] ZUO Jian-guo, LI Wei-zhong, WENG Li-dong. Thermal performance of caprylic acid/1-dodecanol eutectic mixture as phase change material (PCM) [J]. Energy and Buildings, 2011, 43(1): 207-210.
[16] ZHANG Nan, YUAN Yan-ping, CAO Xiao-Ling, DU Yan-xia, ZHANG Zhao-li, GUI Ye-wei. Latent heat thermal energy storage systems with solid–liquid phase change materials: A review [J]. Advanced Engineering Materials, 2018, 20(6): 1700753.
[17] HUANG Jing-yu, LU Shi-lei, KONG Xiang-fei, LIU Shang-bao, LI Yi-ran. Form-stable phase change materials based on eutectic mixture of tetradecanol and fatty acids for building energy storage: preparation and performance analysis [J]. Materials, 2013, 6(10): 4758-4775.
[18] WU Yu-ping, WANG Tao. Hydrated salts/expanded graphite composite with high thermal conductivity as a shape- stabilized phase change material for thermal energy storage [J]. Energy Conversion and Management, 2015, 101: 164-171.
[19] SARI A. Thermal energy storage characteristics of bentonite-based composite PCMs with enhanced thermal conductivity as novel thermal storage building materials [J]. Energy Conversion and Management, 2016, 117: 132-141.
[20] KARAIPEKLI A, SARI A. Development and thermal performance of pumice/organic PCM/gypsum composite plasters for thermal energy storage in buildings [J]. Solar Energy Materials and Solar Cells, 2016, 149: 19-28.
[21] ZHANG Nan, YUAN Yan-ping, YUAN Ya-guang, LI Tian-yu, CAO Xiao-ling. Lauric–palmitic–stearicacid/ expanded perlite composite as form-stable phase change material: Preparation and thermal properties [J]. Energy and Buildings, 2014, 82: 505-511.
[22] LIU Jie-sheng, YU Yuan-yuan, HE Xiang. Research on the preparation and properties of lauric acid/expanded perlite phase change materials [J]. Energy and Buildings, 2016, 110: 108-111.
[23] LI Xiang-yu, CHEN Hui-su, LIU Lin, LU Ze-yu, SANJAYAN J G, DUAN Wen-hui. Development of granular expanded perlite/paraffin phase change material composites and prevention of leakage [J]. Solar Energy, 2016, 137: 179-188.
[24] LU Shi-lei, ZHU Neng, FENG Guo-hui. Eutectic mixtures of capric acid and lauric acid applied in building wallboards for heat energy storage [J]. Energy and Buildings, 2006, 38(6): 708-711.
[25] SARI A. Eutectic mixtures of some fatty acids for latent heat storage: Thermal properties and thermal reliability with respect to thermal cycling [J]. Energy conversion and management, 2006, 47(9, 10): 1207-1221.
[26] RAMAKRISHNAN S, SANJAYAN J, WANG Xiao-min, ALAM M, WILSON J. A novel paraffin/expanded perlite composite phase change material for prevention of PCM leakage in cementitious composites [J]. Applied Energy, 2015, 157: 85-94.
[27] FENG Li-li, ZHAO Wei, ZHENG Jie, FRISCO S, SONG Ping, LI Xing-guo. The shape-stabilized phase change materials composed of polyethylene glycol and various mesoporous matrices (AC, SBA-15 and MCM-41) [J]. Solar Energy Materials and Solar Cells, 2011, 95(12): 3550-3556.
[28] LIAO Li, CAO Qi, LIAO Hong-qing. Investigation of a hyperbranched polyurethane as a solid-state phase change material [J]. Journal of Materials Science, 2010, 45(9): 2436-2441.
[29] ZHANG Dong, TIAN Sheng-li, XIAO De-yuan. Experimental study on the phase change behavior of phase change material confined in pores [J]. Solar Energy, 2007, 81(5): 653-660.
[30] DIACONU B M, CRUCERU M. Novel concept of composite phase change material wall system for year-round thermal energy savings [J]. Energy and Buildings, 2010, 42(10): 1759-1772.
[31] ZHU Na, WU Meng-da, HU Ping-fang, XU Ling-hong, LEI Fei, LI Shan-shan. Performance study on different location of double layers SSPCM wallboard in office building [J]. Energy and Buildings, 2018, 158: 23-31.
[32] ZHANG Wei-yi, ZHANG Xiao-guang, HUANG Zhao-hui, YIN Zhao-yu, WEN Rui-long, HUANG Yao-ting, WU Xiao-wen, MIN Xin. Preparation and characterization of capric-palmitic-stearic acid ternary eutectic mixture/ expanded vermiculite composites as form-stabilized thermal energy storage materials [J]. Journal of Materials Science & Technology, 2018, 34(2): 379-386.
[33] WEN Rui-long, ZHANG Xiao-guang, HUANG Yao-ting, YIN Zhao-yu, HUANG Zhao-hui, FAN Ming-hao, LIU Yan-gai, WU Xiao-wen. Preparation and properties of fatty acid eutectics/expanded perlite and expanded vermiculite shape-stabilized materials for thermal energy storage in buildings [J]. Energy and Buildings, 2017, 139: 197-204.
[34] ZHANG Jian-wu, GUAN Xue-mao, SONG Xiao-xiao, HOU Huan-huan, YANG Zheng-peng, ZHU Jianping. Preparation and properties of gypsum based energy storage materials with capric acid–palmitic acid/expanded perlite composite PCM [J]. Energy and Buildings, 2015, 92: 155-160.
[35] CHUANG O, JEONG S G, KIM S. Preparation of energy efficient paraffinic PCMs/expanded vermiculite and perlite composites for energy saving in buildings [J]. Solar Energy Materials and Solar Cells, 2015, 137: 107-112.
[36] FU Lu-lu, WANG Qing-hao, YE Rong-da, FANG Xiao-ming, ZHANG Zheng-guo. A calcium chloride hexahydrate/ expanded perlite composite with good heat storage and insulation properties for building energy conservation [J]. Renewable Energy, 2017, 114: 733-743.
[37] WEI Hai-ting, LI Xiang-qi. Preparation and characterization of a lauric-myristic-stearic acid/Al2O3-loaded expanded vermiculite composite phase change material with enhanced thermal conductivity [J]. Solar Energy Materials and Solar Cells, 2017, 166: 1-8.
[38] WEI Ting, ZHENG Bai-cun, LIU Juan, GAO Yan-feng, GUO Wei-hong. Structures and thermal properties of fatty acid/expanded perlite composites as form-stable phase change materials [J]. Energy and Buildings, 2014, 68: 587-592.
[39] KARAIPEKLI A, SARI A. Capric–myristic acid/expanded perlite composite as form-stable phase change material for latent heat thermal energy storage [J]. Renewable Energy, 2008, 33(12): 2599-2605.
(Edited by FANG Jing-hua)
中文导读
建筑中应用基于十四醇-十四酸二元共融混合物/膨胀珍珠岩和膨胀蛭石复合相变板材的制备、性能表征及热性能
摘要:以十四醇-十四酸二元低共融混合物作为相变材料,通过自制的真空吸附滚筒分别将其最大程度地吸附到膨胀珍珠岩和膨胀蛭石的微观结构之中制备出相变颗粒,然后使用压模法将相变颗粒分别制备成膨胀珍珠岩和膨胀蛭石复合相变板。通过差示扫描量热仪(DSC)、傅里叶变换红外光谱(FT-IR)、扫描电子显微镜(SEM)和热循环测试分别表征了相变颗粒的热性能,化学稳定性,微观结构和耐久性。结果表明,两种相变颗粒均具有较高的潜热,膨胀珍珠岩相变颗粒能达到110 J/g,膨胀蛭石相变颗粒能达到130 J/g;微观结构致密,没有相变材料泄漏;化学性质稳定,耐久性好。研究结果证明了真空吸附滚筒在复合相变材料制造中的可行性。蓄放热性能实验结果表明,相变板比常见建筑材料具有更好的热惯性,相变板的储热性能随外界风速和温度的升高而呈现非线性变化。因此,在建筑蓄热领域中的应用相变板具有巨大潜力。
关键词:蓄热;相变材料;膨胀珍珠岩;膨胀蛭石;二元共晶混合物
Foundation item: Project(51408184) supported by the National Natural Science Foundation of China; Project(E2017202136) supported by the Natural Science Foundation of Hebei Province, China; Project(BSBE2017-05) supported by Opening Funds of State Key Laboratory of Building Safety and Built Environment and National Engineering Research Center of Building Technology, China; Project(QG2018-3) supported by Hebei Provincial Department of Transportation, China
Received date: 2018-06-19; Accepted date: 2018-12-12
Corresponding author: KONG Xiang-fei, PhD, Associate Professor; Tel: +86-22-60435279; E-mail: xfkong@hebut.edu.cn; ORCID: 0000-0002-1550-4071

