Solidification behaviour of Al-7%Si-Mg casting alloys
CHEN Zhong-wei(陈忠伟), LI Jin-shan(李金山), JIE Wan-qi(介万奇), LIU Lin(刘 林), FU Heng-zhi(傅恒志)
(State Key Laboratory of Solidification Processing,Northwestern Polytechnical University, Xian 710072, China)
Abstract: The effects of Mg content and cooling rate on the solidification behaviour of Al-7%Si-Mg(mass fraction) casting alloys have been investigated using differential scanning calorimetry, differential thermal analysis and microscopy. The Mg contents were selected as respectively 0.00%, 0.35% and 0.70%(mass fraction). DTA curves of Al-7%Si-0.55%Mg(mass fraction) alloy at various cooling rates were accomplished and the alloy melt was cast in different cooling rates. The results indicate that increasing Mg content can lower the liquidus and binary Al-Si eutectic transformation temperatures. Large Fe-rich π-phases (Al8FeMg3Si6) are found in the 0.70% Mg alloys together with some small β-phases (Al5FeSi); in contrast, only β-phases are observed in the 0.35% Mg alloys. The test results of the Al-7%Si-0.55%Mg alloys identify that the liquidus and binary Al-Si eutectic transformation temperatures decrease, and the quantity of ternary Al-Si-Mg2Si eutectic phase decreases as the cooling rate increases.
Key words: Al-Si-Mg casting alloys; solidification; cooling rate; eutectic transformation CLC number: TG244
Document code: A
1 INTRODUCTION
Al-Si alloys are the most widely used materials in the foundry industry due to their light mass, good mechanical properties and castability. Magnesium is introduced to Al-Si alloys in order to increase the tensile strength of the alloys. The most popular Al-Si alloys containing magnesium are A356(Al-7%Si-0.35%Mg, mass fraction) and A357 with a higher magnesium level(0.50%, mass fraction). These alloys are marked by excellent casting characteristics, weldability, pressure tightness and corrosion resistance[1]. These two alloys are heat-treatable due to Mg2Si hardening. The hard particles of Mg2Si are precipitated uniformly throughout the aluminium matrix after solution treatment(T4), followed directly by quenching and aging. Heat treatment provides the variety of tensile strength and other physical properties that are attractive for many applications, including military, aircraft and automotive parts[2].
In general terms, it has been reported that a higher Mg content can increase the yield stress while decrease the ductility[3, 4] and the fracture toughness[5]. Besides its major effect on the age-hardening potential, Mg depresses the eutectic temperature and makes the eutectic Si structure more heterogeneous[6]. The Mg content also affects the type and total volume fraction of Fe-bearing phases[7,8], which are known to have a detrimental effect on the tensile properties[9], especially in Be-free alloys[10]. Bckerud et al[11] studied three types of Al-Si-Mg casting alloys and discussed the solidification characteristics of these alloys under three cooling rates. It was observed that the addition of Mg changed the solidification sequence and the type of Fe-bearing intermetallics. However, it is difficult to conclude the actual effect of Mg because higher Mg alloy (0.56%) contains a small amount of beryllium, which is known to strongly influence the behaviour of Fe. Granger et al[12] in their study found that beryllium-free alloy had an insoluble Fe-bearing phase containing Mg, while the alloy with beryllium did not. Joenoes and Gruzleski[6] studied the effect of Mg content in iron-free synthetic alloys at a constant cooling rate. They found that a small amount of Mg changed the morphology and size distributions of the silicon phase. Caceres et al[13] studied the effect of Mg on the eutectic Si microstructure and mechanical behaviour of Al-Si-Mg casting alloys. It is concluded that the eutectic Si particles are large in the alloys with higher Mg content, and the yield stress increases and the tensile ductility is less with higher Mg content.
This work is aimed at evaluating the effect of Mg content and cooling rate on the solidification behaviour of commercial Al-7%Si-Mg(mass fraction) casting alloys by using DSC, DTA and microscopy.
2 EXPERIMENTAL
2.1 Materials and casting procedures
Commercial unmodified Al-7%Si-0.35%Mg and Al-7%Si-0.70%Mg(mass fraction) casting alloys (similar to the US A356.0 and A357.0) were used in this investigation. A laboratory-prepared Al-7%Si(mass fraction) binary alloy was also used for reference in DSC measurements. The melt was degassed with argon for 20min. The melts of different Mg content alloys were cast in sand molds. Cooling rates measured were about 4.4℃/min. In order to produce a range of cooling rates, the melt of Al-7%Si-0.55%Mg casting alloys was cast in heat-retaining graphite mould, steel mould and water cooled steel mould, respectively. Cooling rates measured were about 22℃/min, 79℃/min and 260℃/min. The chemical compositions of the different alloys, as obtained by inductively coupled plasma-atomic emission spectroscopy, are given in Table 1.
2.2 Thermal analysis
Differential Scanning Calorimetry(DSC) analysis and Differential Thermal Analysis(DTA) were performed on samples of all studied alloys using a TMDSC-2910 instrument. Specimens used were discs with 4mm in diameter. In the study of phase transformation during solidification or remelting, the DSC was run at 10℃/min scanning rate in the range of 500℃ to 700℃. DTA was run at a scanning rate of 20℃/min, 15℃/min and 10℃/min. A high-purity aluminium disc of similar mass to the samples was used as the reference pan.
2.3 Microscopic analysis The Fe-rich intermetallic phases were easily recognized by optical microscopy (OLYMPUS PM-G3).
3 RESULTS
3.1 Effect of Mg addition on solidification behaviour of Al-7Si alloys
Fig.1 shows the DSC curves of Al-7%Si alloy, Al-7%Si-0.35%Mg alloy and Al-7%Si-0.70%Mg alloy. Peak 1 corresponds to the development of aluminium dendrites; Peak 2 represents the main binary eutectic reaction; Peak 3a and Peak 3b are associated with formation of the β-phase (FeSiAl5) and π-phase (Al8FeMg3Si6). Table 2 lists the peak temperature of DSC curves. Table 3 lists solidification reactions observed in Al-7%Si-Mg alloys[11, 14].
DSC tests show that increasing Mg content shifts the liquidus and binary eutectic transforma-tions to a lower temperature. In addition, the Fe-

Fig.1 DSC curves of Al-7%Si-Mg alloys
Table 1 Chemical compositions of alloys (mass fraction, %)

Table 2 DSC remelting characteristics of as-cast Al-7%Si-Mg alloys
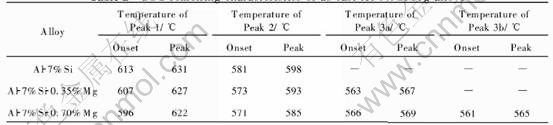
Table 3 Solidification reactions observed in Al-7Si-Mg alloys[11, 14]

rich phases change with increasing Mg content. Fe-rich π-phases (Al8FeMg3Si6) are found in the 0.62% Mg alloys together with some β-phases (Al5FeSi); in contrast, only β-phases are observed in the 0.35% Mg alloys.
3.2 Effect of cooling rate on solidification behaviour of Al-7%Si-0.55%Mg alloy
Fig.2 shows the DTA curves of Al-7%Si-0.55%Mg alloy at various cooling rates. As shown in Table 4, there are mainly four solidification reactions in Al-7%Si-Mg alloys. Peak 1 and Peak 2 represent the development of aluminium dendrites and the main binary eutectic reaction. Peak 3 is associated with formation of ternary eutectic or quaternary reactions and it is mainly the formation reaction of Mg2Si or Fe-rich phases. In Fig.2, Peak 3 shows mainly the formation of ternary Al-Si-Mg2Si eutectic reaction. Only when cooling rate is 10℃/min, there are three solidification reactions for Al-7%Si-0.55%Mg alloy in cooling curves. When the cooling rate is 15℃/min or 20℃/min, there are two solidification reactions for this alloy in cooling curves. The results show that the terna-ry Al-Si-Mg2Si eutectic reaction is depressed when
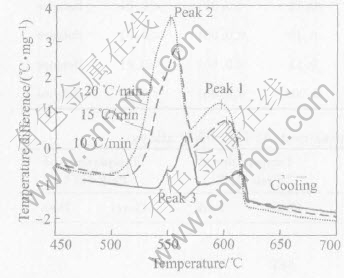
Fig.2 DTA curves of Al-7%Si-0.55%Mg alloy at various cooling rates
Table 4 DSC cooling characteristics of as-cast Al-7%Si-0.55%Mg alloy

cooling rates is increased. When the cooling rate increases, the positions of Peak 1 and Peak 2 shift to lower temperatures. This shows that the formation temperature of α(Al) phase and binary Al-Si eutectic decreases when the cooling rate increases.
Fig.3 shows the DSC curves of Al-7Si-0.55Mg samples cast in heat-retaining graphite mould, steel mould and water cooled steel mould. Peaks 1, 2 and 3 correspond to the melt endothermic peak of α(Al), binary Al-Si eutectic and ternary Al-Mg2Si-Si eutectic. For three DSC curves, temperature difference of Peaks 1 and 2 is small, and temperature difference of Peak 3 is bigger.

Fig.3 DSC curves of Al-7%Si-0.55%Mg alloy cast in various cooling rates
Fig.3(b) shows an enlargement of Peak 3 in Fig.3(a). In the DSC curves, the reaction peaks reflect the special phases changes and the peak area is proportional to the heat of reaction associated with the phase transformation. The values are due to the endothermic reactions of liquid formation during heating. The value of energy absorbed by ternary Al-Mg2Si-Si eutectic phase melt was determined by measuring the area under Peak 3. When the cooling rates are 22℃/min, 79℃/min and 260℃/min (the ratio is 1∶4∶8), the area of endothermic Peak 3 is 2.689J/g, 1.552J/g and 0.317J/g respectively (the ratio is 8.47∶4.89∶1.00). The results show that the formation of ternary Al-Mg2Si-Si eutectic is depressed and the quantity of ternary Al-Mg2Si-Si eutectic decreases as the cooling rate increases.
3.3 Microstructure
Fig.4 compares the as-cast microstructures of the low and high Mg content alloys. The Fe-bearing phase in the low Mg content Al-7%Si-0.35%Mg alloy is apparently mainly β-phases (Al5FeSi), but in the high Mg content Al-7%Si-0.70%Mg alloy the Fe-bearing phase is mainly π-phases (Al8FeMg3Si6) together with some smaller β-phases (Al5FeSi). The morphology and size of Fe-bearing phases change with increasing the Mg content.

Fig.4 As-cast microstructures of Al-7Si-Mg alloys
4 DISCUSSION
4.1 Solidification sequence
The typical solidification sequence noted in the literature for Al-7Si-Mg alloys is presented in Table 3[14]. In stage 1, the primary aluminium dendritic phase nucleates and grows. In stage 2, the main Al-Si binary eutectic reaction(2) takes place. Fe partitions strongly to the liquid phase, enriching it as the fraction of liquid decreases, until the ternary eutectic is reached. Subsequently, the β-phase is partly transformed into the π-phase through a quasi-peritectic reaction(3b)[11]. However, the extent of this peritectic transformation probably depends on the cooling rate and Mg content during solidification. The next stage is the ternary eutectic producing Al, Si and Mg2Si (reaction(4)), and finally a quaternary reaction gives π-Al8FeMg3Si6 in addition to the previous three phases.
The major reactions(1) and (2) are clearly evident from the microstructure and thermal analysis. Reaction(3b) is supported directly by the microstructure in Fig.4. The Al-Si and Al-Si-β-AlFeSi reactions overlap to such an extent that they are merged in the thermal traces. Reaction(3a) is not easy to be observed in DSC curves of Fig.3. Reactions(4) and (5) are consistent with our final microstructure and the remaining peaks on the DSC traces of Fig.3.
4.2 Effect of Mg on solidification temperature
Fig.5 presents a two-dimensional visualization of a section of the complex Al-Si-Mg ternary system[15]. The eutectic temperature of an Al-Si alloy for a given Mg content can roughly be estimated by following the solidification of the alloy itself. For instance, in an Al-7%Si-0%Mg alloy, aluminium solidifies first as a primary phase, and then the melt solidifies along the X-axis until reaching point A. This is the eutectic temperature for the binary alloy (about 577.5℃). When an alloy having a composition at point C is considered, this alloy (Al-7%Si-0.35%Mg) will solidify along the curve CD perpendicular to the contours and will move down to the eutectic valley at point D. This is the eutectic temperature for that particular alloy composition where Al solid solution and Si form as those in a binary eutectic system. Finally, the alloy will reach point B where the remaining liquid solidifies as a ternary eutectic. Similar analysis can also be applied to the Al-7%Si-0.6%Mg alloy (point E) where eutectic solidification eventually occurs at point F. Thus we can estimate the eutectic temperature for all alloys used in the experiments. The experimentally-determined values are somewhat different from those predicted from the phase diagram. The differences are, however, small and are no doubt due to inaccuracies in the phase diagram itself which is generated on the basis of high Mg levels than those used here. The eutectic temperatures measured in this work are probably the best available values for the Al-Si-Mg system at low magnesium levels.

Fig.5 Part of a ternary Al-Si-Mg alloy phase diagram
4.3 Effect of cooling rate on solidification temperatures
The diffusion rate of Si atoms is much slower than that of Mg atoms in aluminium alloy. During solidification process, the content of Si atoms in the liquid phase near the solid/liquid interface increases with cooling rate increasing. Therefore, the solidification path moves from line OS to line OF with the cooling rate increasing as described in Fig.6. Thus, the binary Al-Si eutectic transformation temperature decreases.

Fig.6 Schematic illustration of solidification path
5 CONCLUSIONS
1) Increasing Mg content can lower the liquidus and binary Al-Si eutectic transformation temperatures of the Al-7%Si-Mg casting alloys.
2) Large Fe-rich π-phases (Al8FeMg3Si6) are found in the Al-7%Si-0.70%Mg alloys together with some small β-phases (Al5FeSi); in contrast, only β-phases are observed in the Al-7%Si-0.35%Mg alloys.
3) The liquidus and binary Al-Si eutectic transformation temperatures of the Al-7%Si-0.55%Mg casting alloys decrease, and the quantity of ternary Al-Si-Mg2Si eutectic decreases as the cooling rate increases.
REFERENCES
[1]Hatch J E. Aluminium Properties and Physical Metallurgy [M]. Ohio, Metals Park: ASM, 1984. 320-350.
[2]Haque M M, Bennett G H, Kondic V. Effects of silicon and magnesium additions on strontium modification of aluminium-silicon eutectic-base alloys[J]. Foundry Trade Journal, 1983, 24(3): 387-390.
[3]Shivkumar S, Keller C, Apelian D. Aging behavior in cast Al-Si-Mg alloys[J]. AFS Trans, 1990, 179: 905-911.
[4]Kashyap K T, Murali S, Raman K S. Casting and heat treatment variables of Al-7Si-Mg[J]. Mater Sci Technol, 1993, 9(1): 189-203.
[5]Murali S, Raman K S, Murthy K S S. Effect of Mg, Fe and solidification rates on fracture toughness of Al-7Si-0.3Mg casting alloy[J]. Mater Sci Eng, 1992, A151(1): 1-10.
[6]Joenoes A T, Gruzleski J E. Mg effects on the microstructure of unmodified and modified Al-Si alloys[J]. Cast Met, 1991, 4(2): 62-71.
[7]Closset B, Gruzleski J E. Structure and properties of hypoeutectic Al-Si-Mg alloys modified with pure strontium[J]. Metall Trans A, 1982, 13A(6): 945-951.
[8]Gustafsson G, Thorvaldsson T, Dunlop G L. The influence of Fe and Cr on the microstructure of cast Al-Si-Mg alloys[J]. Metall Trans A, 1986, 17A(1): 45-52.
[9]Tan Y H, Lee S L, Lin Y L. Effects of Be and Fe additions on the microstructure and mechanical properties of A357.0 alloys[J]. Metall Mater Trans A, 1995, 26A(5): 1195-1205.
[10]Verdu C, Cercueil H, Communal S. Microstructural aspects of the damage mechanisms of cast Al-7Si-Mg alloys[J]. Mater Sci Forum, 1996, 217-222(3): 1449-1454.
[11]Bckerud L, Chai G, Tamminen J. Solidification characteristics of aluminium alloys[J]. AFS/SKAN Aluminium, 1990, 2: 128.
[12]Granger D A, Sawtell R R, Kersker M M. Effect of beryllium on the properties of A357.0 casting[J]. AFS Trans, 1984, 92: 579-586.
[13]Caceres C H, Davidson CJ, Griffiths J R, et al. The effect of Mg on the microstructure and mechanical behavior of Al-Si-Mg casting alloys[J]. Metallurgical and Materials Transactions A, 1999, 30A(12): 2611-2618.
[14]Wang Q G, Davidson C J. Solidification and precipitation behaviour of Al-Si-Mg casting alloys[J]. Journal of Materials Science, 2001, 36(3): 739-750.
[15]Phillips H W L. Annotated Equilibrium Diagrams of Some Aluminium Alloy Systems [R]. Institute of Metals. Monograph and Report Series No.25, 1959. 66.
(Edited by YANG Bing)
Foundation item: Project (G2000067202) supported by the National Major Basic Research Program of China
Received date: 2004-09-10; Accepted date: 2004-12-21
Correspondence: CHEN Zhong-wei, PhD; Tel: +86-29-88491764, E-mail: chzw@nwpu.edu.cn