
Characterization of self-assembled nano-phase silane-based particle coating
SHI Hong-wei(史洪微), LIU Fu-chun(刘福春), HAN En-hou(韩恩厚)
State Key Laboratory for Corrosion and Protection, Institute of Metal Research,
Chinese Academy of Sciences, Shenyang 110016, China
Received 22 October 2009; accepted 8 April 2010
Abstract: Self-assembled nano-phase silane-based particle coating was prepared through sol-gel technique. Tetramethoxysilane and 3-glycidoxypropyltrimethoxysilane were used as precursors for the self-assembled sol-gel coatings. The silane colloidal particle size was analyzed by laser particle size measurement. The results indicate that the particle size is in nano-scale and the diameter of particles deceases with increasing dilution times. Gel permeation chromatography proves that the relative molecular mass of macromolecule in a referenced sol solution is 1 220-1 240 amu. A simulation model was proposed to study the siloxane structure. Fourier transform infrared spectra of solution and film prove the disappearing of epoxy bond. The results of solid-state 13C and 29Si nuclear magnetic resonance experiments indicate the formation of Si—O—Si network. Potentiodynamic analysis shows that the self-assembled coating has excellent corrosion resistance. Salt fog tests prove that 2-methyl piperidine as inhibitor significantly improves the corrosion resistance of the self-assembled coating.
Key words: self-assembled coating; particle size; gel permeation chromatography; silane; corrosion resistance
1 Introduction
Due to the unique chemical and electrochemical properties of Cr(VI) compound, it has been widely used for ferrous and nonferrous materials to inhibit corrosion. However, the carcinogenic effect of chromate has generated serious hazardous safety problems. Considering the side-effect of utilization of Cr6+, there is a strong motivation to develop new substitutes for toxic Cr(VI) compound.
For many years, chromate conversion coatings have been used to protect aluminum alloy. With regard to reducing the use of chromate, the initial attempt of chromate replacement for aluminum alloy began in the late of 1970s[1]. Inorganic-organic hybrid coatings have attracted researchers’ interest in the recent years since the combined performance was derived from organic and inorganic groups. The advantage of hybrid sol-gel conversion coatings is attributed to tailorable chemical structure design, creating a way to develop long-live, environmentally compliant coating for protection of aluminum alloy. The self-assembled nano-phased particle coating on aluminum alloy used for aircraft was first proposed by BALBYSHEV et al[2]. They also proposed models of probable molecular structures formed by silane precursors under different hydrolysis conditions. The subsequent research work has been focused on the improvement of coating performance by using different precursors[3-5], doping corrosion inhibitors[6-9], or introducing different surface pretreatment methods before coating[10-11]. Although the above research works involved different aspects, the process from particle to coating film and corresponding alteration of structure was not clear so far.
In this work, the relative molecular mass and particle size of macromolecule in hydrolyzed silane solutions were determined by gel permeation chromatography (GPC) and laser particle size analysis, respectively. Furthermore, the effect of different mole ratios of 3-glycidoxypropyltrimethoxysilane (GPTMS) to tetramethoxysilane (TMOS) or water to silane on particle size were investigated. The objective is to find the model of molecule hydrolyzed from the two precursors. The change of chemical structure of silane solution and coating film (before and after amine curing) were studied using Fourier transform infrared (FT-IR) spectra and nuclear magnetic resonance (NMR), respectively.
Potentiodynamic measurements and salt fog tests were used to investigate the corrosion resistance of the self-assembled coating hydrolyzed on aluminum alloy 2024-T3 after different surface treatments.
2 Experimental
2.1 Preparation of self-assembled nano-phase silane- based particle coating
The standard mixed solution was prepared by hydrolysis of 8.9 mL of TMOS and 42.8 mL of GPTMS (1:3, mole ratio) in 64.8 mL of 0.05 mol/L glacial acetic acid solution. In order to compare particle size under different hydrolysis conditions, mixed solutions of 14.3 mL of GPTMS and 8.9 mL of TMOS (1:1, mole ratio), 42.8 mL of GPTMS and 3 mL of TMOS (9:1, mole ratio) in the same amount of acetic acid solution were also prepared. The solutions were placed in a sealed beaker and vigorously stirred to insure the complete hydrolysis and condensation of the two precursors.
The prepared sol was continuously stirred for 3 d before the addition of curing agent. Then, 1 mL of diethylentriamine (DETA) was added into the standard sol followed by stirring for 5 min. After immersion in the prepared solution for 3 min, de-oxidized substrates were withdrawn and dip-coated at a speed of 10 cm/min. Aluminum alloys 2024-T3 with sizes of 10 mm×10 mm×1.5 mm and 50 mm×10 mm×1.5 mm as substrates were polished with 800 emery paper, and then were de-oxidized by immersing in alkaline solution (NaOH, 12 g/L) for 10 min or a mixed solution (CrO3 0.36 kg, HNO3 0.773 kg, 40% HF 0.087 8 kg, water 8.78 kg) for 90 s. After the dip-coating application, the coupons were transferred to an oven and cured at 100 °C for 5 h. The ultimate film thickness obtained was approximately 4-7 mm. The gel was dried in beaker and then ground into powder for solid-state NMR and FTIR tests.
2.2 Characterization of coating
The sol after 3 d of hydrolysis was used to measure the relative molecular mass and particle size of molecule using methods of GPC and laser particle size analysis, respectively. Zetasizer 3000 (Malvern Instruments Ltd, UK) was employed to perform size distribution measurement.
The 13C and 29Si NMR spectra of the solid-state hybrid were performed using Bruker AV300 spectrometer with the cross-polarization/magic-angle spinning (CP/MAS) technique. FT-IR of the standard solution and film was performed to investigate the change of chemical bonds in the curing process. In addition, morphologies of the self-assembled coating were observed using XL30 type environmental scanning electronic microscope (ESEM) from Philips, Holland.
2.3 Polarization measurements and salt fog tests
Polarization measurements were carried out with Gamry PC4/750 potentiostat coupled to a Gamry corrosion measurement system. The three-electrode cell was equipped with a saturated calomel electrode (SCE), a platinum foil electrode and a dip-coated coupon, as reference, counter, and working electrodes, respectively. Coatings were immersed in 3.5% NaCl solution for 5 min before the test when performing the potentiodynamic scan (PDS). The working area of the coupons exposed to solution was 1 cm2. PDS results were acquired with a constant sweep speed of 1 mV/s.
The corrosion resistance of the coatings was evaluated by the exposure in salt fog atmosphere. The salt fog was generated by 5% NaCl solution at (35±2) °C. Every one day, the coupons with undoped coatings and coatings doped with MPD (NH(CH2)4CHCH3) as corrosion inhibitor were taken out for visual inspection.
3 Results and discussion
3.1 Characterization of structure of silane-based coating
The preparation of self-assembled nano-phase particle coating was composed of forming nano-sized siloxane particles through the hydrolysis by precursors, and forming film through amine cross-linking.
In order to determine the size distribution and the average relative molecular mass of the colloidal particles formed in the hydrolyzed solution, laser particle analysis and GPC were used respectively. After 3 d of hydrolysis, the average relative molecular mass measured by GPC was approximately 1 220-1 240 amu. According to the relative molecular mass of GPTMS (236.3) and TMOS (152.3), the mole ratio of GPTMS to TMOS was approximately 3:3, i.e., the macromolecule containing three TMOS molecules and three GPTMS molecules. The objective of the average relative molecular mass measurement is to simulate the structure of the siloxane macromolecule after the hydrolysis. From the measured relative molecular mass, it was assumed that the silane precursors first formed the small block structure through hydrolysis process. The modeling of chemical structure of hydrolyzed TMOS and GPTMS is presented in the “ball and stick” format, as shown in Fig.1. The hydrolyzed silanols subsequently reacted to generate siloxane bridges, resulting in the increased relative molecular mass. According to the theory of energy minimization proposed by BALBYSHEV et al[2], the primary reaction was the self-polymerization of TMOS. The TMOS molecules first formed 3-6 Si—O unit rings, and then the GPTMS molecules were annexed to the cyclic-TMOS-only structure[2]. Based on the GPC results, the three Si—O linkages were formed by the TMOS molecules and three glycidoxypropyl groups replaced the three hydroxyls in the TMOS rings. The modeling of chemical structure of the above molecule is shown in Fig.2. In this case, the diameter of the siloxane particles was 2.8 nm if the solution was diluted by 16 times of water according to the results of laser particle size measurement.
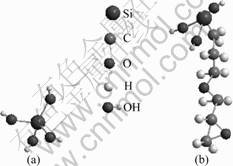
Fig.1 Chemical structures of hydrolyzed TMOS (a) and hydrolyzed GPTMS (b)
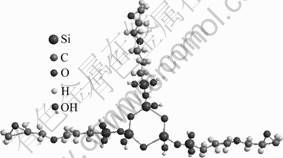
Fig.2 Modelling structure of siloxane molecule by three TMOS and three GPTMS
The diameter of self-assembled particles can be tailorable by alternating the mole ratio of water to silane and TMOS to GPTMS. This design is an alterable method to prepare the nano-structured coating. In our experiment, the alteration of diameter of self-assembled nanoparticles in stepwise-diluted sol solutions with varied ratios of GPTMS to TMOS was investigated. The self-assembled coating is obtained by crosslinking the molecules formed by TMOS and GPTMS with diethylentriamine, as shown in Fig.3. If the amounts-of- substance of TMOS and GPTMS were fixed, the lower the mole ratio of water to silane is, the larger the diameter of siloxane particle is. It was proved through diluting the sol solution by distilled water. Fig.4 presents the apparent particle size of siloxane in sol solution with increasing times of dilution by distilled water. As shown in Fig.4, the original siloxane particle sizes in the standard sol solution, solution with n(GPTMS)/n(TMOS) of 1:1 and solution with n(GPTMS)/n(TMOS) of 9:1 were all lower than 100 nm, indicating that the three sol solutions can form a nano-scale structure before amine was crosslinked. If the original sol solutions were diluted by more than 10 times of distilled water, the diameter of all three the kinds of siloxane particles was less than 3 nm. For the silane solution with n(GPTMS)/ n(TMOS) of 1:1, when diluted with 12 times of water, the average diameter of particles is 1.1 nm, which is the smallest among the three diluted solutions. This result further confirmed that the optimal mole ratio of GPTMS to TMOS was 1:1, as shown in Fig.2. When the mole ratio of GPTMS to TMOS was increased to 9:1, the particle size decreased faster than those with the other two ratios. The possible reason is that high GPTMS content could hinder the self-polymerization of TMOS. Obviously, the particle size of siloxane in all the three sols became smaller and evenly distributed with more addition of distilled water. However, it is unpractical for utilization of self-assembled coating, since too thin film caused by low viscosity of silane solution with much water will not provide adequate corrosion protection and mechanical strength. Therefore, a reasonable water amount is needed to obtain the proper coating thickness.
In many cases, with the addition of corrosion inhibitors with larger molecular structure or less water content, the separated silane particles from coating film can be observed, as shown in Fig.5. A few of the particles had the diameter from 500 to 1 000 nm, whilst for most of the particles around edge of “cave”, the diameter was less than 100 nm. Energy dispersive X-ray spectroscopy (EDX) analysis showed that the spherical part was higher in x(O):x(Si) (1.95) than that (1.74) in the plane film part. Comparatively, the x(C):x(Si) (5.43) is lower than that (6.15) in the plane part. This result indicates that nanometer silane particles are prone to be self-aggregated and form a structure with more rings of Si—O—Si, due to the minimization of energy or existence of a nucleation point. This occurs when water content is less than a certain ratio or stable state of sol system is destroyed. From the above results, it can be seen that when the self-assembled coating is applied, it is important to use compatible corrosion inhibitors and appropriate amount of diluted water in order to avoid cracks or caves and obtain the proper thickness of film.
FT-IR provided the information of SNAP structure before and after diethylentriamine curing. Fig.6 shows the FT-IR spectra of the standard sol solution and the diethylentriamine cured film. As shown in Fig.6(a), there was a peak at 3 406 cm-1 related to hydroxyl group. The band near 2 938 cm-1 was related to the epoxide groups and was due to the stretching CH2. The distinct band at

Fig.3 Self-assembled and amine crosslinking process of silane sol-gel coating

Fig.4 Siloxane particle size in sol solution changed with dilution times by distilled water
1 096 cm-1 was the characteristic of stretching of Si— O—Si. The band at 908 cm-1 was due to the stretching of Si—OH. After amine was cured, clear change appeared in the spectra. The band at 908 cm-1 disappeared, indicating that Si—OH was transformed into Si—O—Si. The peaks at 1 730, 1 633 and 1 384 cm-1 were associated with the C—O, C—C and C—H stretching in epoxide ring[12]. In addition, new bands at 1 112, 1 050 and 449 cm-1 appeared for the cured film, which indicated the increase of ring structure of Si—O—Si. There was a decrease of intensity at band of 1 256 cm-1, which was probably due to the oxidation of epoxy ring[13].
Solid-state 13C and 29Si NMR for diethylentriamine cured film are shown in Figs.7(a) and (b), respectively. In the spectra, distinct peaks for glycidoxypropyl unit in
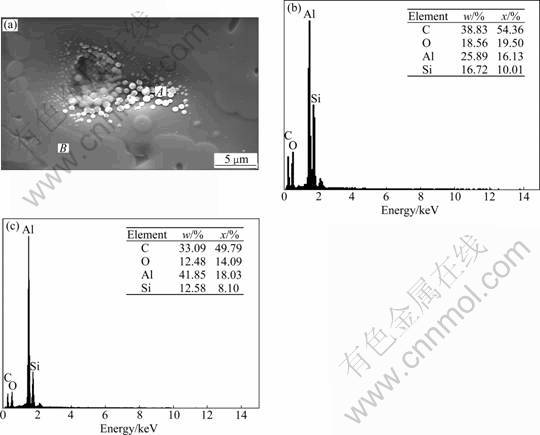
Fig.5 SEM image and EDX analysis of separated silane particle: (a) SEM image; (b) EDS of region A (spherical part) in (a); (c) EDS of region B (plane part) in (a)
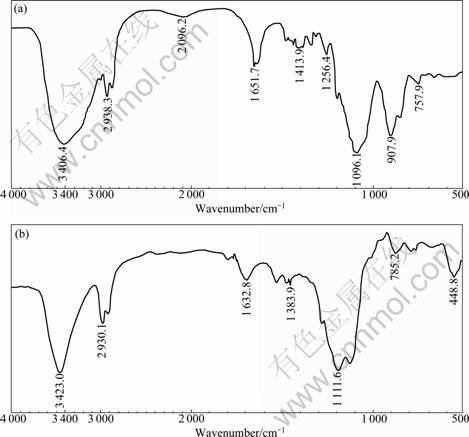
Fig.6 FT-IR spectra of SNAP solution after 3 d of hydrolysis (a) and film (b) after diethylentriamine curing

Fig.7 Solid-state 13C (a) and 29Si CP/MAS NMR (b) spectra of amine cured sol-gel film
GPTMS-TMOS-DETA sol-gel film were observed at 8.63 [Si—CH2], 23.54 [Si—CH2CH2], 73.60 [Si—CH2CH2CH2—O—CH2], and 59.25 [C—OR][14]. 29Si CP/MAS NMR peak distribution is similar with that previously described by GLASER et al[15] for Ormosils. T3, Q3 and Q4 represent R-Si(OSi)3, Si(OSi)3(OH) and Si(OSi)4, respectively. Distinct peaks of silica network units were observed at -65×10-6 (T3), -95×10-6 (Q3) and -109×10-6 (Q4).
3.2 Polarization measurement and salt-spray test
Fig.8 presents the potentiodynamic polarization curves for standard sol-gel film, sol-gel film with n(GPTMS):n(TMOS)=9:1 and bare AA2024-T3. Chemical solution (CrO3 0.36 kg, HNO3 0.773 kg, 40% HF 0.087 8 kg, water 8.78 kg) was used for deoxidizing AA2024-T3 substrate before dip-coating in the sol solution. The electrochemical characteristics obtained from the polarization curves are listed in Table 1. The standard sol-gel film was found to play a barrier for protection of AA2024-T3. The measured corrosion current density decreased significantly from 6.7×10-7 A/cm2 (for AA 2024-T3) to 9.36×10-12 A/cm2. Corrosion potential, φcorr, was found to increase from -588 mV to 439 mV. Alteration of n(GPTMS):n(TMOS)=9:1 slightly

Fig.8 Potentiodynamic polarization curves of referenced sol-gel film (a), sol-gel film with n(GPTMS):n(TMOS)=9:1 (b) and AA2024-T3 (c) in Harrison solution (0.05% NaCl+0.35% (NH4)2SO4)
Table 1 Electrochemical characteristics for self-assembled sol- gel films with n(GPTMS):n(TMOS)=3:1 and n(GPTMS): n(TMOS)=9:1, and AA2024-T3 in Harrison solution (0.05% NaCl+0.35% (NH4)2SO4)

improved the corrosion potential, but did not improve the corrosion current density. In particular, the two sol-gel films both exhibited excellent corrosion resistance.
Figs.9(a) and (b) show morphologies of the standard sol-gel film and sol-gel film with inhibitor of 2-Methyl piperidine (MPD) (1×10-3 mol/L in sol solution) on AA2024-T3 with alkaline washing surface treatment after 12 d of salt-spray test. Figs.9(c) and (d) show the morphologies of the standard sol-gel film and sol-gel film with n(GPTMS):n(TMOS)=9:1 on AA2024-T3 with three-acid surface treatment as did in polarization measurement. For substrate coated with the standard film, severe pitting corrosion can be observed throughout the coating surface, as shown in Fig.9(a). Delamination of the film was also found. As compared with the standard film, the coupons coated with MPD doped film had much higher corrosion resistance, as shown in Fig.9(b). Neither pitting corrosion nor delamination was found. Addition of MPD as an organic inhibitor obviously improved the corrosion resistance of the sol-gel film. The inhibiting effect is probably attributed to the adsorption of the molecule on the metal surface through the NH group, as shown in Fig.10[16]. Therefore, MPD impedes corrosion by blocking the anodic and cathodic reaction [17]. Furthermore, the presence of electropositive CH3

Fig.9 Morphologies of coupons of standard sol-gel film (a), sol-gel film doped with MPD, with alkaline washing surface treatment (b), standard sol-gel film (c) and film with n(GPTMS):n(/TMOS)=9:1 with three-acid surface treatment (d) after exposure for 12 d in salt fog

Fig.10 Model of adsorption of piperidine on aluminum alloy surface
group can intensify the electron density at N atom, and thus reinforce the binding energy with the aluminum alloy surface. In the present work, the inhibition efficiency of MPD in the sol-gel film is high because its low concentration (1×10-3 mol/L) is very low. Some small white corrosion products can be observed on the edge of substrate in Fig.9(c). A continuous white belt of corrosion products can be obviously seen in Fig.9(d), indicating that the sol-gel film was flaked off from the substrate and the corrosion resistance was inferior to that of the standard sol-gel film. From the above results, it can be seen that the corrosion resistance of the sol-gel film doped with MPD was comparable with the standard sol-gel film with three-acid surface treatment. But the former was environmentally compliant since the substrate surface treatment was alkaline washing.
4 Conclusions
1) Molecular simulation was used to study the nanosized macromolecule in silane sol solution for preparing of self-assembled coating. A structure with three GPTMS and three TMOS molecules was derived. It was found that the particle diameter in the three original sol solutions was less than 100 nm and gradually decreased with much diluted water. Moreover, the ultimate diameter of silane particles in the three diluted sol solutions was below 3 nm.
2) FT-IR and NMR results proved the changes of chemical groups in silane structure in solution and after amine curing. These changes were reflected by the formation of ring Si—O—Si network and disappearing of epoxy ring.
3) The polarization measurement demonstrated the excellent corrosion resistance of the self-assembled coating. Salt-spray test indicated that 2-methyl piperidine improved the corrosion resistance of the self-assembled coating.
References
[1] HAMDY A S, BUTT D P, ISMAIL A A. Electrochemical impedance studies of sol-gel based ceramic coatings systems in 3.5% NaCl solution [J]. Electrochimica Acta, 2007, 52: 3310-3316.
[2] BALBYSHEV V N, ANDERSON K L, SINSAWAT A, FARMER B L, DONLEY M S. Modeling of nano-sized macromolecules in silane-based self-assembled nano-phase particle coatings [J]. Prog Org Coat, 2003, 47: 337-341
[3] METROKE T L, APBLETT A. Effect of solvent dilution on corrosion protective properties of Ormosil coatings on 2024-T3 aluminum alloy [J]. Prog Org Coat, 2004, 51: 36-46.
[4] LIU Yan, SUN De-zhi, YOU Hong, JONG Shik-Chung. Corrosion resistance properties of organic-inorganic hybrid coatings on 2024 aluminum alloy [J]. Applied Surface Science, 2005, 246: 82-89.
[5] GUO Xing-hua, AN Mao-zhong, YANG Pei-xia, LI Hai-xian. Effect of sol compositions on corrosion protection of SNAP film coated on magnesium alloy [J]. Chinese Journal of Inorganic Chemistry, 2009, 25(7): 1254-1261.
[6] VOEVODIN N N, BALBYSHEV V N, KHOBAIB M, DONLEY M S. Nanostructured coatings approach for corrosion protection [J]. Prog Org Coat, 2003, 47: 416-423.
[7] KHRAMOV A N, VOEVODIN N N, BALBYSHEV V N, DONLEY M S. Hybrid organo-ceramic corrosion protection coatings with encapsulated organic corrosion inhibitors [J]. Thin Solid Films, 2004, 447/448: 549-557.
[8] LAMAKA S V, ZHELUDKEVICH M L, YASAKAU K A, MONTEMOR M F. TiOx self-assembled networks prepared by templating approach as nanostructured reservoirs for self-healing anticorrosion pre-treatments [J]. Electrochemistry Communications, 2006, 8: 421- 428.
[9] QUINET M, NEVEU B, MOUTARLIER V, AUDEBERT P. Corrosion protection of sol-gel coatings doped with an organic corrosion inhibitor: Chloranil, L. Ricq [J]. Prog Org Coat, 2007, 58: 46-53.
[10] KHRAMOV A N, BALBYSHEV V N, KASTEN L S, MANTZ R A. Sol-gel coatings with phosphonate functionalities for surface modification of magnesium alloys [J]. Thin Solid Films, 2006, 514: 174-181.
[11] LAMAKA S V, ZHELUDKEVICH M L, YASAKAU K A, SERRA R, POZNYAK S K, FERREIRA M G S. Nanoporous titania interlayer as reservoir of corrosion inhibitors for coatings with self-healing ability [J]. Prog Org Coat, 2007, 58: 127-135.
[12] WU K H, CHAO C M, YEH T F, CHANG T C. Thermal stability and corrosion resistance of polysiloxane coatings on 2024-T3 and 6061-T6 aluminum alloy [J]. Surface and Coatings Technology, 2007, 201: 5782-5788.
[13] BERTELSEN C M, BOERIO F J. Linking mechanical properties of silanes to their chemical structure: An analytical study of γ-GPS solutions and films [J]. Prog Org Coat, 2001, 41: 239-246.
[14] METROKE T L, KACHURINA O, KNOBBE E T. Spectroscopic and corrosion resistance characterization of amine and super acid-cured hybrid organic–inorganic thin films on 2024-T3 aluminum alloy [J]. Prog Org Coat, 2002, 44: 185-199.
[15] GLASER R H, WILKES G L. Structure property behavior of polydimethylsiloxane and poly(tetramethylene oxide) modified TEOS based sol-gel materials. 5. Effect of titaniumisorporoxide incorporation [J]. Polym Bull, 1988, 19: 51-57.
[16] SHI Hong-wei, LIU Fu-chun, HAN En-hou. Corrosion protection of AZ91D magnesium alloy with sol-gel coating containing 2-methyl piperidine [J]. Prog Org Coat, 2009, 66: 183-191.
[17] SANKARAPAPAVINASM S, PUSHPANADEN F, AHMED M F. Piperidine, piperidones and tetrahydrothiopyrones as inhibitors for the corrosion of copper in H2SO4 [J]. Corrosion Science, 1991, 32: 193-203.
(Edited by LI Xiang-qun)
Foundation item: Project(51001109) supported by the National Natural Science Foundation of China; Projects(2009BAE70B01, 2009BAE70B02) supported by the National Key Technology R&D Program of China
Corresponding author: LIU Fu-chun; Tel: +86-24-23915895; Fax: +86-24-23894149; E-mail: fcliu@imr.ac.cn
DOI: 10.1016/S1003-6326(09)60397-6