新型钴铝层状双金属氢氧化物/碳球复合物的构建及其超级电容器性能
来源期刊:中国有色金属学报(英文版)2017年第8期
论文作者:黄琪 刘开宇 何方 张水蓉 谢清亮 陈诚
文章页码:1804 - 1814
关键词:钴铝层状双金属氢氧化物;碳球;超级电容器;生长法
Key words:cobalt aluminum-layered double hydroxide; carbon sphere; supercapacitor; growth method
摘 要:介绍了一种构建钴铝层状双金属氢氧化物/碳球(CoAl-LDH/CSs) 复合物的新设计路线。通过生长法使CoAl-LDH生长在CSs上,生长在CSs上的CoAl-LDH薄层由厚度为20 nm的纳米片组成。恒流充放电测试表明,CoAl-LDH/CSs复合物在6 mol/L KOH溶液中以1 A/g的电流密度充放电的比容量为1198 F/g(基于CoAl-LDH/CSs 复合物的质量),甚至在高达10 A/g的大电流密度下仍然呈现出920 F/g的较高比容量。而且该复合物以2 A/g的电流密度充放电循环1000次后仍保留928 F/g的比容量,比容量的保留率为84%,这表明与纯的CoAl-LDH相比,CoAl-LDH/CSs复合物具有较高的比容量、优良的倍率性能和良好的循环稳定性。
Abstract: A new design route was presented to fabricate cobalt aluminum-layered double hydroxide(CoAl-LDH) thin layers which grow on carbon spheres(CSs) through a growth method. The CoAl-LDH thin layers consist of nanoflakes with a thickness of 20 nm. The galvanostatic charge-discharge test of the CoAl-LDH/CSs composite shows a great specific capacitance of 1198 F/g at 1 A/g (based on the mass of the CoAl-LDH/CSs composite) in 6 mol/L KOH solution, and the composite displays an impressive specific capacitance of 920 F/g even at a high current density of 10 A/g. Moreover, the composite remains a specific capacitance of 928 F/g after 1000 cycles at 2 A/g, and the specific capacitance retention is 84%, indicating that the composite has high specific capacitance, excellent rate capability and good cycling stability in comparison to pristine CoAl-LDH.
Trans. Nonferrous Met. Soc. China 27(2017) 1804-1814
Qi HUANG1, Kai-yu LIU1,2, Fang HE1, Shui-rong ZHANG1, Qing-liang XIE1, Cheng CHEN1
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. College of Chemistry and Materials Science, Longyan University, Longyan 364012, China
Received 10 May 2016; accepted 22 February 2017
Abstract: A new design route was presented to fabricate cobalt aluminum-layered double hydroxide(CoAl-LDH) thin layers which grow on carbon spheres(CSs) through a growth method. The CoAl-LDH thin layers consist of nanoflakes with a thickness of 20 nm. The galvanostatic charge-discharge test of the CoAl-LDH/CSs composite shows a great specific capacitance of 1198 F/g at 1 A/g (based on the mass of the CoAl-LDH/CSs composite) in 6 mol/L KOH solution, and the composite displays an impressive specific capacitance of 920 F/g even at a high current density of 10 A/g. Moreover, the composite remains a specific capacitance of 928 F/g after 1000 cycles at 2 A/g, and the specific capacitance retention is 84%, indicating that the composite has high specific capacitance, excellent rate capability and good cycling stability in comparison to pristine CoAl-LDH.
Key words: cobalt aluminum-layered double hydroxide; carbon sphere; supercapacitor; growth method
1 Introduction
It is always desired to develope supercapacitor electrode materials with high specific capacitance, excellent rate capability and long cycle life [1]. There are two types of supercapacitors based on the capacitive behavior: electric double layer capacitors (EDLCs) and pseu-docapacitors (PCs), which mainly result from ions stored at the electrode/electrolyte interface and fast surface redox reactions, respectively. EDLCs normally use carbon-based active electrode materials [2,3]. Carbon materials present abundant resources, low cost, high stability, good conductivity and lightweight design. However, they have a small specific capacitance (theoretical capacitance is 280 F/g) in spite of excellent rate capability and long cycle life [4]. Therefore, the resulted low energy density cannot meet the needs of practical application. As in the case of PCs, the most extensively investigated active pseudocapacitive materials include various transition metal oxides (RuO2 [5], Co3O4 [6], and NiCo2O4[7]) and hydroxides (Ni(OH)2 [8], Co(OH)2 [9]), and conducting redox polymers [10,11]. Transition metal oxides and hydroxides have high electrochemical activity, and exhibit good pseudocapacitive performance as well. Their theoretical capacitance is several times higher than that of carbon materials [12,13]. However, transition metal oxides and hydroxides have a low conductivity and poor cycle life due to their limited velocity of ion diffusion and electron transfer compared with carbon materials [14,15]. Low conductivity and poor cycle life would extremely affect the practical application of supercapacitors. To deal with the aforementioned problems, it may be a good way for combining transition metal oxides/hydroxides active materials with carbon materials to take the advantage of pseudo-capacitance and double layer capacitance [16,17].
The carbon spheres (CSs), which belong to EDLCs have great potentialities to be applied in the field of supercapacitors and are being researched heavily in the 21st century, because of their morphology and structure, chemical thermodynamics stability, high conductivity and other unique advantages [18-20]. Layered double hydroxides (LDHs) (belonging to PCs) are classical inorganic layered materials which can be described by the general formula  (Among them, M2+, e.g., Mg2+, Fe2+, Co2+, Ni2+ or Zn2+, is divalent cation; M3+, e.g., Al3+, Cr3+, Ga3+or Fe3+, is trivalent cation; and An- is the interlayer anion which compensates for the positive charge of the brucite-like layers). The easy tunability of metal ions without changing the structure and anion exchange properties of LDHs makes them attractive alternatives for applications in catalysis [21], biology [22], adsorption [23], energy storage and conversion [24].
(Among them, M2+, e.g., Mg2+, Fe2+, Co2+, Ni2+ or Zn2+, is divalent cation; M3+, e.g., Al3+, Cr3+, Ga3+or Fe3+, is trivalent cation; and An- is the interlayer anion which compensates for the positive charge of the brucite-like layers). The easy tunability of metal ions without changing the structure and anion exchange properties of LDHs makes them attractive alternatives for applications in catalysis [21], biology [22], adsorption [23], energy storage and conversion [24].
Recently, LDHs and carbon materials composites have been investigated widely as promising capacitive materials because of high redox activity, enhanced electrochemical performance, low cost, and environmental friendliness [4,25,26]. SU et al [27] prepared CoAl-LDH/MWCNT by urea-induced homogeneous precipitation. When the added MWNTs content is 10%, the specific capacitance increases to 342.4 F/g and remains at a value of 304 F/g until the 400th cycle at 2 A/g. FANG et al [28] prepared CoAl-LDH/GO by a microwave-assisted reflux method, and a maximum specific capacitance of 772 F/g was obtained at 1 A/g in 6 mol/L KOH solution for the composite containing 12.9% GO. Up to now, CoAl- LDH and carbon materials composites still face the defects of low conductivity, small specific capacitance, inferior rate capability, and inferior cycling stability, which are restricting the wider applications of supercapacitors.
In this work, the carbon spheres were prepared by a simple hydrothermal reaction with glucose solution, and then the surface of carbon spheres was coated by CoAl-LDH nanosheets through a growth method for the first time. Finally, CoAl-LDH/CSs composite material was prepared. The three-dimensional carbon ball structure formed by the growth of CoAl-LDH nanosheets on CSs is a key factor to make the full use of pseudo-capacitance and double layer capacitance. Due to the addition of carbon spheres, the electronic conductivity and the transmission rate of ions on the electrode surface can be improved; the CoAl-LDH agglomeration can be effectively prevented; and the specific surface area of the composite can be improved. These can in turn improve the electrochemical performance of electrode material.
2 Experimental
2.1 Material preparation
Preparation of carbon spheres (CSs): 1 mol/L glucose solution reacted at 180 °C for 24 h in the autoclave of 100 mL. Then, the product was prepared by centrifugation, washed three times with H2O and ethanol and dried at 80 °C for 12 h under vacuum.
Preparation of AlOOH sol: 11.3 g aluminum isopropoxide was dissolved in 100 mL H2O, stirred for 20 min at 85 °C water bath, then 1 mol/L HNO3 solution was added dropwise to adjust the pH value to be 3-4. The solution was stirred in 85 °C water bath for 2 h, cooled to room temperature naturally, dried under vacuum and ground into AlOOH powder. 5.8 g AlOOH powder was dissolved in 107 mL H2O, stirred in 84 °C water bath for 1 h. Then, 9.5 mL HNO3 (1 mol/L) solution was added dropwise, refluxed for 6 h and cooled to room temperature. AlOOH sol was prepared.
Synthesis of CoAl-LDH/CSs composite: 0.3 g CSs were dissolved in 10 mL AlOOH sol, stirred for 2 h, got by centrifugal precipitation, washed once with ethanol and dried at room temperature. This step was repeated 10 times to get the AlOOH/CSs nanocomposite. Then, 0.1 g AlOOH/CSs nanocomposite was dispersed in 70 mL H2O and stirred for 10 min. 0.01 mol Co(NO3)2·6H2O and 0.3 g urea were added and stirred for 20 min. Then, solution was transferred into 100 mL autoclave at 100 °C for 24 h, cooled naturally to room temperature, collected by centrifugal, washed 3 times with H2O and ethanol, respectively, and dried at 60 °C under vacuum overnight to get CoAl-LDH/CSs composite. The process is shown in Scheme 1. CoAl-LDH was synthesized by the same method using different raw materials.
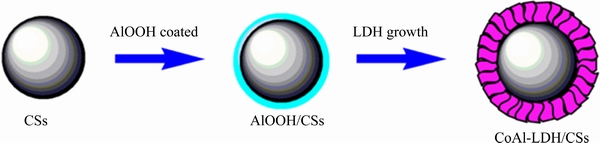
Scheme 1 Schematic illustration of formation of CoAl-LDH/CSs composite
2.2 Material characterization
The structures of the obtained samples were characterized by X-ray diffraction (XRD, Rigaku) using Cu Kα radiation at 40 kV and 250 mA, λ=1.5406  . Fourier transform-infrared measurements (FT-IR) (Thermo Electron Scientific Instruments, Nicolet 6700, America) were recorded on KBr pellets with a PE Paragon 1000 spectrophotometer. SEM investigations were performed using a Quanta FEG 250 field emission scanning electron microscope (FEI, Electron Optics, B.V). TEM and HRTEM (TECNAI G2 F20 S-TWIN) were used to study the themorphologies and microstructure properties. N2 adsorption/desorption isotherms were measured at a liquid nitrogen temperature (-196 °C) using a physisorption/chemisorptions analyzer (Quantachrome Nova Surface Area Analyzer). Prior to adsorption, the samples were dried and degassed by heating in a vacuum to 200 °C for 5 h. The pore volumes were estimated from the desorption branch of the isotherms, applying the BJH method in the software package Quantachrome NovaWin.
. Fourier transform-infrared measurements (FT-IR) (Thermo Electron Scientific Instruments, Nicolet 6700, America) were recorded on KBr pellets with a PE Paragon 1000 spectrophotometer. SEM investigations were performed using a Quanta FEG 250 field emission scanning electron microscope (FEI, Electron Optics, B.V). TEM and HRTEM (TECNAI G2 F20 S-TWIN) were used to study the themorphologies and microstructure properties. N2 adsorption/desorption isotherms were measured at a liquid nitrogen temperature (-196 °C) using a physisorption/chemisorptions analyzer (Quantachrome Nova Surface Area Analyzer). Prior to adsorption, the samples were dried and degassed by heating in a vacuum to 200 °C for 5 h. The pore volumes were estimated from the desorption branch of the isotherms, applying the BJH method in the software package Quantachrome NovaWin.
2.3 Electrochemical measurement
The electrochemical properties of the samples were investigated on a three-electrode system, in which the samples were used for the fabrication of working electrode, a Hg/HgO electrode was used as the reference electrode, a platinum foil was used as the counter electrode, and 6 mol/L KOH solution was used as the electrolyte. The working electrodes were prepared by mixing active material (2 mg), acetylene black and polytetra fluoroethylene (PTFE) at a mass ratio of 80:15:5 with addition of a small amount of ethanol. After coating the above slurry on Ni foam (8 mm × 8 mm), the mixture was dried at 60 °C overnight under vacuum. Before the test, the working electrodes were soaked in 6 mol/L KOH for 12 h. The cyclic voltammograms (CV) were recorded on a CHI 660D electrochemistry workstation at room temperature (25±2) °C in 6 mol/L KOH solution. The galvanostatic charge and discharge tests were recorded on Land battery test system (Wuhan, China). The electrochemical impedance spectroscopy (EIS) tests of all samples were examined on PARSTAT 2273-type electrochemical system (Princeton Applied Research) in 6 mol/L KOH solution and performed with an AC amplitude of 5 mV in the frequency range of 10 mHz-100 kHz.
3 Results and discussion
3.1 Microstructure characterization
The XRD patterns of CSs, CoAl-LDH and CoAl-LDH/CSs composite are shown in Fig. 1. The XRD pattern of CSs has an obvious absorption peak at 24.8 °(002) featuring a basal spacing of 0.359 nm, which indicates that a carbon material was synthesized. The diffraction peaks of CoAl-LDH and CoAl-LDH/CSs composite are fully consistent with the (003), (006), (002), (012), (015), (018), (110), (202) crystal surfaces of the compound Co6Al2CO3(OH)16·H2O (JCPDS: 51-0045 ) structure in R3m space group [29], which demonstrates that the sample phase prepared is of high purity. As shown in the figure, the two diffraction peaks (003) and (006) are specially strong, while the other peaks present relative weak intensities, which indicates that the crystal growth is in a certain direction. The d-spacing of (003) reflection is 0.749 nm according to the Bragg’s law, which is consistent with the reported literatures [25,30,31], showing 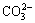 ions and water molecules are inserted in the interlayer space. The intensity of diffraction peaks of CoAl-LDH/CSs composite is weaker than those of CoAl-LDH, which shows that the crystallinity of the composite is low. The diffraction peak (002) of CSs was also observed in the CoAl-LDH/CSs composite, indicating that the composite materials have been synthesized successfully [32-35]. It is confirmed by SEM and TEM, as discussed in details below.
ions and water molecules are inserted in the interlayer space. The intensity of diffraction peaks of CoAl-LDH/CSs composite is weaker than those of CoAl-LDH, which shows that the crystallinity of the composite is low. The diffraction peak (002) of CSs was also observed in the CoAl-LDH/CSs composite, indicating that the composite materials have been synthesized successfully [32-35]. It is confirmed by SEM and TEM, as discussed in details below.

Fig. 1 XRD patterns of CSs, CoAl-LDH and CoAl-LDH/CSs composite

Fig. 2 FT-IR spectra of CSs, CoAl-LDH and CoAl-LDH/CSs composite
The FT-IR spectra of CSs, CoAl-LDH and CoAl-LDH/CSs composite are shown in Fig. 2, and the main spectral absorption range is between 500 and 4000 cm-1. In the IR spectra of CSs, CoAl-LDH and CoAl-LDH/CSs composite, the peaks at 3456 cm-1 correspond to the O—H stretching vibrations of water molecules and the hydrogen bonding of OH groups. The weak band at 1635 cm-1 is due to the bending mode of water molecules [28]. The peaks at 1360 and 770 cm-1 are attributed to the ν3 vibration and bending modes of the interlayer anion (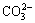 ), respectively, which was released from urea hydrolysis during the hydrothermal procedure [36,37]. These analyses indicate that water molecules and
), respectively, which was released from urea hydrolysis during the hydrothermal procedure [36,37]. These analyses indicate that water molecules and 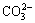 ions have been successfully inserted in inter-layer space of CoAl-LDH during the assembly process. Other absorption bands below 800 cm-1 belong to stretching and bending modes of the metal oxygen bond (M—O) in the hydrotalcite-like lattice [28].
ions have been successfully inserted in inter-layer space of CoAl-LDH during the assembly process. Other absorption bands below 800 cm-1 belong to stretching and bending modes of the metal oxygen bond (M—O) in the hydrotalcite-like lattice [28].
In order to check the pore structure and specific structural characteristics of CSs, CoAl-LDH and CoAl-LDH/CSs composite, the N2 adsorption/desorption isotherms and the pore size distribution are shown in Fig. 3. All of the curves show typical type IV isotherms with an H3-type hysteresis loop (p/p0>0.4) [29,38], identifying that all samples have mesopores. The specific surface area of the CoAl-LDH/CSs composite (994.876 m2/g) is higher than that of the pristine CoAl-LDH (23.840 m2/g) and CSs (5.930 m2/g), which indicates that the specific surface area of the composite is increased with the addition of CSs. LI et al [39] fabricated the Fe3O4@C@Ni-Al LDH composite which also has ultra-high surface area (792 m2/g). The total pore volume of the CoAl-LDH/CSs composite is increased to 1.305 cm3/g, but the total pore volumes of CoAl-LDH and CSs are 0.066 cm3/g and 0.02 cm3/g, respectively. The larger pore volume of the composite material is produced in the presence of CSs, which reduces the aggregation of CoAl-LDH nanosheets. It can be seen from the pore size distribution curves of all samples that CoAL-LDH shows pore size distribution of 7.736 nm, and CSs show pore size distribution of 7.383 nm, while the introduction of CSs gives rise to narrow particle size distribution (4.375 nm) of the CoAl-LDH/CSs composite. According to the paper reported, the 2-5 nm pore size distribution which is larger than the size of two solvated ions is beneficial to the optimization of the capacitor performance [40]. A more efficient transport route to the internal voids is provided by unique mesoporous structure, and this is very important for the electrochemistry performance of supercapacitors.

Fig. 3 Gas (N2) adsorption-desorption isotherm loop and pore size distribution for synthesized CoAl-LDH (a), pristine CSs (b) and CoAl-LDH/CSs composite (c)
The SEM image of CSs prepared are shown in Fig. 4(a). It can be seen from the figure that the diameter of carbon spheres ranges from 200 to 300 nm. The SEM image of pristine CoAl-LDH is shown in Fig. 4(b). It can be seen from the chart that the CoAl-LDH nanosheets exhibit irregular hexagonal flakes; CoAl-LDH nanosheets stack; and the average transverse size of CoAl-LDH nanosheets is 1-2 μm. From Fig. 4(c), in AlOOH/CSs nanocomposite, CSs are covered by AlOOH sol uniformly. Compared with the original carbon spheres, the surface of AlOOH/CSs nanocomposite is more rough. This is mainly due to the coverage of AlOOH on CSs surface. The SEM images of CoAl-LDH/CSs composite are shown in Fig. 4(d). It can be seen from the chart that CSs and CoAl-LDH are composed, and CoAl-LDH nanosheets grow on the surface of carbon spheres to form nanostructure. This can in turn effectively avoid the agglomeration of hydrotalcite, and make full use of both the good conductivity of carbon material and the good pseudo- capacitive performance of CoAl-LDH.

Fig. 4 SEM images of CSs (a), pristine CoAl-LDH (b), AlOOH/CSs (c) and CoAl-LDH/CSs composite (d)
In order to observe the microstructure of the synthesized CoAl-LDH/CSs nanocomposite, TEM images are shown in Fig. 5. The combination of CoAl-LDH and carbon ball shows a loose layer morphology in Fig. 5(a), due to the addition of CSs which can prevent the aggregation of CoAl-LDH. The images show that the CoAl-LDH have grown by a growth method. TEM images show that those hexagonal platelet-like CoAl-LDH flakes in the composite are about 20 nm in thickness, which are indicated in Fig. 5(b). From Fig. 5(c), the dashed frame shows the location of Fig. 5(d). The clear lattice between adjacent fringes is observed though the high-resolution TEM (HRTEM) visualization of CoAl-LDH/CSs composite from Fig. 5(d). The inter-planar distance of CoAl-LDH corresponding to the d003 value of rhombohedral CoAl-LDH was measured to be 0.75 nm, which is consistent with the measured values of XRD.
3.2 Electrochemical properties
The electrochemical behaviors of the as-prepared electrode materials were investigated by CV, galvanostatic charge-discharge measurements and EIS.
The specific capacitances can be calculated according to Eq. (1) [25,29]:
 (1)
(1)
where C (F/g), m (g), v (V/s), Vc and Va (V) and I (A) are the specific capacitance, the mass of the active materials in the electrode, the potential scan rate, the high and low potential limits of the CV tests, and the instant current on CV curves, respectively.
Figure 6 shows the CV curves of the CoAl-LDH and CoAl-LDH/CSs composite at a scanning rate of 20 mV/s. It clearly presents that the CV area of CoAl-LDH/CSs composite is significantly larger than that of CoAl-LDH, indicating that the specific capacitance of CoAl-LDH/CSs composite is larger, and that carbon spheres can increase the conductivity and the specific surface area of CoAl-LDH/CSs composite. The increase of conductivity can enhance the electron transmission of oxidation-reduction reaction, while the increase of surface area is beneficial to Faraday reaction between electrolyte and electrode materials; therefore, the improvement of conductivity and surface area can improve the utilization of active material of electrode surface.

Fig. 5 TEM images (a, b, c) and high-resolution TEM image (d) of CoAl-LDH/CSs composite

Fig. 6 CV curves of CoAl-LDH and CoAl-LDH/CSs composite at scanning rate of 20 mV/s
CV curves of CoAl-LDH/CSs composite with different scanning rates from 10 to 50 mV/s are illustrated in Fig. 7. A pair of obvious peaks at 0.325 V and 0.476 V are clearly presented, which are attributed to the oxidation-reduction reaction of the pseudocapacitive active substance Co(OH)2 in alkaline electrolyte. With increasing scan rate, there is only one oxidation peak in the electron transfer process. This can be explained by fact that the intermediate product CoOOH in the redox process is not stable; it only exists for a very short period of time and then converts to CoO2 easily. However, when the scanning rate decreases, two pairs of redox peaks can be observed, indicating that the electrode materials are CoOOH and CoO2. The electrochemical reactions in different oxidation states of Co are summarized as [41]
Co(OH)2+OH-=CoOOH+H2O+e (2)
CoOOH+OH-=CoO2+H2O+e (3)
From Fig. 7, it is very obvious that the oxidation- reduction reaction system of CoAl-LDH/CSs composite is quasi reversible (Ea,c>59 mV) and all of CV curves are similar. With the increase of scanning rate, the peak current density becomes larger and the potential difference (ΔEa,c) gradually increases. Meanwhile, the oxygen peak has a positive transfer while the reduction peak has a negative transfer, identifying that the reversibility of electrode material is not very good.

Fig. 7 Cyclic voltammetry curves of CoAl-LDH/CSs composite at different scanning rates
Galvanostatic discharge characteristics of CoAl-LDH and CoAl-LDH/CSs composite at different current densities are shown in Fig. 8. The discharge specific capacitance of the sample (CCD) can be calculated based on the charge-discharge curves according to Eq. (4) [36]:
 (4)
(4)
where I is the charge-discharge current (A), t is the discharge time (s), ΔV is the potential window, and m is the mass of the active materials (g).

Fig. 8 Galvanostatic charge-discharge characteristics of CoAl- LDH and CoAl-LDH/CSs composite at 1 A/g (a) and discharge performance of CoAl-LDH/CSs composite at different current densities (b)
The calculated discharge specific capacitance of CoAl-LDH and CoAl-LDH/CSs composite at different current densities, according to Eq. (4), is shown in Fig. 9. The specific capacitances of the CoAl-LDH/CSs composites are 1198, 1136, 1040, 928 and 920 F/g at different discharge current densities of 1, 2, 4, 8 and 10 A/g, respectively, while they are 620, 596, 536, 464, and 440 F/g for CoAl-LDH at the corresponding current densities. It is quite obvious that the specific capacitance of CoAl-LDH/CSs composite is higher. The CoAl-LDH/ CSs composite has high specific capacitance compared with the previously reported CoAl-LDH based materials (Table 1). In addition, our tests show that when the discharge current density increases from 1 to 10 A/g, the specific capacitance of the composite electrode still keeps at 76.8%. In contrast, pure CoAl-LDH keeps 71.0% of the specific capacitance, which indicates that the CoAl-LDH/CSs composite has better rate capability. There are three reasons for the good capacitance of the CoAl-LDH/CSs composite: firstly, carbon spheres can provide a good substrate for the conductivity of CoAl-LDH nanosheets, and improve the electron transfer capability and promote oxidation-reduction reaction of ion on electrode surface. Secondly, carbon spheres can prevent the accumulation of CoAl-LDH, increase the specific surface area of electrode material, increase the contact of electrolyte and electrode material, and increase the utilization of active material. Finally, CoAl-LDH and carbon spheres form a three-dimensional spherical structure, which is beneficial to improving the stability of composite material, so as to improve the cycle performance and rate performance of electrode material.

Fig. 9 Specific capacitance of CoAl-LDH and CoAl-LDH/CSs composite electrode at different current densities
Table 1 Comparison of specific capacitance of CoAl-LDH based electrodes in literatures

In order to test the cycling performance of CoAl-LDH and CoAl-LDH/CSs composite, charge- discharge experiments were also carried out at the current density of 2 A/g. From Fig. 10, the specific capacitance of composite material remains at 928 F/g after 1000 cycles at the current density of 2 A/g. Meanwhile, the capacitance retention of composite material is 84%, which is significantly higher than that of pure CoAl-LDH (508 F/g, the capacitance retention is 73.8%). There are several reasons which might contribute to that CoAl-LDH/CSs composite electrode has higher capacitance and better stability than pure CoAl-LDH: the CoAl-LDH and carbon spheres are composed, and it greatly improves conductivity and is good for electron conduction on the electrode surface. In addition, carbon spheres can suppress agglomeration of CoAl-LDH and improve the specific surface area of the composite, resulting in increased utilization of active material. It can be seen from the chart that the specific capacitance decreases in the circulation process due to the fact that volume expansion of electrode material causes the material to fall off from the collector. The addition of CSs can prevent volume expansion and shedding of CoAl-LDH nanosheets [48].

Fig. 10 Cycling performance of CoAl-LDH and CoAl-LDH/ CSs composite at 2 A/g
In order to determine the role of CoAl-LDH and CoAl-LDH/CSs composite materials in the electrode materials, the AC impedance test of pure CoAl-LDH and CoAl-LDH/CSs composite materials was further carried out. The Nyquist curve of Fig. 11 mainly consists of two parts including semicircle in the high frequency region and lies in the low frequency region. In the high frequency region, the distance from a starting point to the origin is the system impedance (Re), and the diameter of the semicircle can be seen as the charge transfer resistance (Rct). In the low frequency region, the tilt of the straight line corresponds to Webb impedance (W), and it may be because OH- in the electrolyte diffuses on the active surface of materials. From Fig. 11, the radius of CoAl-LDH/CSs composite is less than that of pure CoAl-LDH, which shows that the addition of carbon spheres greatly improves the conductivity of electrode material and prevents the stacking of CoAl-LDH nanosheets. This increases the specific surface area of the composite and promotes the electronic transmission on the surface of the electrode material. So, the addition of carbon spheres enhances the capacitor performance of the composite material. Carbon spheres can also reduce the electrochemical polarization, and improve electrode material rate performance and cycle stability [47].

Fig. 11 Nyquist plot for CoAl-LDH and CoAl-LDH/CSs composite
4 Conclusions
1) The growth of CoAl-LDH nanosheets on CSs forms a three-dimensional carbon ball structure, including the ordered CoAl-LDH nanosheet arrays and the high electroconductivity substrates. The large specific surface area of CoAl-LDH/CSs composite can promote the interaction between the electrolyte and the active material.
2) The CoAl-LDH/CSs composite presents high specific capacitance, excellent rate capability and good cycling stability. It also possesses a high specific capacitance of 1198 F/g at 1 A/g and 920 F/g at 10 A/g. Moreover, a high specific capacitance of 928 F/g remains in this composite after 1000 cycles at 2 A/g and capacitance retention of 84%.
3) The design route to produce CoAl-LDH/CSs composite might provide a new sight to develope other materials with high electrochemical performance for supercapacitors.
References
[1] LEI Z B, CHRISTOV N, ZHAO X S. Intercalation of mesoporous carbon spheres between reduced graphene oxide sheets for preparing high-rate supercapacitor electrodes [J]. Energy & Environmental Science, 2011, 4(5): 1866-1873.
[2] LI Z, XU Z W, TAN X H, WANG H L, HOLT C M B. STEPHENSON T, OLSEN B C., MITLIN D. Mesoporous nitrogen-rich carbons derived from protein for ultra-high capacity battery anodes and supercapacitors [J]. Energy & Environmental Science, 2013, 6(3): 871-878.
[3] DOU Yuan-yun, LUO Min, LIANG Sen, ZHANG Xue-ling, DING Xiao-yi, LIANG Bin. Flexible free-standing graphene-like film electrode for supercapacitors by electrophoretic deposition and electrochemical reduction [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(5): 1425-1433.
[4] MALAK P A, VIX G C, FRACKOWIAK E. Carbon/layered double hydroxide (LDH) composites for supercapacitor application [J]. Energy & Fuels, 2010, 24(6): 3346-3351.
[5] YUAN Chang-zhou, CHEN Li, GAO Bo, SU Ling-hao, ZHANG Xiao-gang. Synthesis and utilization of RuO2·xH2O nanodots well dispersed on poly(sodium 4-styrene sulfonate) functionalized multi-walled carbon nanotubes for supercapacitors [J]. Journal of Materials Chemistry, 2009, 19(2): 246-252.
[6] XIONG Sheng-lin, YUAN Chang-zhou, ZHANG Xiao-gang, XI Bao-juan, QIAN Yi-tai. Controllable synthesis of mesoporous Co3O4 nanostructures with tunable morphology for application in supercapacitors [J]. Chemistry – A European Journal, 2009, 15(21): 5320-5326.
[7] ZHANG G Q, WU H B, HOSTER H E, CHAN P M B, LOU X W. Single-crystalline NiCo2O4 nanoneedle arrays grown on conductive substrates as binder-free electrodes for high-performance supercapacitors [J]. Energy & Environmental Science, 2012, 5(11): 9453-9456.
[8] YANG Guan-wu, XU Cai-ling, LI Hu-lin. Electrodeposited nickel hydroxide on nickel foam with ultrahigh capacitance [J]. Chem Commun (Camb), 2008, 48(48): 6537-6539.
[9] KONG Ling-bin, LANG Jun-wei, LIU Min, LUO Yong-chun, KANG Long. Facile approach to prepare loose-packed cobalt hydroxide nano-flakes materials for electrochemical capacitors [J]. Journal of Power Sources, 2009, 194(2): 1194-1201.
[10] FANG Y P, LIU J W, YU D J, WICKSTED J P, KALKAN K, TOPAL C, FLANDERS B N, WU J, LI J. Self-supported supercapacitor membranes: Polypyrrole-coated carbon nanotube networks enabled by pulsed electrodeposition [J]. Journal of Power Sources, 2010, 195(2): 674-679.
[11] HAN Yong-qin, HAO Liang, ZHANG Xiao-gang. Preparation and electrochemical performances of graphite oxide/polypyrrole composites [J]. Synthetic Metals, 2010, 160(21-22): 2336-2340.
[12] GUADAGNINI L, MIGNANI A, STTACAVE E, TONELLI D. Ni(OH)2 versus Ni/Al layered double hydroxides as matrices to immobilize glucose oxidase [J]. Electrochimica Acta, 2010, 55(3): 1217-1220.
[13] LI Wen-rong, CHEN De-hong, LI Zheng, SHI Yi-feng, WAN Ying, HUANG Jun-jie, YANG Jian-jun, ZHAO Dong-yuan, JIANG Zhi-yu. Nitrogen enriched mesoporous carbon spheres obtained by a facile method and its application for electrochemical capacitor [J]. Electrochemistry Communications, 2007, 9(4): 569-573.
[14] YU Chang, YANG Juan, ZHAO Chang-tai, FAN Xiao-ming, WANG Gang, QIU Jie-shan. Nanohybrids from NiCoAl-LDH coupled with carbon for pseudocapacitors: understanding the role of nano- structured carbon [J]. Nanoscale, 2014, 6(6): 3097-3104.
[15] LU Z Y, ZHU W, LEI X D, WILLIAMS G R, O'HARE D, CHANG Z, SUN X M, DUAN X. High pseudocapacitive cobalt carbonate hydroxide films derived from CoAl layered double hydroxides [J]. Nanoscale, 2012, 4(12): 3640-3643.
[16] GAO Z, WANG J, LI Z S, YANG W L, WANG B, HOU M J, HE Y, LIU Q, MANN T, YANG Piao-ping, ZHANG Mi-lin, LIU Lian-he. Graphene Nanosheet/Ni2+/Al3+ layered double-hydroxide composite as a novel electrode for a supercapacitor [J]. Chemistry of Materials, 2011, 23(15): 3509-3516.
[17] LIU Ming-xian, GAN Li-hua, XIONG Wei, XU Zi-jie, ZHU Da-zhang, CHEN Long-wu. Development of MnO2/porous carbon microspheres with a partially graphitic structure for high performance supercapacitor electrodes [J]. Journal of Materials Chemistry A, 2014, 2(8): 2555-2562.
[18] MA Fang-wei, ZHAO Hui, SUN Li-ping, LI Qiang, HUO Li-hua, XIA Tian, GAO Shan, PANG Guang sheng, SHI Zhan, FENG Shou-hua. A facile route for nitrogen-doped hollow graphitic carbon spheres with superior performance in supercapacitors [J]. Journal of Materials Chemistry, 2012, 22(27): 13464-13468.
[19] ZHOU Jian-hua, HE Jian-ping, ZHANG Chuan-xiang, WANG Tao, SUN Dun, DI Zhi-yong, WANG Dao-jun. Mesoporous carbon spheres with uniformly penetrating channels and their use as a supercapacitor electrode material [J]. Materials Characterization, 2010, 61(1): 31-38.
[20] HUANG C W, HSU C H, KUO P L, HSIEH C T, TENG H. Mesoporous carbon spheres grafted with carbon nanofibers for high-rate electric double layer capacitors [J]. Carbon, 2011, 49(3): 895-903.
[21] SELS B, DE V D, BUNTINX M, PIERARD F, KIRSCH D, MESMAEKER A, JACOBS P. Layered double hydroxides exchanged with tungstate as biomimetic catalysts for mild oxidative bromination [J]. Nature, 1999, 400(6747): 855-857.
[22] CHOY J, CHOI S, OH J, PARK T. Clay minerals and layered double hydroxides for novel biological applications [J]. Applied Clay Science, 2007, 36(1-3): 122-132.
[23] MILLANGE F, WALTON R I, LEI L X, O'HARE D. Efficient separation of terephthalate and phthalate anions by selective ion-exchange intercalation in the layered double hydroxide Ca2Al(OH)6NO3 2H2O [J]. Chemistry of Materials, 2000, 12(7): 1990-1994.
[24] NING Fan-yu, SHAO Ming-fei, ZHANG Cheng-long, XU Si-min, WEI Min, DUAN Xue. Co3O4@ layered double hydroxide core/shell hierarchical nanowire arrays for enhanced supercapacitance performance [J]. Nano Energy, 2014, 7: 134-142.
[25] ZHANG Luo-jiang, ZHANG Xiao-gang, SHEN Lai-fa, GAO Bo, HAO Liang, LU Xiang-jun, ZHANG Fang, DING Bing, YUAN Chang-zhou. Enhanced high-current capacitive behavior of graphene/CoAl-layered double hydroxide composites as electrode material for supercapacitors [J]. Journal of Power Sources, 2012, 199: 395-401.
[26] NIU Yu-lian, LI Rui-yi, LI Zai-jun, FANG Yin-jun, LIU Jun-kang. High-performance supercapacitors materials prepared via in situ growth of NiAl-layered double hydroxide nanoflakes on well- activated graphene nanosheets [J]. Electrochimica Acta, 2013, 94: 360-366.
[27] SU Ling-hao, ZHANG Xiao-gang, LIU Yang. Electrochemical performance of Co–Al layered double hydroxide nanosheets mixed with multiwall carbon nanotubes [J]. Journal of Solid State Electrochemistry, 2008, 12(9): 1129-1134.
[28] FANG Ji-hong, LI Min, LI Qian-qian, ZHANG Wei-feng, SHOU Qing-liang, LIU Fu, ZHANG Xiao-bin, CHENG Ji-peng. Microwave-assisted synthesis of CoAl-layered double hydroxide/ graphene oxide composite and its application in supercapacitors [J]. Electrochimica Acta, 2012, 85: 248-255.
[29] XU Jie, GAI Shi-li, HE Fei, NIU Na, GAO Peng, CHEN Yu-jin, YANG Piao-ping. A sandwich-type three-dimensional layered double hydroxide nanosheet array/graphene composite: fabrication and high supercapacitor performance [J]. Journal of Materials Chemistry A, 2014, 2(4): 1022-1031.
[30] SU Ling-hao, ZHANG Xiao-gang. Effect of carbon entrapped in Co-Al double oxides on structural restacking and electrochemical performances [J]. Journal of Power Sources, 2007, 172(2): 999-1006.
[31] MIYATA S. Anion-exchange properties of hydrotalcite-like compounds [J]. Clays Clay Miner, 1983, 31(4): 305-311.
[32] YUAN Ding-sheng, CHEN Jing-xing, ZENG Jiang-hua, TAN San-xiang. Preparation of monodisperse carbon nanospheres for electrochemical capacitors [J]. Electrochemistry Communications, 2008, 10(7): 1067-1070.
[33] ZHI Ming-jia, XIANG Cheng-cheng, LI Jian-tian, LI Ming, WU Nian-qiang. Nanostructured carbon-metal oxide composite electrodes for supercapacitors: A review [J]. Nanoscale, 2013, 5(1): 72-88.
[34] MA Xiao-mei, LIU Ming-xian, GAN Li-hua, ZHAO Yun-hui, CHEN Long-wu. Synthesis of micro- and mesoporous carbon spheres for supercapacitor electrode [J]. Journal of Solid State Electrochemistry, 2013, 17(8): 2293-2301.
[35] FAN Lei, TANG Le, GONG Hui-fang, YAO Zhi-heng, GUO Rong. Carbon-nanoparticles encapsulated in hollow nickel oxides for supercapacitor application [J]. Journal of Materials Chemistry, 2012, 22(32): 16376-16381.
[36] YANG J, YU C, FAN X M, LING Z, QIU J S, GOGOTSI Y. Facile fabrication of MWCNT-doped NiCoAl-layered double hydroxide nanosheets with enhanced electrochemical performances [J]. Journal of Materials Chemistry A, 2013, 1(6): 1963-1968.
[37] LIU Z P, MA R Z, OSADA M, IYI N, EBINA Y, TAKADA K, SASAKI T. Synthesis, anion exchange, and delamination of Co-Al layered double hydroxide: Assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies [J]. Journal of the American Chemical Society, 2006, 128(14): 4872-4880.
[38] ZHANG L J, WANG J, ZHU J J, ZHANG X G, KWAN S H, HUI K N. 3D porous layered double hydroxides grown on graphene as advanced electrochemical pseudocapacitor materials [J]. Journal of Materials Chemistry A, 2013, 1(32): 9046-9053.
[39] LI Lei, LI Ru-min, GAI Shi-li, HE Fei, YANG Piao-ping. Facile fabrication and electrochemical performance of flower-like Fe3O4@C@layered double hydroxide (LDH) composite [J]. Journal of Materials Chemistry A, 2014, 2(23): 8758-8765.
[40] SIMON P, GOGOTSI Y. Materials for electrochemical capacitors [J]. Nature Materials, 2008, 7(11): 845-854.
[41] HAN Jing-bin, DOU Yi-bo, ZHAO Jing-wen, WEI Min, EVANS D G, DUAN Xue. Flexible CoAl LDH@PEDOT core/shell nanoplatelet array for high-performance energy storage [J]. Small, 2013, 9(1): 98-106.
[42] ZHAO Jing-wen, LU Zhen-zhi, SHAO Ming-fei, YAN Dong-peng, WEI Min, EVANS D G, DUAN Xue. Flexible hierarchical nanocomposites based on MnO2 nanowires/CoAl hydrotalcite/carbon fibers for high-performance supercapacitors [J]. RSC Advances, 2013, 3(4): 1045-1049.
[43] WANG Lei, WANG Dong, DONG Xin-yi, ZHANG Zhi-jun, PEI Xian-feng, CHEN Xin-jiang, CHEN Biao, JIN Jian. Layered assembly of graphene oxide and Co-Al layered double hydroxide nanosheets as electrode materials for supercapacitors [J]. Chem Commun (Camb), 2011, 47(12): 3556-3558.
[44] PAN Guo-xiang, XIA Xin-hui, LUO Jing-shan, CAO Feng, YANG Zhi-hong, FAN Hong-jin. Preparation of CoAl layered double hydroxide nanoflake arrays and their high supercapacitance performance [J]. Applied Clay Science, 2014, 102: 28-32.
[45] WU Xiao-liang, JIANG Li-li, LONG Cong-lai, WEI Tong, FAN Zhuang-jun. Dual support system ensuring porous Co-Al hydroxide nanosheets with ultrahigh rate performance and high energy density for supercapacitors [J]. Advanced Functional Materials, 2015, 25(11): 1648-1655.
[46] HUANG Zong-chuan, WANG Sen-lin, WANG Jian-peng, YU Ya-ming, WEN Jing-jing, LI Rui. Exfoliation-restacking synthesis of coal-layered double hydroxide nanosheets/reduced graphene oxide composite for high performance supercapacitors [J]. Electrochimica Acta, 2015, 152: 117-125.
[47] CHENG J P, FANG J H, LI M, ZHANG W F, LIU F, ZHANG X B. Enhanced electrochemical performance of CoAl-layered double hydroxide nanosheet arrays coated by platinum films [J]. Electrochimica Acta, 2013, 114: 68-75.
[48] HUANG S, ZHU G N, ZHANG C, TJIU W W, XIA Y Y, LIU T X. Immobilization of Co-Al layered double hydroxides on graphene oxide nanosheets: growth mechanism and supercapacitor studies [J]. ACS Appl Mater Interfaces, 2012, 4(4): 2242-2249.
黄 琪1,刘开宇1,2,何 方1,张水蓉1,谢清亮1,陈 诚1
1. 中南大学 化学化工学院,长沙 410083;
2. 龙岩学院 化学与材料学院,龙岩 364012
摘 要:介绍了一种构建钴铝层状双金属氢氧化物/碳球(CoAl-LDH/CSs) 复合物的新设计路线。通过生长法使CoAl-LDH生长在CSs上,生长在CSs上的CoAl-LDH薄层由厚度为20 nm的纳米片组成。恒流充放电测试表明,CoAl-LDH/CSs复合物在6 mol/L KOH溶液中以1 A/g的电流密度充放电的比容量为1198 F/g(基于CoAl-LDH/CSs 复合物的质量),甚至在高达10 A/g的大电流密度下仍然呈现出920 F/g的较高比容量。而且该复合物以2 A/g的电流密度充放电循环1000次后仍保留928 F/g的比容量,比容量的保留率为84%,这表明与纯的CoAl-LDH相比,CoAl-LDH/CSs复合物具有较高的比容量、优良的倍率性能和良好的循环稳定性。
关键词:钴铝层状双金属氢氧化物;碳球;超级电容器;生长法
(Edited by Xiang-qun LI)
Foundation item: Project (21471162) supported by the National Natural Science Foundation of China; Project (2015H6016) supported by the Science and Technology Project of Fujian Province, China
Corresponding author: Kai-yu LIU; Tel: +86-731-88879616; E-mail: kaiyuliu309@163.com
DOI: 10.1016/S1003-6326(17)60203-6

