Removal of astrazon golden yellow 7GL from colored wastewater using chemically modified clay
来源期刊:中南大学学报(英文版)2017年第4期
论文作者:Meltem CAKMAK Seyda TASAR Veyis SELEN Dursun OZER Ahmet OZER
文章页码:743 - 753
Key words:adsorption; astrazon golden yellow 7GL (AGY-7GL); chemically modified clay (CMC); thermodynamic and kinetic parameters
Abstract: Chemically modified clay (CMC) was used as an adsorbent for the removal of Astrazon Golden Yellow 7GL (AGY-7GL), which is a basic dye from wastewater. For this purpose, the chemically modified clay was first characterized by determining zero point of charge (pHzpc), and using BET, SEM and FTIR. Then effects of operational parameters on adsorption of AGY-7GL were studied in a batch system. The effect of various parameters such as contact time (0-180 min), pH (2-8), temperature (293-323 K), CMC concentration (0.075-0.5 mg/L) and initial AGY-7GL concentration (75-250 mg/L) were investigated on the adsorption efficiency and capacity adsorption of CMC for the removal of AGY-7GL. Thermodynamic and kinetic parameters were calculated from the results of the adsorption experiment. The evaluation of kinetic models shows that this data best fits the pseudo-second-order model. It is determined that the adsorption equilibrium data works very well with the nonlinear Freundlich isotherm model. Thermodynamic parameters such as △H0 (19.0 kJ/mol), △G0 (-28.8 kJ/mol) and △S0 (0.148 kJ/mol) were also determined. According to the experimental results, it is concluded that CMC could be used as an alternative low cost potential adsorbent for the removal of AGY-7GL from wastewater.
Cite this article as: Meltem  , Veyis SELEN, Dursun
, Veyis SELEN, Dursun  ZER, Ahmet
ZER, Ahmet  ZER. Removal of astrazon golden yellow 7GL from colored wastewater using chemically modified clay [J]. Journal of Central South University, 2017, 24(4): 743-753. DOI: 10.1007/s11771-017-3476-y.
ZER. Removal of astrazon golden yellow 7GL from colored wastewater using chemically modified clay [J]. Journal of Central South University, 2017, 24(4): 743-753. DOI: 10.1007/s11771-017-3476-y.
J. Cent. South Univ. (2017) 24: 743-753
DOI: 10.1007/s11771-017-3476-y
Meltem  , Veyis SELEN, Dursun
, Veyis SELEN, Dursun  ZER, Ahmet
ZER, Ahmet  ZER
ZER
Department of Chemical Engineering, Firat University, 23279 Elazig, Turkey
 Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Abstract: Chemically modified clay (CMC) was used as an adsorbent for the removal of Astrazon Golden Yellow 7GL (AGY-7GL), which is a basic dye from wastewater. For this purpose, the chemically modified clay was first characterized by determining zero point of charge (pHzpc), and using BET, SEM and FTIR. Then effects of operational parameters on adsorption of AGY-7GL were studied in a batch system. The effect of various parameters such as contact time (0-180 min), pH (2-8), temperature (293-323 K), CMC concentration (0.075-0.5 mg/L) and initial AGY-7GL concentration (75-250 mg/L) were investigated on the adsorption efficiency and capacity adsorption of CMC for the removal of AGY-7GL. Thermodynamic and kinetic parameters were calculated from the results of the adsorption experiment. The evaluation of kinetic models shows that this data best fits the pseudo-second-order model. It is determined that the adsorption equilibrium data works very well with the nonlinear Freundlich isotherm model. Thermodynamic parameters such as △H0 (19.0 kJ/mol), △G0 (-28.8 kJ/mol) and △S0 (0.148 kJ/mol) were also determined. According to the experimental results, it is concluded that CMC could be used as an alternative low cost potential adsorbent for the removal of AGY-7GL from wastewater.
Key words: adsorption; astrazon golden yellow 7GL (AGY-7GL); chemically modified clay (CMC); thermodynamic and kinetic parameters
1 Introduction
Dyes and pigments, which are generally complex aromatic molecular with synthetic structural origins, contain double bonds and various functional groups [1]. It is well known that dyes are persistent (stable structure), recalcitrant (i.e., biologically non-degradable in natural bodies of water), and are classified as structural, such as disperse, azo, diazo, neutral, basic, acidic, metal complex and anthraquinone-based dyes [2]. Today there are more than ten thousand different pigments and dyes that have been widely used for coloring in various industries such as food, cosmetics, leather, paper and especially textiles [3, 4]. As a result of the dyeing processes, more than 700000 t of dyes and pigments are discharged in effluent from various industries in the world every year [5]. The colored wastewater impairs the aesthetic nature of the receiving environment, reduces the light penetration through the water’s surface and reduces the solubility of gases. This pollution affects the photosynthetic activity of aquatic life [6]. If untreated wastewater is discharged in a natural water reserve, it will pose a serious environmental hazard [2-7]. Untreated wastewater in a natural water reserve can cause cancer, allergic dermatitis, mutations and skin irritations; therefore, removal of dyes and pigments from waste effluents is becoming one of the most important issues for water ecosystems [8]. Different types of physicochemical treatments (such as chemical oxidation [9], membrane separation [10], coagulation and flocculation [11], reverse osmosis [12], adsorption [13-17], ultrafiltration, Fenton treatment, electrochemical oxidation, and electrocoagulation [2]) have been tried to treat the colored wastewater. However, none of these methods are successful in completely removing the color from wastewater. Among these methods, adsorption is the best and most effective technique [18]. The costs of the adsorbent and adsorption capacity used in the adsorption process are the most important factors affecting the process costs. As reported in the literature, the adsorption capacities of natural adsorbents such as agricultural wastes, fly ash, wood sawdust, peanut shells and sugar beet pulp are not high enough in studies involving the removal of dyes and heavy metal ions from wastewater [8, 17, 19-24]. Therefore, it is necessary to develop effective, inexpensive and easily available adsorbents to improve the efficiency and effectiveness of the adsorption process.
The aim of this research is to investigate the adsorption efficiency, kinetic and thermodynamic parameters of AGY-7GL on chemically modified clay. For this purpose, the adsorbent was first characterized with Brunauer-Emmett-Teller (BET), scanning electron microscopy (SEM), fourier transform infrared (FTIR) and zero point charge (pHzpc) analyses. Next, effects of operational parameters on adsorption of Astrazon Yellow 7GL were studied in a batch operating conditions. The efficiency of adsorption was measured using various parameters such as initial pH of solution and contact time, adsorbent concentration, initial dye concentration and temperature for the removal of AYG-7GL. Furthermore, the thermodynamic and kinetic parameters were calculated from the adsorption experiment results and compared within literature.
2 Material and methods
2.1 Preparation of adsorbent and characterization
Preparation of the adsorbent. The natural clay used in this work was provided by a local industry in  , Turkey. The clay was dried in an oven at 80 oC and then the clay was sieved. The fraction between 149 and 315 mm was activated with 3 eq/L H2SO4 solution at 97 °C for 24 h. After the activation process, to remove the free sulfuric acid, the samples were washed with distilled water until the pH of supernatant remained constant at approximately 5.0. Activated clay was stored for the duration of the study.
, Turkey. The clay was dried in an oven at 80 oC and then the clay was sieved. The fraction between 149 and 315 mm was activated with 3 eq/L H2SO4 solution at 97 °C for 24 h. After the activation process, to remove the free sulfuric acid, the samples were washed with distilled water until the pH of supernatant remained constant at approximately 5.0. Activated clay was stored for the duration of the study.
Zeta potential of adsorbent. The pH of zero charge pHzpc was measured using pH drift methods. The pH solutions (50 mL) of 0.1 mol/L NaCI were adjusted to between 2 and 12 by adding either NaOH or HCI. After the adsorbent (0.05 g) was added to the prepared solution, the pH stabilized during a 24 h period, and then the final pH was measured. A graph was then plotted between pHfinal versus pHinitial. The figure was used to obtain the pHzpc of the adsorbent at which the initial and final pH values were equal.
BET analysis of activated clay. BET analysis was performed by the adsorption of N2 at 77 K using surface area analyzer (Micromeritics ASAP 2020). Before measuring the adsorption of N2, firstly, activated clay samples (CMC) before and after adsorption of AGY-7GL were subjected to degassing for 18 h at a final pressure of 133.32×10-4 Pa. The total pore volume (Vp) was estimated from the volume of N2 (as liquid) held at a relative pressure (P/P0) of 0.95.
FTIR analysis of activated clay. Activated clay samples and the adsorbent were first dried in the oven at 80 oC and then mixed with KBr to form pellets for Fourier transform infrared (FTIR) spectroscopy analysis. The FTIR spectra were recorded using a FTIR spectrometer (ATI Unicam Mattson 1000) by averaging 16 scans. IR absorbance data were obtained between 400 and 4000 cm-1 by averaging 16 scans.
SEM analysis of activated clay. The surface morphology of activated clay was investigated using a scanning electron microscope.
Preparation of colored wastewater. The stock solution of Astrazon Golden Yellow 7GL was prepared with a concentration of 1000 mg/L. The pH values of the solutions were adjusted to the desired values ranging from 2.0 to 8.0 using 0.1 mol/L solutions of NaOH and HCI. All working solutions and dilutions were prepared using distilled water.
2.2 Experimental procedure
The effect of pH. The effect of pH was studied using 600 mL of AGY-7GL solution with a concentration of 100 mg/L. The pH of the solution was adjusted in the range of 2.0-8.0 using 0.1 mol/L NaOH and 0.1 mol/L HCI solutions. The 600 mL solution with a dye concentration of 100 mg/L was placed in a 1000 mL flask, and a 0.25 g/L sample of activated clay was mixed into the 600 mL solution. The mixture was then shaken by a magnetic stirrer at 293 K and 400 r/min for 180 min. At the end of the any contact time, the adsorbent was centrifuged from the aqueous phase. The residual concentration of dye in the filtrate was analyzed using a spectrophotometer (Chebious Optimum One UV/Vis).
The effect of contact time and temperature. 600 mL of solution containing 100 mg of dye/L was placed in a flask, and 0.25 g of activated clay was added to the flask. The mixtures were mixed at 400 r/min at different contact times ranging from 0 to 180 min at 293, 303, 313 and 323 K, separately. Then, for each mixture, the adsorbent was centrifuged and the supernatant was analyzed.
The effect of adsorbent concentration. All the experiments were conducted using 600 mL of the solution that had a concentration of 100 mg of dye/L at the determined optimum pH. Different concentrations of activated clay, i.e., 0.075, 0.15, 0.25, 0.35, and 0.5 g/L, were mixed with the solutions in a 1000 mL flask, and the mixtures were shaken for 180 min at 293 K and 400 r/min. The dye concentrations in the centrifuged were then analyzed.
The effect of the initial concentration of dye in the solution. The adsorption of dye by activated clay was conducted by shaking 0.25 g of activated clay in 600 mL of solutions that contained various initial concentrations of dye, ranging from 75 to 250 mg dye/L at optimum pH. A mechanical stirrer shook the mixtures for the determined optimum contact time (180 min) at 293 K and 400 r/min. Each mixture was separated after mixing, and the supernatant was analyzed.
Calculated adsorption yield and capacity. All the experiments were performed several times, and the mean values of the experimental results were determined. The reproducibility of the experimental results was found to be within ±5%.
The adsorption efficiency of CMC and the maximum capacity of the adsorbent, qe (mg/g), which is expressed as the quantity of dye adsorbed using the activated clay, is calculated using Eqs. (1) and (2), respectively.
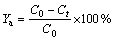 (1)
(1)
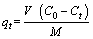 (2)
(2)
where C0 is the initial concentration of AGY-7GL (mg/L), Ct is the concentration at any time t, (mg/L), V is the volume of the solution (L), and M is the mass of the CMC (g).
3 Results and discussion
3.1 BET analysis results
Single point BET surface area, the average pore diameter and the pore volume of the samples (activated clay, which was analyzed before and after adsorption experiment) was determined by the nitrogen adsorption method. The values are 73.57 m2/g, 65.36  and 0.120231 cm3/g for before adsorption activated clay; 28.72 m2/g, 108.23
and 0.120231 cm3/g for before adsorption activated clay; 28.72 m2/g, 108.23  and 0.077726 cm3/g for after adsorption activated clay, respectively, using a Micromeritics ASAP 2020 apparatus by nitrogen adsorption methods.
and 0.077726 cm3/g for after adsorption activated clay, respectively, using a Micromeritics ASAP 2020 apparatus by nitrogen adsorption methods.
3.2 FTIR analysis results
The functional groups on the surface of CMC before and after adsorption of AGY-7GL were qualitatively analyzed using FTIR, and the major peaks are presented in Table 1. Researchers have reported similar peaks for clay in Refs. [25, 26].
The IR spectrum can be used for clay mineral identification from the mineral fingerprint. The fingerprint gives important and unique information about the mineral, such as the family, the specimen, structure, nature of isomorphic substituents, degree of regularity, crystalline and non-crystalline impurities of minerals, and distinction of molecular water from constitutional hydroxyl [25]. In light of this information and the two peaks, OH stretching of inner hydroxyl groups (3620 cm-1) and OH stretching of water (3435 cm-1), it was concluded that the clay samples used in the study are Montnorillonite minerals.
3.3 SEM analysis of adsorbent
The SEM images of CMC before and after adsorption of AGY-7GL are given in Figs. 1(a) and (b).According to SEM images shown in Fig. 1(a), it is determined that CMC surface is porous and rough. It is clearly seen from the SEM image in Fig. 1(b) that after the adsorption the surface pores of CMC were covered by AGY-7GL. It is evident that upon adsorbing the AGY-7GL, the adsorbent structure has changed. It is clearly seen that the CMC surface structure has changed after adsorption of AGY-7GL. In conclusion, it is determined that the SEM images confirm the BET analysis results.
Table 1 FTIR spectrum of CMC
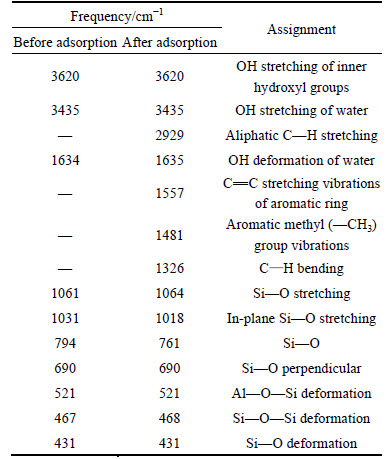
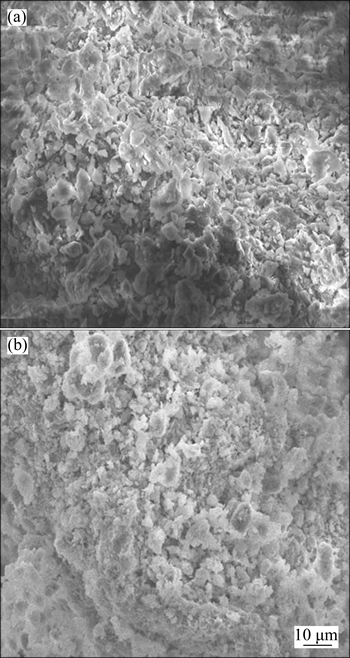
Fig. 1 SEM images of CMC before (a) and after (b) adsorption
3.4 Zero potential of adsorbent
The zero potential of adsorbent (pHzpc) is a significant important parameter because the pHzpc value is used to determine the linear pH range sensitive [27]. If pH
zpc (pH of solution is below the zero charge point), the surface of the adsorbent can attract anions from the solution because the surface of the adsorbent is positively charged. In the present case, at pH>pHzpc (the pH of the solution is above the zero charge point) the surface of the adsorbent can attract cations from the solution because the surface of the adsorbent is negatively charged [28]. According to previous research, pHzpc is determined using pH drift method [27-29]. In this work, the value of the pHzpc of CMC is determined from the plot of final pH against initial pH (Fig. 2). From the results of the pHzpc measurement (Fig. 2), the zero point charge of CMC is found to be 6.0. According to experimental results, if a solution has a pH>6.0, the CMC possesses a negatively charged surface, which is favorable for adsorbing cationic dye (basic dye). However, if a solution has a pH<6.0, the CMC possesses a positively charged surface, which is favorable for adsorbing anionic dye (acidic dye) [27].
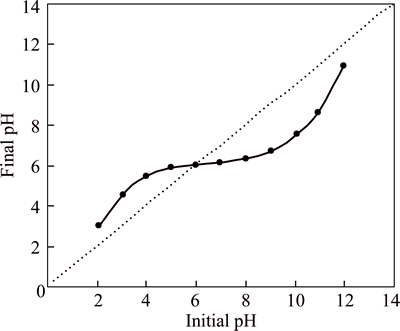
Fig. 2 Zeta potential of CMC
3.5 Effect of initial pH of solution
The initial pH of the colored waste water is a very significant parameter. The pH of the colored wastewater is affected by the surface charges of the adsorbent, because during the adsorption process, ions degree of ionization/dissociation and concentration of the counter of the adsorbent are changed with the pH value of solution [30]. The role of the H+ concentration in the removal of dye was examined using CMC at different initial pH values, ranging from 2.0 to 8.0, as shown in Fig. 3. The experimental results prove that the adsorption capacity of CMC is low (average of about 269 mg/g) for the pH at 2.0. The adsorption capacity increases linearly up to a maximum value of about 319 mg/g as the pH of the solution increases to 6.0. Thus, the later experiments were conducted at a pH value of 6.0. The experimental result is consistent with pHzpc of the CMC (pHzpc=6) and other researchers reports in Refs. [30, 31]. Figure 3 shows the relationship between the adsorption yield and capacity of AGY-7GL and contact time. Figure 3(a) indicates that the adsorption of the AGY-7GL is relatively rapid in the initial 60 min due to the higher availability of the uncovered active sites on the surface of CMC and the driving force, which allows fast transfer of AGY-7GL to the surface of CMC particles.
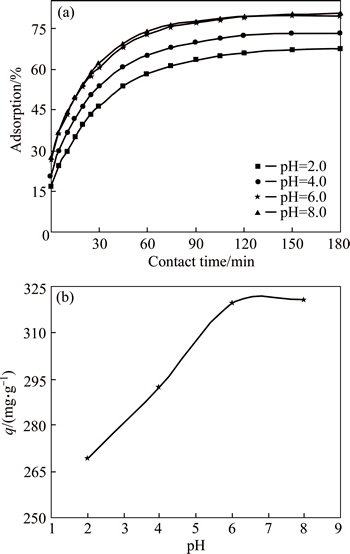
Fig. 3 Effects of contact time on adsorption yield of dye (a) and initial pH of solution on adsorption capacity of dye (b) (Conditions: initial dye concentration of 100 mg/L; contact time of 180 min; CMC concentration of 0.25 g/L; temperature of 293 K).
After this initial stage, with further increasing time, the adsorption efficiency did not change significantly because both uncovered active sites and the driving force of AGY-7GL decrease. Thus, the diffusion rate becomes slower. Therefore, 105 min could be considered the equilibrium time for adsorption of AGY-7GL. Therefore, the optimal equilibrium is 105 min for the dye experiments.
3.6 Effect of initial concentration of dye in solution
According to previous research, the initial dye concentration has an important impact on adsorption. The experimental data was analyzed (Fig. 4), and it is determined that when the initial dye concentrations are increased in the range of 75-250 mg/L, the dye removal efficiency decreases from 86% to 46%, and adsorption capacity increases from 228 to 460 mg/g at 293 K, respectively. Because at lower initial dye concentrations, the ratio of the initial concentrations of dye to the available surface area is low; subsequently, the fractional sorption becomes independent of the initial dye concentration.
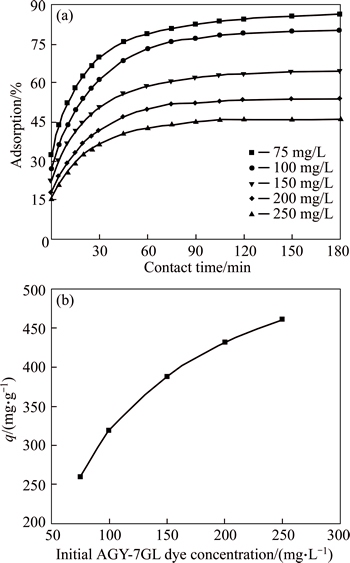
Fig. 4 Effects of initial dye concentration of solution on adsorption yield of dye (a) and adsorption capacity of dye (b) (Conditions: CMC concentration of 0.25 g/L; pH of 6; temperature of 293 K; contact time of 180 min)
However, at higher dye concentrations, the sites available for sorption become fewer compared to the concentrations of dye present, and hence, the removal of dye is strongly dependent upon the initial dye concentration [32]. The initial dye concentration creates a significant important driving force to overcome all mass transfer resistances between solid and liquid phases [33].
3.7 Effect of adsorbent concentration
The concentration of an adsorbent significantly influences the efficiency of adsorption. Figure 5 presents the experimental results obtained in this work. The amount of AGY-7GL adsorbed decreases from 486 mg/g for an absorbent mass of 0.075 g/L, 194 mg/g for an absorbent mass of 0.50 g/L, whereas the removal of AGY-7GL dye increases from 36.5% to 97.2 % when the adsorbent mass increases from 0.075 to 0.50 g/L. This is due to the increase in surface area resulting from the increase in adsorbent mass, thus increasing the number of active adsorption sites [17].
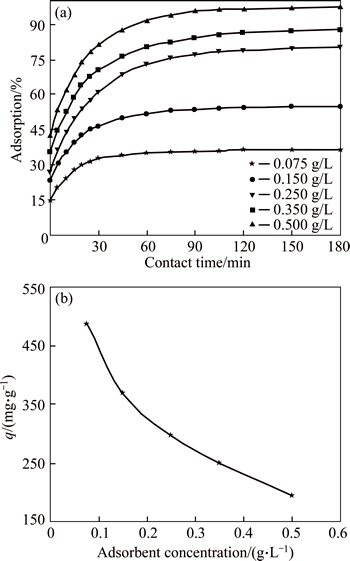
Fig. 5 Effects of CMC concentration of solution on adsorption yield of dye (a) and adsorption capacity of dye (b) (Conditions: initial dye concentration of 100 mg/L; pH of 6; temperature of 293 K; contact time of 180 min)
The amount of dye adsorbed per unit mass of adsorbent decreases with increasing adsorbent mass due to the reduction in effective surface area. This may be also attributed to overlapping or aggregation of adsorption sites, resulting in a decrease in total surface area of adsorbent available to the dye and an increase in diffusion path length [34, 35].
3.8 Effect of temperature
Adsorption processes are affected significantly by temperature. Figure 6 shows that the equilibrium uptake of dye molecules by the adsorbent decreases as the temperature decreases to 293 K. It is determined that about 319 and 371 mg/g dye ion of CMC are adsorbed at equilibrium at 293 and 323 K, respectively. This result may be explained by the diffusion rate. Because it is determined that the diffusion rate increases with increasing mobility of AGY-7GL molecules, and the mobility of the AGY-7GL molecules increases with increasing adsorption temperatures [36]. An increase in the temperature decreases the viscosity of the solution, thereby increasing the rate of diffusion of the adsorbate molecules across the external boundary layer and within the internal pores of the adsorbent particles [37]. Additionally, the increasing removal of dye with increasing temperature shows the endothermic nature of the adsorption process.
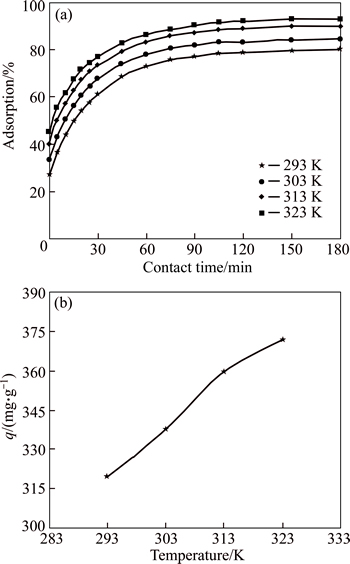
Fig. 6 Effects of temperature of solution on adsorption yield of dye (a) and adsorption capacity of dye (b) (Conditions: initial dye concentration of 100 mg/L; pH of 6; contact time of 180 min; CMC concentration of 0.25 g/L)
3.9 Kinetic analysis of adsorption process
When designing a process and an adsorption reactor, it is important to know the rate of the adsorption process, which is time-dependent. For this aim, the adsorption data were analyzed with the pseudo-first-order kinetic model and the pseudo-second-order kinetic model.
The pseudo-first-order expression of Lagergren is given as [38]
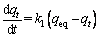 (3)
(3)
The integration of Eq. (3) for the boundary conditions t=0 to t=t results in the following:
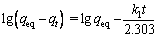 (4)
(4)
The pseudo-second-order model is given by [39]
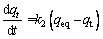 (5)
(5)
The linearized integrated form of Eq. (5) is given by
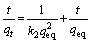 (6)
(6)
where t (min) is the contact time, k1 (min-1) and k2 (g/(mg·min)) are the pseudo-first-order and the pseudo-second-order rate constants, respectively; qeq (mg/g) and qt (mg/g) are the amounts of adsorbate on the surface of the adsorbent at equilibrium and at any contact time, respectively. The values of the adsorption rate constant (k1) for dye adsorption on CMC can be determined from the plot of lg(qeq-qt) against t (Fig. 7). The rate parameters, k2 and qeq, can be obtained from the plot of (t/qt) versus t (Fig. 8).
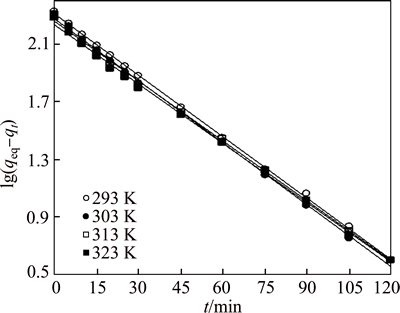
Fig. 7 Pseudo first-order kinetic plots for removal of AGY-7GL
Table 2 provides the values of k1, qec1, k2, qec2 and the correlation coefficient R2. The results indicate that the pseudo-second-order kinetic model is more representative than the pseudo-first-order kinetic model for simulating the kinetic data. For this reason, to determine the activation energy of the adsorption process for dye molecules by CMC, pseudo-second-order rate constants and Arrhenius equation Eq. (7) are used:
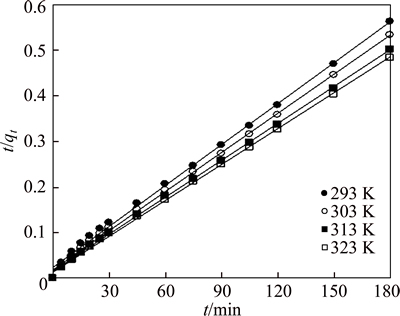
Fig. 8 Pseudo second-order kinetic plots for removal of AGY-7GL
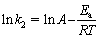 (7)
(7)
where Ea (J/mol) is the activation energy, A (g/(mg·min)) is the Arrhenius constant, k2 (g/(mg·min)) is the rate constant of adsorption, T (K) is the temperature of the solution and R (8.314 J/(mol·K)) is the ideal gas constant. To calculate the activation energy (Ea) for the adsorption process, lnk2 is plotted versus 1/T (not shown). The activation energy value (Ea) is calculated as about 8.5 kJ/mol, indicating that physical adsorption mechanisms occur.
3.10 Thermodynamic analysis of adsorption process
For the design of an adsorption system, the equilibrium adsorption isotherm is fundamentally important due to equilibrium studies of adsorption that were used to determine the capacity of the adsorbent. The relationship between the initial concentration and the amount of adsorbent is known as the degree of the adsorbent affinity for the adsorbate, which determines its distribution between the solid and liquid phases [40]. In the present research, the Langmuir and Freundlich models are used to explain the experimental data.
The Langmuir model [41] is obtained under the ideal assumption of totally homogenous adsorption surface and represented as
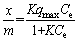 (8)
(8)
Equation (8) can be written in linear form as
 (9)
(9)
where Ce (mg/L) is the equilibrium solute concentration, qmax (mg/g) is the maximum sorption capacity, K (L/mg) is the Langmuir constant, and (x/m=qe) (mg/g) is the amount of AYG-7GL adsorbed at equilibrium.
The Freundlich isotherm is applied under the assumption of a heterogeneous adsorption surface and active sites with different energies involved [42]. The model is represented as [43]
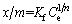 (10)
(10)
Equation (10) can be written in linear form as
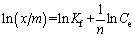 (11)
(11)
where Kf and n are Freundlich constants (capacity and intensity, respectively) that are characteristic of the adsorption system.
Figures 9 and 10 show the experimental data fitted to the nonlinear and linear forms of the Langmuir and Freundlich isotherms for the adsorption of dye molecules by CMC, respectively. The nonlinear and linear isotherm constants were obtained using Polymath 6.1 software and Microsoft Excel, respectively. The correlation coefficients (R2) and the isotherm constants are given in Table 3 for linear and nonlinear Langmuir and Freundlich sorption models. It is determined that the nonlinear Freundlich isotherm better represents the equilibrium adsorption of AGY-7GL by CMC. It is observed that all n values are found to be greater than 1 and in the range of 2-10, indicating that the AGY-7GL molecules are favorably adsorbed by CMC at all temperatures, and the value of n represents that adsorption is good [44]. Since the Freundlich constant (Kf) increases with increasing temperature, this result shows that AGY-7GL molecules are easily adsorbed by the CMC.
Thermodynamic studies are used to decipher any reaction in a better way [45]. Thermodynamic studies were performed and the parameters, such as change in enthalpy (△H0), free energy change (△G0), and entropy (△S0), are determined at 293, 303, 313 and 323 K, respectively. The Langmuir isotherm is used to calculate these thermodynamic parameters using the following equations:
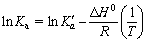 (12)
(12)
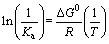 (13)
(13)
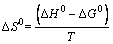 (14)
(14)
where K is the adsorption equilibrium constant obtained from the Langmuir isotherm (L/mol), R is the universal gas constant (8.314 J/(mol·K)), and T is absolute temperature. The intercept and the slope of the plots of ln K versus 1/T are used to determine the △S0 and △H0 values.
Table 2 Kinetic parameters for removal of dye molecules by CMC
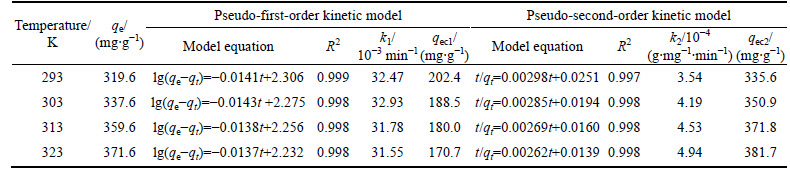
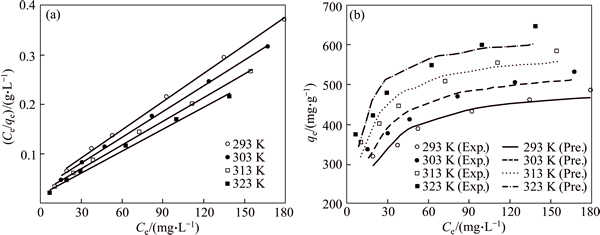
Fig. 9 Comparison of experimental and predicted Langmuir adsorption isotherms linear (a) and nonlinear (b) obtained at different fixed temperature (Conditions: 600 mL of AGY-7GL solution at various concentrations; 0.15 g CMC; pH of 6.0; contact time of 180 min)
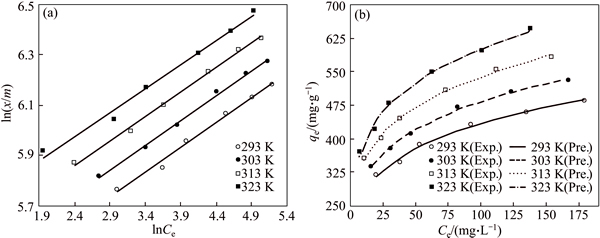
Fig. 10 Comparison of experimental and predicted Freundlich adsorption isotherms in linear (a) and nonlinear (b) forms obtained at different fixed temperature (Conditions: 600 mL of AGY-7GL solution at various concentrations; 0.15 g CMC; pH of 6.0; contact time of 180 min)
Table 3 Linear and nonlinear Langmuir and Freundlich isotherm constants for removal of dye molecules
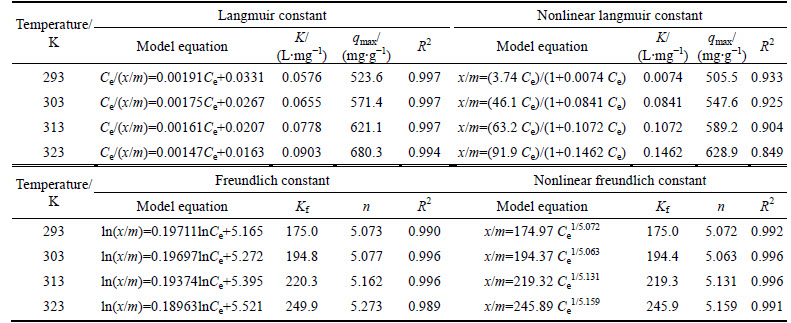
Table 4 provides the thermodynamic parameters that are calculated for the process. The enthalpy (△H0) is determined to be 19.0 kJ/mol. The positive values of △H0 and the negative values of △G0 reflect the feasible and endothermic nature of the adsorption process, such that adsorption can occur spontaneously. It is indicated that the adsorption process is physical because △H0, △G0 and activation energy (Ea) values are less than 20, 84 and 40 kJ/mol, respectively. The positive values of △S0 indicate the affinity of the CMC for the AGY-7GL molecules [46].
3.11 Comparison adsorption capacity of adsorbent
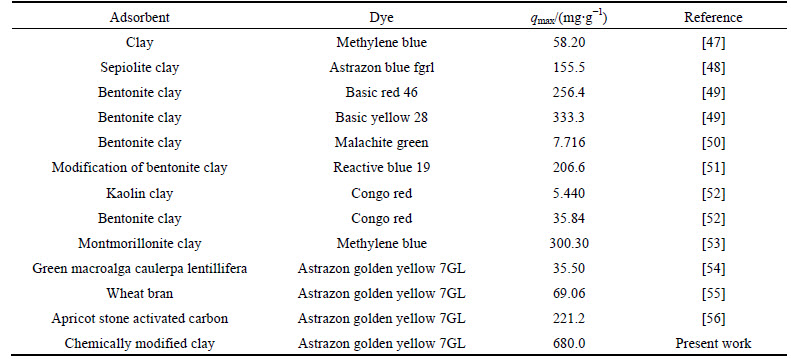
Table 5 summarizes the comparison of maximum adsorption capacities of various adsorbents including CMC for AGY-7GL and the maximum adsorption capacities of clay minerals (sepiolite, bentonite, kaolin and montmorillonite clay) including CMC for other basic dyes.
Table 4 Thermodynamic parameters for removal of dye molecules
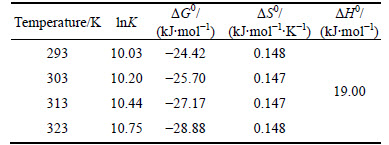
Table 5 indicates that CMC can be used as a low- cost, eco-friendly and effective alternative adsorbent for the adsorption of dye molecules from colored wastewater. Further study should be conducted to investigate the feasibility of using CMC as adsorbents for toxic heavy metals, mixed dye and colored wastewater from the textile industry.
4 Conclusions
This work investigates the use of CMC for the adsorption of dye (AGY-7GL) from an aqueous solution.
1) The experimental results and comparison data indicate that CMC can be used as a low-cost, eco- friendly and effective alternative adsorbent for the adsorption of basic dye molecules from colored wastewater.
2) The adsorption process of AGY-7GL on CMC fit the pseudo-second-order kinetic model by taking into account the correlation coefficient value. Calculated activation energy value (Ea) is 8.5 kJ/mol.
3) The nonlinear Freundlich isotherm is a better fit than the other model, based on the values of the correlation coefficients (R2). The maximum Langmuir adsorbent capacity (qmax) is approximately 680.3 mg/g.
Table 5 Maximum adsorption capacities of some adsorbents used for dyes
4) The positive value of △S°indicates the affinity of the adsorbent for the dye molecules. The positive values of △H°and the negative values of △G°reflect the feasible and endothermic nature of the adsorption process, such that adsorption can occur spontaneously.
References
[1] GONG R M, LI M, YANG C, SUN Y Z, CHEN J. Removal of cationic dyes from aqueous solution by adsorption on peanut hull [J]. Journal of Hazardous Materials, 2005, 121(1-3): 247-250.
[2] CHHABRA M, MISHRA S, SREEKRISHNAN T R. Combination of chemical and enzymatic treatment for efficient decolorization/ degradation of textile effluent: High operational stability of the continuous process [J]. Biochemical Engineering Journal, 2015, 93: 17-24.
[3] DIZGE N, AYDINER C, DEMIRBAS E, KOBYA M, KARA S. Adsorption of reactive dyes from aqueous solutions by fly ash: Kinetic and equilibrium studies [J]. Journal of Hazardous Materials, 2008, 150(3): 737-746.
[4] LEE J W, CHOI S P, THIRUVENKATACHARI R, SHIM W G, MOON H. Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes [J]. Dyes and Pigments, 2006, 69(3): 196-203.
[5] AKSAKAL O, UCUN H. Equilibrium, kinetic and thermodynamic studies of the biosorption of textile dye (Reactive Red 195) onto Pinus sylvestris L [J]. Journal of Hazardous Materials, 2010, 181(1-3): 666-672.
[6] GONZALEZ J A, VILLANUEVA M E, PIEHL L L, COPELLO G J. Development of a chitin/graphene oxide hybrid composite for the removal of pollutant dyes: Adsorption and desorption study [J]. Chemical Engineering Journal, 2015, 280: 41-48.
[7] ANNADURAI G, LING L Y, LEE J F. Adsorption of reactive dye from an aqueous solution by chitosan: Isotherm, kinetic and thermodynamic analysis [J]. Journal of Hazardous Materials, 2008, 152(1): 337-346.
[8] AMIN N K. Removal of direct blue-106 dye from aqueous solution using new activated carbons developed from pomegranate peel: Adsorption equilibrium and kinetics [J]. Journal of Hazardous Materials, 2009, 165(1-3): 52-62.
[9] TURGAY O, ERSOZ G, ATALAY S, FORSS J, WELANDER U. The treatment of azo dyes found in textile industry wastewater by anaerobic biological method and chemical oxidation [J]. Separation and Purification Technology, 2011, 79(1): 26-33.
[10] KIM T H, PARK C, KIM S. Water recycling from desalination and purification process of reactive dye manufacturing industry by combined membrane filtration [J]. Journal of Cleaner Production, 2005, 13(8): 779-786.
[11] MOGHADDAM S S, MOGHADDAM M R A, ARAMI M. Coagulation/flocculation process for dye removal using sludge from water treatment plant: Optimization through response surface methodology [J]. Journal of Hazardous Materials, 2010, 175(1-3): 651-657.
[12] NATARAJ S K, HOSAMANI K M, AMINABHAVI T M. Nanofiltration and reverse osmosis thin film composite membrane module for the removal of dye and salts from the simulated mixtures [J]. Desalination, 2009, 249(1): 12-17.
[13] DULMAN V, CUCU-MAN S M. Sorption of some textile dyes by beech wood sawdust [J]. Journal of Hazardous Materials, 2009, 162(2-3): 1457-1464.
[14] MAHMOODI N M, SALEHI R, ARAMI M, BAHRAMI H. Dye removal from colored textile wastewater using chitosan in binary systems [J]. Desalination, 2011, 267(1): 64-72.
[15] AHMED S A S, KHALIL L B, EL-NABARAWY T. Removal of reactive blue 19 dye from aqueous solution using natural and modified orange peel [J]. Carbon Letters, 2012, 13(4): 212-220.
[16] SELEN V, OZER D, OZER A. A study on the removal of Cr(VI) ions by sesame (sesamum indicum) stems dehydrated with sulfuric acid [J]. Arabian Journal for Science and Engineering, 2014, 39(8): 5895-5904.
[17] CICEK F, OZER D, OZER A, OZER A. Low cost removal of reactive dyes using wheat bran [J]. Journal of Hazardous Materials, 2007, 146(1, 2): 408-416.
[18] ZHANG Z Y, ZHANG Z B, FERNANDEZ Y, MENENDEZ J A, NIU H, PENG J H, ZHANG L B, GUO S H. Adsorption isotherms and kinetics of methylene blue on a low-cost adsorbent recovered from a spent catalyst of vinyl acetate synthesis [J]. Applied Surface Science, 2010, 256(8): 2569-2576.
[19] AKSU Z, ISOGLU I A. Us of agricultural waste sugar beet pulp for the removal of Gemazol turquoise blue-G reactive dye from aqueous solution [J]. Journal of Hazardous Materials, 2006, 137(1): 418-430.
[20] SILVA J P, SOUSA S, RODRIGUES J, ANTUNES H, PORTER J J, GONCALVES I, FERREIRA-DIAS S. Adsorption of acid orange 7 dye in aqueous solutions by spent brewery grains [J]. Separation and Purification Technology, 2004, 40(3): 309-315.
[21] WANG S B, BOYJOO Y, CHOUEIB A, ZHU Z H. Removal of dyes from aqueous solution using fly ash and red mud [J]. Water Research, 2005, 39(1): 129-138.
[22] SANTHY K, SELVAPATHY P. Removal of reactive dyes from wastewater by adsorption on coir pith activated carbon [J]. Bioresource Technology, 2006, 97(11): 1329-1336.
[23] OZER A, AKKAYA G, TURABIK M. Biosorption of Acid Blue 290 (AB 290) and Acid Blue 324 (AB 324) dyes on Spirogyra rhizopus [J]. Journal of Hazardous Materials, 2006, 135(1-3): 355-364.
[24] KARAGOZ S, TAY T, UCAR S, ERDEM M. Activated carbons from waste biomass by sulfuric acid activation and their use on methylene blue adsorption [J]. Bioresource Technology, 2008, 99(14): 6214- 6222.
[25] MADEJOVA J, KOMADEL P. Baseline studies of the clay minerals society source clays: Infrared methods [J]. Clays and Clay Minerals, 2001, 49(5): 410-432.
[26] LIU H M, YUAN P, QIN Z H, LIU D, TAN D Y, ZHU J X, HE H P. Thermal degradation of organic matter in the interlayer clay-organic complex: A TG-FTIR study on a montmorillonite/12-aminolauric acid system [J]. Applied Clay Science, 2013, 80-81: 398-406.
[27] DANISH M, HASHIM R, IBRAHIM M N M, SULAIMAN O. Characterization of physically activated acacia mangium wood-based carbon for the removal of methyl orange dye [J]. Bioresources, 2013, 8(3): 4323-4339.
[28] LOPEZ-RAMON M V, STOECKLI F, MORENO-CASTILLA C, CARRASCO-MARIN F. On the characterization of acidic and basic surface sites on carbons by various techniques [J]. Carbon, 1999, 37(8): 1215-1221.
[29] JIA Y F, XIAO B, THOMAS K M. Adsorption of metal ions on nitrogen surface functional groups in activated carbons [J]. Langmuir, 2002, 18(2): 470-478.
[30] YAO Y J, HE B, XU F F, CHEN X F. Equilibrium and kinetic studies of methyl orange adsorption on multiwalled carbon nanotubes [J]. Chemical Engineering Journal, 2011, 170(1): 82-89.
[31] WANG P F, CAO M H, WANG C, AO Y H, HOU J, QIAN J. Kinetics and thermodynamics of adsorption of methylene blue by a magnetic graphene-carbon nanotube composite [J]. Applied Surface Science, 2014, 290: 116-124.
[32] VIJAYARAGHAVAN K, YUN Y S. Bacterial biosorbents and biosorption [J]. Biotechnology Advances, 2008, 26(3): 266-291.
[33] AKSU Z, TEZER S. Equilibrium and kinetic modelling of biosorption of Remazol Black B by Rhizopus arrhizus in a batch system: Effect of temperature [J]. Process Biochemistry, 2000, 36(5): 431-439.
[34] OZER D, DURSUN G, OZER A. Methylene blue adsorption from aqueous solution by dehydrated peanut hull [J]. Journal of Hazardous Materials, 2007, 144(1, 2): 171-179.
[35] FRANCA A S, OLIVEIRA L S, FERREIRA M E. Kinetics and equilibrium studies of methylene blue adsorption by spent coffee grounds [J]. Desalination, 2009, 249(1): 267-272.
[36] ALKAN M, CELIKCAPA S, DEMIRBAS O, DOGAN M. Removal of reactive blue 221 and acid blue 62 anionic dyes from aqueous solutions by sepiolite [J]. Dyes and Pigments, 2005, 65(3): 251-259.
[37] NASUHA N, HAMEED B H, DIN A T M. Rejected tea as a potential low-cost adsorbent for the removal of methylene blue [J]. Journal of Hazardous Materials, 2010, 175(1-3): 126-132.
[38] PANDAY K K, PRASAD G, SINGH V N. Copper(II) removal from aqueous-solutions by fly-ash [J]. Water Research, 1985, 19(7): 869-873.
[39] HO Y S, MCKAY G. Pseudo-second order model for sorption processes [J]. Process Biochemistry, 1999, 34(5): 451-465.
[40] GIL A, ASSIS F C C, ALBENIZ S, KORILI S A. Removal of dyes from wastewaters by adsorption on pillared clays [J]. Chemical Engineering Journal, 2011, 168(3): 1032-1040.
[41] WAHAB M A, JELLALI S, JEDIDI N. Ammonium biosorption onto sawdust: FTIR analysis, kinetics and adsorption isotherms modeling [J]. Bioresource Technology, 2010, 101(14): 5070-5075.
[42] GAO J F, ZHANG Q, SU K, CHEN R N, PENG Y Z. Biosorption of acid yellow 17 from aqueous solution by non-living aerobic granular sludge [J]. Journal of Hazardous Materials, 2010, 174(1-3): 215-225.
[43] LIU Y, LIU Y. Biosorption isotherms, kinetics and thermodynamics [J]. Separation and Purification Technology, 2008, 61(3): 229-242.
[44] ALVER E, METIN A U. Anionic dye removal from aqueous solutions using modified zeolite: Adsorption kinetics and isotherm studies [J]. Chemical Engineering Journal, 2012, 200: 59-67.
[45] PANNEERSELVAM P, MORAD N, TAN K A. Magnetic nanoparticle (Fe3O4) impregnated onto tea waste for the removal of nickel(II) from aqueous solution [J]. Journal of Hazardous Materials, 2011, 186(1): 160-168.
[46] AL-DEGS Y S, EL-BARGHOUTHI M I, EL-SHEIKH A H, WALKER G M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon [J]. Dyes and Pigments, 2008, 77(1): 16-23.
[47] GURSES A, DOGAR C, YALCIN M, ACIKYILDIZ M, BAYRAK R, KARACA S. The adsorption kinetics of the cationic dye, methylene blue, onto clay [J]. Journal of Hazardous Materials, 2006, 131(1-3): 217-228.
[48] KARAGOZOGLU B, TASDEMIR M, DEMIRBAS E, KOBYA M. The adsorption of basic dye (Astrazon Blue FGRL) from aqueous solutions onto sepiolite, fly ash and apricot shell activated carbon: Kinetic and equilibrium studies [J]. Journal of Hazardous Materials, 2007, 147(1, 2): 297-306.
[49] TURABIK M. Adsorption of basic dyes from single and binary component systems onto bentonite: Simultaneous analysis of Basic Red 46 and Basic Yellow 28 by first order derivative spectrophotometric analysis method [J]. Journal of Hazardous Materials, 2008, 158(1): 52-64.
[50] TAHIR S S, RAUF N. Removal of a cationic dye from aqueous solutions by adsorption onto bentonite clay [J]. Chemosphere, 2006, 63(11): 1842-1848.
[51] OZCAN A, OMEROGLU C, ERDOGAN Y, OZCAN A S. Modification of bentonite with a cationic surfactant: An adsorption study of textile dye Reactive Blue 19 [J]. Journal of Hazardous Materials, 2007, 140(1, 2): 173-179.
[52] VIMONSES V, LEI S M, JIN B, CHOWD C W K, SAINT C. Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials [J]. Chemical Engineering Journal, 2009, 148(2, 3): 354-364.
[53] ALMEIDA C A P, DEBACHER N A, DOWNS A J, COTTET L, MELLO C A D. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay [J]. Journal of Colloid and Interface Science, 2009, 332(1): 46-53.
[54] PUNJONGHARN P, MEEVASANA K, PAVASANT P. Influence of particle size and salinity on adsorption of basic dyes by agricultural waste: Dried Seagrape (Caulerpa lentillifera) [J]. Journal of Environmental Sciences-China, 2008, 20(6): 760-768.
[55] SULAK M T, DEMIRBAS E, KOBYA M. Removal of Astrazon Yellow 7GL from aqueous solutions by adsorption onto wheat bran [J]. Bioresource Technology, 2007, 98(13): 2590-2598.
[56] DEMIRBAS E, KOBYA M, SULAK M T. Adsorption kinetics of a basic dye from aqueous solutions onto apricot stone activated carbon [J]. Bioresource Technology, 2008, 99(13): 5368-5373.
(Edited by FANG Jing-hua)
Cite this article as: Meltem  , Veyis SELEN, Dursun
, Veyis SELEN, Dursun  ZER, Ahmet
ZER, Ahmet  ZER. Removal of astrazon golden yellow 7GL from colored wastewater using chemically modified clay [J]. Journal of Central South University, 2017, 24(4): 743-753. DOI: 10.1007/s11771-017-3476-y.
ZER. Removal of astrazon golden yellow 7GL from colored wastewater using chemically modified clay [J]. Journal of Central South University, 2017, 24(4): 743-753. DOI: 10.1007/s11771-017-3476-y.
Received date: 2015-12-24; Accepted date: 2016-04-11
Corresponding author: Veyis SELEN, Assistant Professor, PhD; Tel: +90-424-2370000; E-mail: vselen@firat.edu.tr

