文章编号:1004-0609(2013)06-1661-05
纳米SiO2空心微球对重金属离子的吸附特性
刘 纯1, 2,殷恒波2,石莉萍1,王爱丽2,吴占敖3,吴 刚3,姜 涛3,沈玉堂2,姜廷顺2
(1. 北华大学 化学与生物学院,吉林132013;
2. 江苏大学 化学化工学院,镇江 212013;
3. 中国人民解放军第359医院,镇江 212001)
摘 要:选用具有高比表面、强吸附能力的二氧化硅纳米空心微球作吸附剂,对Pb2+、Cu2+及Cr3+的吸附性能进行测试。利用Langmuir 线性方程对吸附平衡数据进行拟合。结果表明:二氧化硅纳米空心微球对重金属离子具有较高的饱和吸附量,分别为1.256、0.681、0.563 mmol/g。饱和吸附量与重金属离子的共价指数及电荷密度有很强的相关性,随着共价指数增加或电荷密度的降低,饱和吸附量增大;吸附常数与重金属离子电场强度(有效核电荷数)有关,随着有效核电荷数的增加,吸附常数增大。此吸附过程具有化学吸附特征,再生的吸附剂可以重复使用。
关键词:纳米SiO2空心微球;重金属离子吸附;Langmuir 线性方程
中图分类号:O613.72;O647.31 文献标志码:A
Adsorbability characteristic of hollow SiO2 nanospheres for heavy metal ions
LIU Chun1, 2, YIN Heng-bo2, SHI Li-ping1, WANG Ai-li2, WU Zhan-ao3, WU Gang3,
JIANG Tao3, SHEN Yu-tang2, JIANG Ting-shun2
(1. School of Chemistry and Biology, Beihua University, Jilin 132013, China;
2. Faculty of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, China;
3. Chinese PLA 359 Hospital, Zhenjiang 212001, China)
Abstract: The adsorbability of hollow SiO2 nanospheres of high adsorption capacity and large specific surface area for Pb2+, Cu2+, and Cr3+ ions was investigated. The absorbtion equilibrium data were fitted by Langmuir linear equation. The results show that hollow SiO2 nanospheres have high adsorption capacity for heavy metal ions, the adsorption capacities for Pb2+, Cu2+ and Cr3+ ions are 1.256, 0.681, and 0.563 mmol/g, respectively. As the fitting data shown, there is a strong correlation between the adsorption capacity and ion characteristics (covalent index and charge density). The adsorption capacity increases with the increase in covalent index or decrease in charge density. The adsorption constant is related to electric field intensity of ion (effective nuclear charge), and the adsorption constant increases with the increase in effective nuclear charge of ion. This adsorption process should belong to chemisorption. The regenerated adsorbent can be reused many times.
Key words: hollow SiO2 nanosphere; heavy metal ion adsorbtion; Langmuir linear equation
含重金属的废水主要来源于采矿、金属加工、制革、药品、橡胶、塑料以及木材加工等行业[1]。富含重金属的废水污染水源及土壤,可直接危及动植物的生存,还可经生物链被生物吸附和富集,最终在人体内积累并造成对骨骼、肝、神经等的毒害,且其毒性不易消除,因而成为备受关注的重大环保课题。
目前,用于处理含重金属废水的方法主要有化学沉积法、离子交换法、吸附法、膜滤法、混凝和絮凝法、浮选法、电化学处理方法等[2],而在低浓度的重金属离子的去除方面,吸附法是公认的、较为有效的技术。由于活性炭比较高的价格限制了其应用,因此研制低价、高效的吸附剂便成为了吸附法的核心问题。
目前,非活性碳吸附材料研究主要集中在天然吸附剂[3-6]、生物吸附材料[7-10]、螯合吸附剂[11-14]、无机吸附材料[15-18]等方面。其中,具有介孔壳的无机空心材料因其成本低、制备工艺简单、吸附速度快、吸附时间短等优点,而被格外关注。
本文作者将对二氧化硅纳米空心微球吸附Pb2+、Cu2+、Cr3+的性能进行测试、比较,利用Langmuir模型对平衡数据进行拟合,分析离子属性参数与饱和吸附量及吸附常数的关系,并对其吸附机理进行了深入地分析。
1 实验
1.1 试剂
偏硅酸钠、十六烷基三甲基溴化铵(CTAB)、过硫酸钾、硫酸、氢氧化钠、无水乙醇、硝酸铅、硝酸铜、硝酸铬均为中国国药集团出品分析纯试剂。苯乙烯和α-甲基丙烯酸为国药集团出品化学纯试剂,在使用前进行减压蒸馏提纯。实验用水均为蒸馏水。
1.2 二氧化硅空心微球的制备
苯乙烯与甲基丙烯酸(体积比为10:1)共聚微球(PSM)乳液的合成是利用无皂乳液聚合的方法[19-21]。以PSM为模板、硅酸钠为前驱体,包覆量80%(质量分数)的二氧化硅空心微球制备方法[21]:2.5 g PSM模板转入到2 L大烧杯中,加蒸馏水200 mL、超声分散20 min。水浴温度80 ℃,加入CTAB 0.25 g,搅拌陈化1h。然后,用氢氧化钠溶液(10%,质量分数)调pH值为7.0,在不断搅拌下,利用蠕动泵控速缓慢滴加0.1 mol/L偏硅酸钠溶液300 mL,同时蠕动泵控速滴加硫酸溶液(1%,质量分数)维持反应系统的pH值为7.0。滴加完前驱体后,将pH值调整为9.5,不断搅拌下陈化3 h。之后,减压过滤,用蒸馏水洗涤产品至电导率低于20 mS/m,经120 ℃烘干4 h。合成的核-壳复合材料,从室温开始程序升温加热到550 ℃(1 ℃/min),于550 ℃保持氧化4 h,制得中空二氧化硅微球。
1.3 样品表征
扫描电子显微镜(SEM, JSM 7001F)和透射电子显微镜(TEM, Philips Tecnnai-12, operating at 120 kV)用来分析模板以及二氧化硅空心球的形貌。
空心二氧化硅微球的氮气吸附/脱附等温线由吸附分析仪(Quntachrome Corporation, NOVA2000e)得到,比表面积和孔径分布分别通过BET和BJH模型来计算。
1.4 重金属离子吸附实验
在25 ℃下,取浓度100 mg/L的硝酸铅溶液300 mL于500 mL烧杯中,称取二氧化硅空心球0.1 g左右,迅速加到溶液中,开始搅拌计时,分别于1、3、6、10、20、50、100、180 min取样,滤液用原子吸收(TAS-986)法测定吸附离子Cu2+和Cr3+的浓度。
按上述操作,改用硝酸铜及硝酸铬溶液进行实验,测试对Cu2+、Cr3+的吸附性能。
回收用过的吸附剂,用少量1 mol/L的硝酸溶液搅拌、浸泡1 h,减压过滤、洗涤;重复3次,最后洗至pH=7.0,经120 ℃烘干2 h,继续按上述方法进行吸附实验。
2 结果与讨论
2.1 二氧化硅空心微球表征
PSM模板粒径为362.4 nm。二氧化硅空心微球的SEM与TEM分析表明,空心球粒径约367.6 nm,壁厚约22.0 nm(见图1)。氮气吸附/脱附实验表明,二氧化硅空心微球的比表面、孔径和孔容分别为291.347 m2/g、3.415 5 nm和0.386 cm3/g。
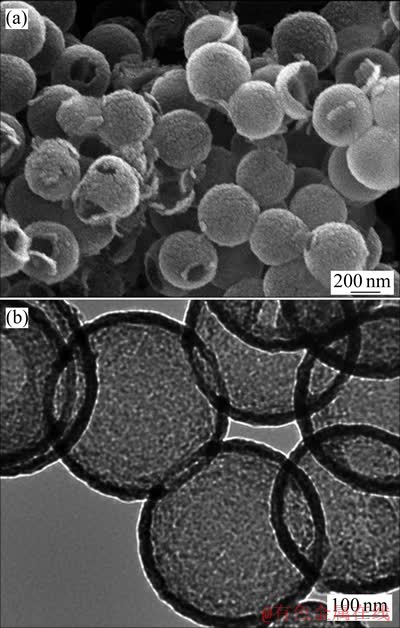
图1 二氧化硅空心球的SEM像及TEM像
Fig. 1 SEM(a) and TEM(b) images of hollow silica nanospheres
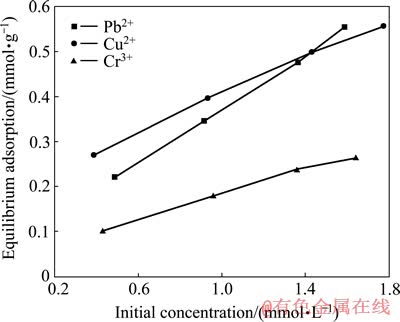
图2 重金属离子初始浓度对平衡吸附量的影响
Fig. 2 Effect of initial concentration of heavy metal ions on equilibrium adsorption
2.2 重金属离子吸附
二氧化硅空心微球加入Pb2+、Cu2+、Cr3+溶液后便很快分散均匀,快速达到吸附平衡,3h后溶液浓度基本不变。符合Langmuir单层吸附模型的特征。随着Pb2+、Cu2+、Cr3+初始浓度的增加,平衡吸附量均增加(见图2)。
平衡吸附量qe(mmol/g)可用下式计算:
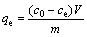 (1)
(1)
式中:c0、ce分别为初始浓度和平衡浓度,mmol/L;V 为溶液体积,L;m为吸附剂质量,g。
将平衡数据用Langmuir线性方程拟合,可得到饱和吸附量(吸附容量)qmax和吸附常数b。Langmuir方程的线性表达式:
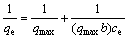 (2)
(2)
式中:qe为平衡吸附量,mmol/g;ce为平衡浓度,mmol/L;qmax 为饱和吸附量,mmol/g;b为Langmuir常数,g/mmol。
以1/qe为横坐标对1/ce作直线,通过斜率和截距即可求出qmax和b值(见表1)。由表1可知,二氧化硅中空微球对3种离子的吸附能力由大到小依次为Pb2+、Cu2+、Cr3+,这除了与离子的电荷密度有关外,还与离子的其它与电性作用相关属性有关。
表2列出了3种离子与电性作用相关的属性。表中r为离子半径[22],单位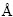 ;Xm为电负性;
;Xm为电负性;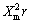 为共价指数,反映共价相互作用相对于离子相互作用的重要性;
为共价指数,反映共价相互作用相对于离子相互作用的重要性;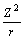 、
、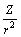 是与离子极化有关的参数,反映离子极化能力的强弱[23];Z*为离子的有效核电荷数,依据Slater’s Rule计算;
是与离子极化有关的参数,反映离子极化能力的强弱[23];Z*为离子的有效核电荷数,依据Slater’s Rule计算;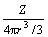 是离子的电荷密度。
是离子的电荷密度。
将这些属性与qmax变化规律关联可以看出:饱和吸附量qmax与Z/(4πr3/3)和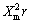 最具相关性:随着被吸附的离子电荷密度的减小,已吸附的离子间排斥小,吸附阻力减小,吸附量加大;这也可以用来解释图2中,随着初始浓度的增加,Pb2+(电荷密度最小)平衡吸附量增加较快的现象;当离子
最具相关性:随着被吸附的离子电荷密度的减小,已吸附的离子间排斥小,吸附阻力减小,吸附量加大;这也可以用来解释图2中,随着初始浓度的增加,Pb2+(电荷密度最小)平衡吸附量增加较快的现象;当离子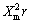 增大时,金属离子与吸附剂结合的共价性增强,造成原子轨道的重叠,使其具有部分共价键的属性,利于吸脱附平衡向吸附方向移动而增大吸附量。
增大时,金属离子与吸附剂结合的共价性增强,造成原子轨道的重叠,使其具有部分共价键的属性,利于吸脱附平衡向吸附方向移动而增大吸附量。
表1 线性拟合结果
Table 1 Results of linear fitting
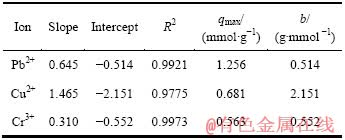
表2 不同离子参数的特征
Table 2 Parameters characteristics of different ions

对比表1和2还可看出:Langmuir常数b(g·mmol-1) 与离子的有效核电荷数Z*有明显的相关性。随着Z*增加,Langmuir常数b增大。这种现象可由Langmuir模型进行解释,由Langmuir模型可知:b=k1/k2表示吸附能力强弱,k1、k2分别为吸附和解吸速率常数。当被吸附离子电场强度较高时,吸附速度较快,k1较大,而脱附也越加困难;k2越小,b也相应增大。另外,离子电场强度较高,吸附后电场强度降低放出的能量也越高,也是对b增大的一种解释。
利用离子半径和qmax可计算出被吸附离子Pb2+、Cu2+、Cr3+的总截面积分别为34.206、6.308、5.068 m2/g,与吸附剂比表面积(291.347 m2/g)相比,最多也只占其1/10左右,这可用以下几个原因来解释:一是组成空心球二氧化硅有一部分是以硅氧四面体形式存在的,其中的氧原子键合趋近饱和,不易与被吸附离子结合,因而吸附点较少;二是被吸附的离子由于电荷密度上的差异(见表2),相互间作用力不同,电荷密度越高,相互作用越强,吸附点间距就越大,有效吸附点也就越少;三是溶液中固体的吸附较为复杂,被吸附的除了离子外,还有其溶剂化层,甚至是靠静电作用吸附的反离子,这也是吸附离子总截面远小于比表面的一个原因。另外,这种现象还进一步说明了:在吸附剂上对目标离子的吸附是有选择的单层吸附,具有化学吸附的特征,应该属于化学吸附。
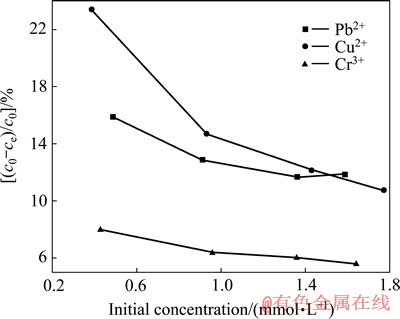
图3 重金属离子的初始浓度对去除效率的影响
Fig. 3 Effect of initial concentration of heavy metal ions on removal efficiency
图3所示为重金属离子的初始浓度对去除效果的影响。从图3中可以看出,重金属离子浓度较低时的去除效果好于重金属离子浓度较高时的;浓度对不同离子的去除效果影响不同,在重金属离子浓度较低时,离子去除率由大到小依次为Cu2+、Pb2+、Cr3+;在重金属离子浓度较高时,离子去除率由大到小依次为Pb2+、Cu2+、Cr3+。
这种差异的产生是因为:在低浓度区吸附过程为主导,此时离子的电场强度(Z)为主要影响因素;而在高浓度区,由于吸附量的增大,吸附结合力强度和阻力的问题变成了矛盾的主要方面,因此共价指数(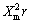 )和离子电荷密度(Z/(4πr3/3))成了主要影响因素。
)和离子电荷密度(Z/(4πr3/3))成了主要影响因素。
利用硝酸浸泡可使吸附剂表面形成带正电溶胶层(pH值小于其等电点,其等电点约为3),而与已吸附的重金属离子之间排斥力加大,削弱了其与吸附剂的结合力而被洗脱;洗脱液可经富集,再利用化学沉淀法,回收重金属。脱附、洗涤、干燥再生后的吸附剂,再次用于吸附实验,吸附能力没有明显下降,吸附剂可多次重复再生、使用。
3 结论
1) 利用Langmuir模型对吸附平衡数据进行拟合,结合吸附数据与离子属性相关性分析,发现重金属离子Pb2+、Cu2+、Cr3+在二氧化硅空心球上的吸附具有单层化学吸附的特征。
2) 吸附剂的饱和吸附量qmax主要和离子与吸附剂结合的共价指数及电荷密度有关,共价指数越高,电荷密度越小,饱和吸附量qmax越大。
3) 吸附常数b主要受离子的电场强度,即离子的有效核电荷数影响,在重金属离子浓度较低时离子的电场强度是影响除去效果的主要因素;而重金属离子浓度较高时,离子的电场强度作用不明显,主要影响因素是共价指数和离子电荷密度。
4) 在重金属离子浓度较低时,离子去除率由大到小依次为Cu2+、Pb2+、Cr3+;在重金属离子浓度较高时,离子去除率由大到小依次为Pb2+、Cu2+、Cr3+。吸附剂经脱附、洗涤、干燥后,可多次重复再生、使用。
REFERENCES
[1] SRIVASTAVA N K, MAJUMDER C B. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater[J]. Journal of Hazardous Materials, 2008, 151: 1-8.
[2] FU Feng-lian, WANG Qi. Removal of heavy metal ions from wastewaters: A review[J]. Journal of Environmental Management, 2011, 92: 407-418.
[3] 黄 凯, 李一飞, 焦树强, 朱鸿民. 柠檬酸活化赤泥对亚甲基蓝染料废水的吸附净化作用[J]. 中国有色金属学报, 2011, 21(12): 3182-3188.
HUANG Kai, LI Yi-fei, JIAO Shu-qiang, ZHU Hong-min. Adsorptive removal of methylene blue dye wastewater from aqueous solution using citric acid activated red mud[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(12): 3182-3188.
[4] XING Sheng-tao, ZHAO Mei-qing, MA Zi-chuan. Removal of heavy metal ions from aqueous solution using red loess as an adsorbent[J]. Journal of Environmental Sciences, 2011, 23(9): 1497-1502.
[5] SASSI M, BESTANI B, SAID A H, BENDERDOUCHE N, GUIBAL E. Removal of heavy metal ions from aqueous solutions by a local dairy sludge as a biosorbant[J]. Desalination, 2010, 262: 243-250.
[6] WANG Li-hua, LIN Chun-i. The removal of heavy metal ions from spiked aqueous solutions using solid wastes-Comparison of sorption capability[J]. Journal of the Taiwan Institute of Chemical Engineers, 2010, 41: 585-590.
[7] 郭学益, 梁 莎, 肖彩梅, 田庆华. MgCl2改性柑橘皮对水溶液中重金属离子的吸附性能[J]. 中国有色金属学报, 2011, 21(9): 2270-2276.
GUO Xue-yi, LIANG Sha, XIAO Cai-mei, TIAN Qing-hua. Adsorption of heavy metal ions from aqueous solutions by MgCl2 modified orange peel[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(9): 2270-2276.
[8] PEJIC B M, VUKCEVIC M M, PAJIC-LIJAKOVIC I D, LAUSEVIC M D, KOSTIC M M. Mathematical modeling of heavy metal ions (Cd2+, Zn2+and Pb2+) biosorption by chemically modified short hemp fibers[J]. Chemical Engineering Journal, 2011, 172: 354-360.
[9] 郭学益, 公琪琪, 梁 沙, 田庆华, 肖彩梅. 改性柿子生物吸附剂对铜和铅的吸附性能[J]. 中国有色金属学报, 2012, 22(2): 599-603.
GUO Xue-yi, GONG Qi-qi, LIANG Sha, TIAN Qing-hua, XIAO Cai-mei. Adsorption properties of modified persimmon biosorbent on Cu2+ and Pb2+[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(2): 599-603.
[10] 苏艳蓉, 柴立元, 杨志辉, 尤翔宇, 朱咏华. Pannonibacter phragmitetus T1菌对Pb2+的吸附特性[J]. 中国有色金属学报, 2011, 21(12): 3211-3217.
SU Yan-rong, CHAI Li-yuan, YANG Zhi-hui, YOU Xiang-yu, ZHU Yong-hua. Biosorption characteristics of Pb2+ by Pannonibacter phragmitetus T1[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(12): 3211-3217.
[11] AN Fu-qiang, GAO Bao-jiao, DAI Xin, WANG Min, WANG Xiao-hua. Efficient removal of heavy metal ions from aqueous solution using salicylic acid type chelate adsorbent[J]. Journal of Hazardous Materials, 2011, 192: 956-962.
[12] BAI Lan, HU Hui-ping, FU Weng, WAN Jia, CHENG Xi-liang, LEI Zhu-ge, XIONG Lei, CHEN Qi-yuan. Synthesis of a novel silica-supported dithiocarbamate adsorbent and its properties for the removal of heavy metal ions[J]. Journal of Hazardous Materials, 2011, 195: 261-275.
[13] ZHANG Lin-zi, ZHAO Yong-hong, BAI Ren-bi. Development of a multifunctional membrane for chromatic warning and enhanced adsorptive removal of heavy metal ions: Application to cadmium[J]. Journal of Membrane Science, 2011, 379: 69-79.
[14] MIZOGUCHI K, IDA J, MATSUYAMA T, YAMAMOTO H. Straight-chained thermo responsive polymer with high chelating group content for heavy metal ion recovery[J]. Separation and Purification Technology, 2010, 75: 69-75.
[15] HUANG Ji-quan, CAO Yong-ge, LIU Zhu-guang, DENG Zhong-hua, TANG Fei, WANG Wen-chao. Efficient removal of heavy metal ions from water system by titanate nanoflowers[J]. Chemical Engineering Journal, 2012, 180: 75-80.
[16] ZHANG Dong, ZHANG Chun-li, ZHOU Pin. Preparation of porous nano-calcium titanate microspheres and its adsorption behavior for heavy metal ion in water[J]. Journal of Hazardous Materials, 2011, 186: 971-977.
[17] LI Shou-zhu, YUE Xiu-li, JING Yuan-miao, BAI Shan-shan, DAI Zhi-fei. Fabrication of zonal thiol- functionalized silica nanofibers for removal of heavy metal ions from wastewater[J]. Colloids and Surfaces A: Physicochem Eng Aspects, 2011, 380: 229-233.
[18] 刘雪岩, 杨丽君, 金燕利, 张 蕾, 徐天赐, 李 娜. 纳米TiO2对镉(Ⅱ)的吸附性能[J]. 中国有色金属学报, 2011, 21(12): 2971-2977.
LIU Xue-yan, YANG Li-jun, JIN Yan-li, ZHANG Lei, XU Tian-ci, LI Na. Adsorption properties of nano-TiO2 for Cd(Ⅱ)[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(12): 2971-2977.
[19] AGRAWAL M, PICH A, GUPTA S, ZAFEIROPOULOS N E, SIMON P, STAMM M. Synthesis of novel tantalum oxide sub- micrometer hollow spheres with tailored shell thickness[J]. Langmuir, 2008, 24: 1013-1018.
[20] LIU Chun, GE Chen, WANG Ai-li, YIN Heng-bo, REN Min, ZHANG Yun-sheng, YU Long-bao, JIANG Ting-shun. Synthesis of porous hollow silica spheres using functionalized polystyrene latex spheres as templates[J]. Korean J Chem Eng, 2011, 28(6): 1458-1463.
[21] LIU Chun, YIN Heng-bo, WANG Ai-li1, WU Zhan-ao, WU Gang, JIANG Tao, SHEN Yu-tang, JIANG Ting-shun. Size-controlled preparation of hollow silica spheres and glyphosate release[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(5): 1161-1168.
[22] PAULING L. The nature of chemical bond and the structure of molecules and crystals[M]. 3rd ed. New York: Cornell University Press, 1960.
[23] MCCLOSKEY J T, NEWMAN M C, CLARK S B. Predicting the relative toxicity of metal ions using ion characteristics: Microtox (R) biolumineseence assay[J]. Environmental Toxicology and Chemistry, 1996, 15(10): 1730-1737.
(编辑 李艳红)
基金项目:江苏省教育厅基金资助项目(11KJB530002, CX10B-259Z);吉林省教育厅“十二五”规划项目(吉科教合字[2011]第140号)
收稿日期:2012-05-30;修订日期:2013-03-08
通信作者:殷恒波,教授,博士;电话:0511-88787591;E-mail: yin@ujs.edu.cn