文章编号:1004-0609(2010)11-2260-09
298.15 K下Li-Ti-H2O系φ—pH图及Li4Ti5O12的合成
邱文顺1,李运姣1,赵中伟1,霍广生1,习小明2,陈盼盼1
(1. 中南大学 冶金科学与工程学院,长沙 410083;2. 长沙矿冶研究院,长沙 410012)
摘 要:运用双参数模型和同系线性规律对Li2Ti3O7、Li4Ti5O12和Li4TiO4的标准生成吉布斯自由能进行估算,并绘制在298.15 K时,不同离子浓度下Ti-H2O系和Li-Ti-H2O系的φ—pH图。结果表明:在水溶液中,Li4Ti5O12在pH为4.1~13.7之间具有较大的热力学稳定区域,从热力学角度预测了从水溶液合成Li4Ti5O12 的可能性,并通过实验在pH为9~10之间,采用TiCl4水溶液强制水解法制备出纯相尖晶石Li4Ti5O12,验证水溶液中合成Li4Ti5O12的可行性。
关键词:Li-Ti-H2O系;Li4Ti5O12;热力学;合成
中图分类号:TM912.9 文献标志码:A
φ—pH diagram of Li-Ti-H2O system at 298.15 K and synthesis of Li4Ti5O12
QIU Wen-shun1, LI Yun-jiao1, ZHAO Zhong-wei1, HUO Guang-sheng1, XI Xiao-ming2, CHEN Pan-pan1
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Changsha Research Institute of Mining and Metallurgy, Changsha 410012, China)
Abstract: The standard Gibbs free energies of the formation of Li2Ti3O7,Li4Ti5O12 and Li4TiO4 were estimated using two-parameter model and congeneric linear rule. The φ—pH diagrams of Ti-H2O and Li-Ti-H2O systems at different concentrations were drawn at 298.15 K. The results show that Li4Ti5O12 has a thermodynamically stable area in a wide pH range between 4.1 and 13.7 in aqueous solution. This indicates a great possibility for the synthesis of Li4Ti5O12. The pure spinel Li4Ti5O12 is successfully obtained from an aqueous TiCl4 solution by forced hydrolysis at pH between 9 and 10. This indicates that the synthesis of Li4Ti5O12 in an aqueous solution is feasible.
Key words: Li-Ti-H2O system; Li4Ti5O12; thermodynamics; synthesis
具有层状结构的石墨是目前商品化锂离子电池的负极材料,其理论比容量高、循环性能好。但由于碳电极与金属锂的电极电位相近,当电池过充时,碳电极表面金属锂枝晶的形成易造成电池短路,从而引起着火和爆炸,安全性能有待进一步提高。随着新型锂离子电池正极材料LiFePO4的推广与应用,寻找能与之匹配的碳负极替代材料已成为当今锂离子电池研究领域的热门课题之一。
尖晶石型Li4Ti5O12是目前倍受人们青睐的锂离子电池负极材料之一[1-2]。由于Li4Ti5O12与其嵌Li产物Li7Ti5O12结构相似、晶格常数相近,其在锂离子的嵌入与脱出过程中结构基本不发生改变而具有优良的循环性能和使用寿命,被公认为“零应变”材料[3-4];且其电极电位比金属锂的电极电位高,可防止锂枝晶的形成,大大提高电池的安全性能。
目前,尖晶石Li4Ti5O12的合成方法很多,主要包括以TiO2为钛源的固相反应法和以钛有机醇盐为钛源的溶胶-凝胶法等[5]。与固相反应法相比,软化学合成方法因具有对实验设备要求简单、化学组成易于控制和生产过程操作弹性大等优点,在无机材料合成化学中占有重要地位。作为工业生产尖晶石Li4Ti5O12的原材料,由于TiCl4和TiOSO4等钛的无机盐原料来源广泛、价格便宜,比钛的有机醇盐具有更大的优势。已有研究[6-7]提出以钛冶金中间产品TiCl4为钛源,以LiOH为中和剂兼锂源,利用水解反应,在Ti(Ⅳ)水解生成水合TiO2沉淀的同时,使Li+嵌入其晶格中,成功地合成了尖晶石Li4Ti5O12。由于钛属于稀有金属,其有关化合物的热力学数据研究还很不完善。仅KELSALL和ROBBINS[8]对Ti-H2O系及Ti-F(-Fe)-H2O系进行了热力学分析,考察浸出钛铁矿的热力学条件。然而,作为新型先进材料的锂钛复合氧化物种类繁多而且复杂,其热力学研究还很不完善,有关生成自由能等热力学数据基本处于空白阶段,这就使得Li-Ti-O系化合物(如Li4Ti5O12等)的合成因缺乏必要的热力学指导而具有一定的盲目性。
本文作者在查阅大量文献的基础上,对Li-Ti-H2O系进行热力学分析,在运用双参数模型和同系线性规律对Li2Ti3O7、Li4Ti5O12和Li4TiO4的标准生成焓和标准生成吉布斯自由能进行估算的基础上,绘制了Ti-H2O系和Li-Ti-H2O系φ—pH图,对水溶液中合成Li4Ti5O12的条件进行预测,并通过实验验证水溶液中合成Li4Ti5O12的可行性。
1 Li-Ti-H2O系的热力学分析
1.1 锂钛复合氧化物标准生成吉布斯自由能的估算
在298.15 K下,已报道的与Li-Ti-H2O系有关的稳定化合物或离子的热力学参数见表1。所涉及的锂
表1 Li-Ti-H2O系中各反应组分在298.15 K下的热力学 数据
Table 1 Thermodynamic data of species in Li-Ti-H2O system at 298.15 K

钛复合氧化物Li4Ti5O12、Li4TiO4和Li2Ti3O7的吉布斯自由能函数至今未见报道。本文作者利用估算精度高、误差小的双参数模型[10-11]先估算出锂钛复合氧化物标准生成焓(所用参数见表2),然后根据化学中广泛应用的同系线性规律[12]估算所缺乏的标准生成吉布斯自由能值。估算时采用的其它热力学参数列于表3和4。
表2 298.15 K下锂钛复合氧化物标准生成焓估算所用参 数[10-11]
Table 2 Parameters used for estimation of standard enthalpies of formation for lithium-titanium complex oxides at 298.15 K[10-11]

对于二元复合氧化物aMmOx?bNnOy,可将其看作是简单氧化物MmOx和NnOy反应而成,a和b分别为其反应系数。令反应aMmOx+bNnOy=aMmOx·bNnOy的焓变为?rHΘ,则aMmOx?bNnOy的标准生成焓?fHmΘ与?rHΘ的关系为
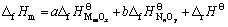 (1)
(1)
式中: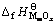 和
和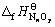 分别为MmOx和NnOy的标准生成焓,kJ·mol-1。
分别为MmOx和NnOy的标准生成焓,kJ·mol-1。
假定aMmOx·bNnOy的焓变?rHΘ由两部分组成,一部分为简单氧化物的贡献,另一部分为简单氧化物间的相互作用,其表达式如下:
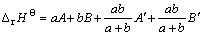 (2)
(2)
式中:A和A′为MmOx的参数;B和B′为NnOy的参数。
由于每个氧化物均含有两个参数,因此,将此模型称为“线性双参数模型”。模型中的参数可利用已知二元复合氧化物的?rHΘ通过回归方法求得。当该模型用于估算的|?rHΘ|>100 kJ·mol-1时,误差较小;而对于|?rHΘ|<100 kJ·mol-1的数据则偏差较大,此时可采用对数双参数模型:
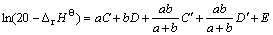 (3)
(3)
式中:C和C′为MmOx的参数;D和D′为NnOy的参数;E为常数。
由表2所列数据,结合式(1)~(3),求得锂钛复
表3 298.15 K时锂钛复合氧化物标准生成吉布斯自由能估算所用参数[13]
Table 3 Parameters used for estimation of standard Gibbs free energies of formation for lithium-titanium complex oxides at 298.15 K[13]
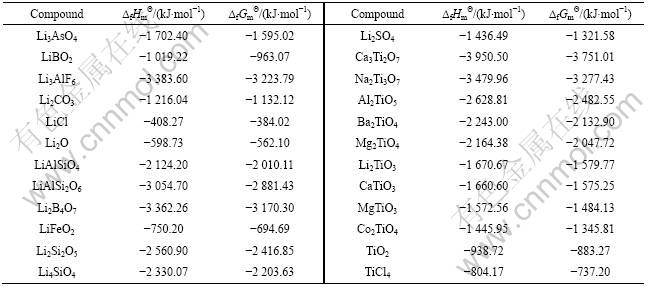
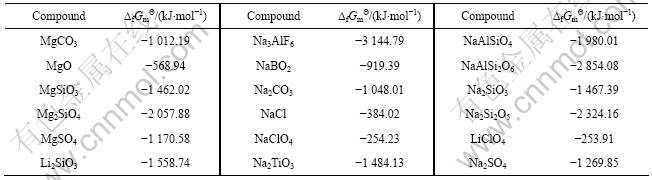
表4 298.15 K时锂钛复合氧化物标准生成吉布斯自由能估算所用参数[13]
Table 4 Parameters used for estimation of standard Gibbs free energies of formation for lithium-titanium complex oxides at 298.15 K
合氧化物(Li2Ti3O7、Li4Ti5O12、Li4TiO4)的标准生成焓分别如下:
Li2O+3TiO2 Li2Ti3O7
Li2Ti3O7
 (Li2Ti3O7)=-3 558.09 kJ?mol-1 (4)
(Li2Ti3O7)=-3 558.09 kJ?mol-1 (4)
2Li2O+5TiO2 Li4Ti5O12
Li4Ti5O12
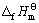 (Li4Ti5O12)=-6 170.06 kJ?mol-1 (5)
(Li4Ti5O12)=-6 170.06 kJ?mol-1 (5)
2Li2O+TiO2 Li4TiO4
Li4TiO4
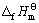 (Li4TiO4)=-2 342.39 kJ?mol-1 (6)
(Li4TiO4)=-2 342.39 kJ?mol-1 (6)
根据同系线性规律,首先将锂钛复合氧化物看作钛化合物估算其标准生成吉布斯自由能。利用表3的热力学数据,将各种钛化合物的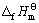 对
对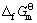 作图(见图1),可以看出数据点几乎落在一条直线上,说明
作图(见图1),可以看出数据点几乎落在一条直线上,说明
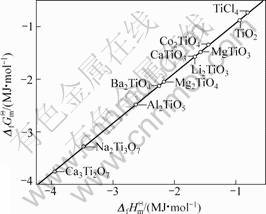
图1 各种钛盐的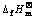 与
与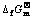 的线性关系
的线性关系
Fig.1 Linear relationship between 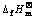 and
and 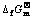 of various titanium salts
of various titanium salts
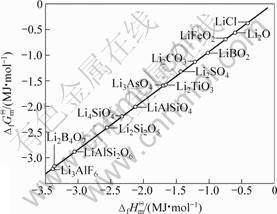
图2 各种锂盐的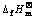 与
与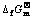 的线性关系
的线性关系
Fig.2 Linear relationship between 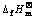 and
and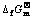 of various lithium salts
of various lithium salts
两函数之间存在线性关系,其直线方程如下
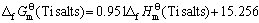 (7)
(7)
其线性相关系数R=0.999 3。利用双参数模型估算所得到的各种锂钛复合氧化物的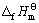 值,可计算求得Li2Ti3O7、Li4Ti5O12、Li4TiO4的标准生成吉布斯自由能分别为
值,可计算求得Li2Ti3O7、Li4Ti5O12、Li4TiO4的标准生成吉布斯自由能分别为
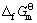 (Li4Ti5O12)=-5 855.43 kJ·mol-1 (8)
(Li4Ti5O12)=-5 855.43 kJ·mol-1 (8)
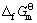 (Li2Ti3O7)=-3 370.19 kJ·mol-1 (9)
(Li2Ti3O7)=-3 370.19 kJ·mol-1 (9)
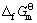 (Li4TiO4)=-2 213.48 kJ·mol-1 (10)
(Li4TiO4)=-2 213.48 kJ·mol-1 (10)
若将锂钛复合氧化物视为锂化合物重新进行估算, 将各种锂化合物的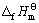 对
对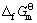 作图,得一条直线(见图2),其方程如下:
作图,得一条直线(见图2),其方程如下:
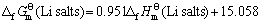 (11)
(11)
其线性相关系数R=0.999 9,结合双参数模型估算的各种锂钛复合氧化物的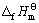 值,可以得到:
值,可以得到:
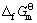 (Li4Ti5O12)=-5 849.52 kJ·mol-1 (12)
(Li4Ti5O12)=-5 849.52 kJ·mol-1 (12)
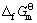 (Li2Ti3O7)= -3 366.87 kJ·mol-1 (13)
(Li2Ti3O7)= -3 366.87 kJ·mol-1 (13)
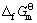 (Li4TiO4)= -2 211.36 kJ·mol-1 (14)
(Li4TiO4)= -2 211.36 kJ·mol-1 (14)
由此可见,将锂钛复合氧化物视为钛化合物或锂化合物估算所得的标准生成吉布斯自由能数值的误差很小,其平均值分别如下:
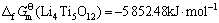 (15)
(15)
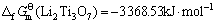 (16)
(16)
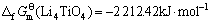 (17)
(17)
上述两种途径对标准生成吉布斯自由能的估算所得数值误差虽然很小,但均有赖于双参数模型估算标准生成焓。为检验建立在统计学基础上的双参数模型的可靠性,采用另外一种完全不依赖双参数模型的方法分别估算Li2Ti3O7和Li4TiO4的标准生成吉布斯自由能。
首先,根据同系线性规律,利用表3所列的热力学数据,将各种锂化合物与其对应的镁化合物标准生成吉布斯自由能作图(见图3),得到拟合直线方程如下:
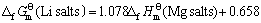 (18)
(18)
其线性相关系数R=0.997 0。由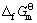 (Mg2TiO4)= -2 047.72 kJ·mol-1,求得:
(Mg2TiO4)= -2 047.72 kJ·mol-1,求得:
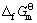 (Li4TiO4)=-2 207.02 kJ·mol-1 (19)
(Li4TiO4)=-2 207.02 kJ·mol-1 (19)
此值与双参数模型估算值(式(17))相比,绝对误差为5.39 kJ·mol-1,相对误差仅0.24%。
其次,将各种锂化合物与其对应的钠化合物标准生成吉布斯自由能对应作图(见图4),可以看出数据点排成一条直线,两函数之间具有良好的线性关系。其直线方程如下:
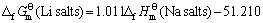 (20)
(20)
其线性相关系数R=0.999 2。由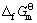 (Na2Ti3O7)=
(Na2Ti3O7)=

图3 镁盐与锂盐的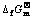 间的同系线性关系
间的同系线性关系
Fig.3 Congeneric linear relationship between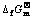 of magnesium salts and
of magnesium salts and 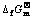 of lithium salts
of lithium salts
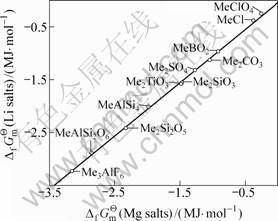
图4 钠盐与锂盐的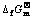 间的同系线性关系
间的同系线性关系
Fig.4 Congeneric linear relationship between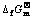 of sodium salts and
of sodium salts and 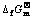 of lithium salts
of lithium salts
-3 277.43 kJ·mol-1,求得:
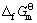 (Li2Ti3O7)=-3 363.58 kJ·mol-1 (21)
(Li2Ti3O7)=-3 363.58 kJ·mol-1 (21)
其与双参数模型估算值(见式(16))相比,绝对误差为-4.94 kJ·mol-1,相对误差仅-0.15%。
综上所述,取不同估算方法所得结果的平均值,可得298.15 K下有关锂钛复合氧化物的标准生成吉布斯自由能如下:
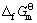 (Li4Ti5O12)=-5 852.48 kJ·mol-1 (22)
(Li4Ti5O12)=-5 852.48 kJ·mol-1 (22)
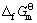 (Li2Ti3O7)=-3 366.05 kJ·mol-1 (23)
(Li2Ti3O7)=-3 366.05 kJ·mol-1 (23)
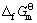 (Li4TiO4)=-2 209.72 kJ·mol-1 (24)
(Li4TiO4)=-2 209.72 kJ·mol-1 (24)
1.2 热力学平衡计算
对于金属-水系中可能存在的反应,可以分为以下3种类型:
1) 无电子得失的水解-中和反应;
2) 有电子得失的氧化-还原反应;
3) 氧化还原与水解中和反应共存。
第1类反应可表示为:2Mn++nH2O=M2On+2nH+,其pH与其离子活度之间的关系通过平衡常数K求得:
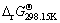 =-2.303RT lgK (25)
=-2.303RT lgK (25)
令H+离子活度系数为1,则有 ,于是有:
,于是有:
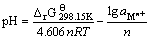 (26)
(26)
第2类反应为半电池还原-氧化反应:aA+bB+ze= cC+dD,其电位计算公式如下:
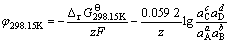 (27)
(27)
第3类反应可表示为aA+bB+ze+hH+=cC+dD,在H+离子活度系数为1时,其电位计算公式表示如下:
 (28)
(28)
基于上述3类反应的电位φ的计算方法,根据逐级反应原理以及Ti-H2O系与Li-Ti-H2O系中可能存在的反应,可得到在298.15 K下体系内各平衡反应的φ和pH表达式,如表5所列。
1.3 Ti-H2O系和Li-Ti-H2O系的φ—pH图
为绘制Ti-H2O系和Li-Ti-H2O系φ—pH图,作了以下假设:
1) 以离子浓度代替离子活度;
2) 在水溶液中,Ti2+不能稳定存在[8],因此不予考虑;
3) 水溶液中能稳定存在的Ti3+、TiO2+和TiO22+等离子,不考虑其络合作用;
4) 对钛的氧化物仅考虑TiO2和Ti2O3,不考虑非计量比的Ti3O5和Ti4O7等;
5) 不考虑Li2Ti3O7的存在。虽然锂钛复合氧化物能稳定存在的有4种,即Li4TiO4、Li2TiO3、Li4Ti5O12和Li2Ti3O7。但是Li2Ti3O7只在高温下存在,温度降低则会分解为尖晶石Li4Ti5O12和TiO2[14]。
基于以上假设,列出有关反应及其φ和pH的关系(见表5)。根据表5中φ和pH的关系,在考虑无水钛氧化物(Anhydrous oxide)和水合钛氧化物(Hydrous oxide)两种情况下分别绘制了Ti-H2O和Li-Ti-H2O系在不同离子浓度(0.1 mol·L-1和1.0 mol·L-1)的φ—pH图,如图5和6所示。
由图5(a)可知,在考虑钛氧化物为无水状态时,TiO2占据φ—pH图中绝大部分区域,表明其结构相当稳定。即使在pH值为-2的强酸性条件下也看不到TiO2+离子的优势区域。而Ti2O3的稳定区域很小,且只在还原性气氛下存在,在酸性溶液中则溶解得到Ti3+。在更低的电位下,Ti2O3和Ti3+被还原成TiH2。
然而,在考虑水合钛氧化物时,由于水合TiO2的稳定性比无水TiO2的差,因此,认为水合钛氧化物更有可能是离子水解时生成的产物。在101 325 Pa的H2和O2下,当[H+]≥1.0 mol·L-1时水溶液中出现了
表5 Ti-H2O系和Li-Ti-H2O系的平衡反应式及在298.15 K时的φ—pH计算式
Table 5 Equilibrium reactions of Ti-H2O and Li-Ti-H2O systems and φ—pH equations at 298.15 K

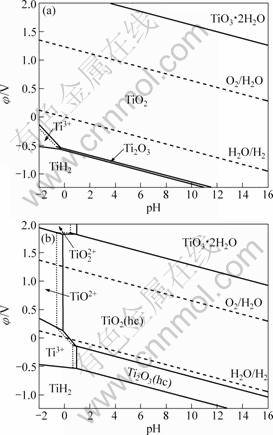
图5 298.15 K时Ti-H2O系在不同离子浓度下的φ—pH图
Fig.5 φ—pH diagrams of Ti-H2O system at different ions concentrations at 298.15 K (Solid line: [Ti3+]=[TiO2+]= [TiO22+]=0.1 mol?L-1; dashed line: [Ti3+]=[TiO2+]=[TiO22+]=1.0 mol?L-1): (a) Anhydrous oxide,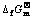 (TiH2)= -80.30 kJ·mol-1; (b) Hydrous oxide,
(TiH2)= -80.30 kJ·mol-1; (b) Hydrous oxide,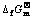 (TiH2)= -80.30 kJ·mol-1
(TiH2)= -80.30 kJ·mol-1
TiO2+和Ti3+离子,如图5(b)所示。与图5(a)相比,Ti3+离子和水合Ti2O3的区域扩大。在更高的电位下则出现TiO22+离子和TiO3·2H2O的稳定区。HTiO3-作为更高碱性条件下存在的钛阴离子,因其平衡线在pH=17.0处(见表5),因此不在此φ—pH图上显示。
如图6(a)所示,在考虑无水钛氧化物的情况下,在Ti-H2O中引入Li+后,在pH值为11.5~13.4(离子浓度0.1 mol·L-1)之间的水溶液中出现了Li4Ti5O12稳定区域。且随着pH值的增大,稳定区域依次为Li2TiO3和Li4TiO4。Li4TiO4作为更高碱性条件下存在锂钛复合氧化物,因其平衡线在pH=18.0,因此,其稳定区域在此φ—pH图上未显示。
在考虑水合TiO2的情况下,若不考虑锂钛复合氧化物的水合态,对于如下反应式:
4Li++5TiO2(hc)+2H2O=Li4Ti5O12(hc)+4H+ (29)
则有pH=-4.1383-lg[Li+],该pH值远远负于TiO2+
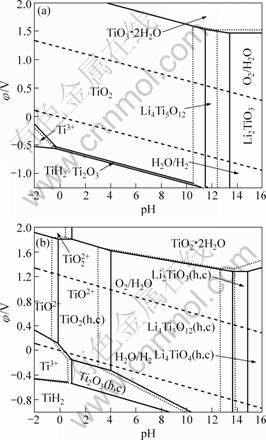
图6 298.15 K时Li-Ti-H2O系在不同离子浓度下的φ—pH图
Fig.6 φ—pH diagrams of Li-Ti-H2O system at different ions concentrations at 298.15 K (Solid line: [Li+]=[Ti3+]=[TiO2+]= [TiO22+]=0.1 mol?L-1; dashed line: [Li+]=[Ti3+]=[TiO2+]= [TiO22+]=1.0 mol?L-1): (a) Anhydrous oxide,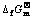 (TiH2)= -80.30 kJ·mol-1; (b) Hydrous oxide,
(TiH2)= -80.30 kJ·mol-1; (b) Hydrous oxide,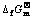 (TiH2)= -80.30 kJ·mol-1
(TiH2)= -80.30 kJ·mol-1
离子与水合TiO2的平衡pH值(pH=-0.592 8-0.5× lg [TiO2+]),这就意味着Li4Ti5O12的稳定区域将吞噬整个水合TiO2的稳定区域,这与实验事实不相符。
因此,在考虑水合TiO2的情况下,对锂钛复合氧化物相应地也考虑其水合状态[8]。无水TiO2的标准生成吉布斯自由能为 888.80 kJ·mol-1,比2个结合水的水合TiO2的-821.30 kJ·mol-1[15]要负67.50 kJ·mol-1。因此,假定水合Ti化合物每失去一个结合水,其吉布斯自由能变化为-33.75 kJ·mol-1。对水合态的Li4Ti5O12、Li2TiO3和Li4TiO4的结合水数依次考虑为5、1和0,结合无水态锂钛复合氧化物的标准生成吉布斯自由能,从而得到水合Li4Ti5O12、Li2TiO3和Li4TiO4的标准生成吉布斯自由能分别为-5 683.73、-1 544.05和 -2 209.72 kJ·mol-1。由此绘制298.15 K下水合氧化物Li-Ti-H2O系的φ—pH图,如图6(b)所示。与无水状态相比,水合TiO2不如无水TiO2稳定,因此更有利于Li+的嵌入,得到Li4Ti5O12的产物。其稳定区域很大,在pH为4.1~13.7范围内均可稳定存在。同时,在较高pH(>13.7)下出现了Li2TiO3的稳定区,在更高的pH(>14.8)下出现了Li4TiO4的稳定区。
2 Li4Ti5O12的水解合成验证
由图6(b)可知,在水溶液中,以TiO2+存在的Ti(Ⅳ)先水解得到水合TiO2(TiO2·xH2O)。由于结构的不稳定性,在一定的Li+活(浓)度及pH值下,Li+能嵌入水合TiO2中,生成Li4Ti5O12。从热力学角度考虑,当Li+活(浓)度为0.1 mol·L-1、pH=4.1时,溶液中由水合TiO2嵌Li生成Li4Ti5O12的反应恰好达到平衡,此时,溶液中水合TiO2和Li4Ti5O12两相共存。之后,随着pH的增大,进入Li4Ti5O12的稳定区;pH继续增大至13.7处后,进入Li2TiO3相稳定区;当pH达到14.8时,开始出现Li4TiO4的稳定区。
TiO2具有两种最典型的晶体结构:金红石(Rutile)和锐钛矿(Anatase)。晶体结构的不同使金红石和锐钛矿表现出不同的物理化学性质。相比而言,锐钛矿具有较小的密度,其原子的排列要比金红石疏松得多,因而,更有利于嵌入Li+而形成Li4Ti5O12[16]。在pH较低(pH<4.1)时,Ti(Ⅳ)水解生成金红石TiO2,由于其结构紧密,Li+不易嵌入而不能得到Li4Ti5O12,随着pH的增加,结构紧密的金红石量减少,原子排列疏松的锐钛矿增多[17],使得Li+离子的嵌入变易,Li+的嵌入量逐渐增多,形成的Li4Ti5O12相也越来越多。然而,随着pH的继续升高,Li+离子嵌入量继续增大,最终得到Li与Ti摩尔比更高的Li2TiO3,甚至出现Li4TiO4。因此,根据上述φ—pH图,25 ℃时,在TiCl4水解合成Li4Ti5O12的实验中,当离子浓度为1.0 mol·L-1时,pH应控制在3.0~12.8之间为宜。
在90 ℃和不断搅拌的条件下,将TiCl4(分析纯,天津市科密欧化学试剂有限公司生产)水溶液加入到LiOH?H2O(分析纯,天津市科密欧化学试剂有限公司生产)溶液中,充分反应3 h,控制反应体系平衡pH分别为9.09、10.15和11.31,得到白色沉淀经过滤后于105 ℃下真空干燥得到前躯体,分析Li和Ti的含量,得前驱体中Li与Ti摩尔比(n(Li)/n(Ti),见表6)。在Li4Ti5O12中,按化学计量其Li与Ti摩尔比为0.8,而合成的前驱体中,n(Li)/n(Ti)>0.9,表明前驱体中已嵌入足够量的Li。将前驱体在空气中于800 ℃下热处理6 h的样品,采用日本理学D/max-rA X射线衍射仪(Rigaku公司生产,日本)进行物相分析,其结果如图7所示。其中在pH=9.0和10.15下合成的两个样品的特征峰与Li4Ti5O12标准卡片上的主要特征峰完全吻合,表明在此实验条件下已经获得晶型结构完整的Li4Ti5O12产物。应用MDI Jade 软件对X射线衍射数据进行拟合,并根据式(30)计算Li4Ti5O12物相的纯度(P),其结果列于表6中。从图7和表6可以看出,在pH为9~10下合成的样品Li4Ti5O12纯度较高(Li4Ti5O12含量大于98%),XRD谱上未检测到其他的杂相峰;但在较高的pH(pH为11.31)下获得的样品以尖晶石Li4Ti5O12为主,含有一定量的Li2TiO3杂相。纯度的计算公式如下:
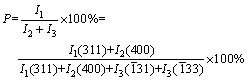 (30)
(30)
式中:I1(311)为Li4Ti5O12的(311)晶面的峰强度;I2(400)为Li4Ti5O12的(400)晶面的峰强度;I3( 31)为I(Li2TiO3)的(
31)为I(Li2TiO3)的( 31)晶面的峰强度;I3(
31)晶面的峰强度;I3( 33)为I(Li2TiO3)的(
33)为I(Li2TiO3)的( 33)晶面的峰强度。
33)晶面的峰强度。
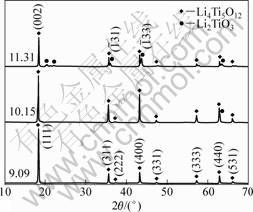
图7 不同平衡pH时产物的XRD谱
Fig.7 XRD patterns of products at different equilibrium pH
表6 前驱体Li/Ti摩尔比及溶液平衡pH值对产物Li4Ti5O12相纯度的影响
Table 6 Effect of molar ratio of Li to Ti and solution equilibrium pH on phase purity (P) of Li4Ti5O12 product

上述实验在pH为9~10 范围内合成了尖晶石Li4Ti5O12,验证了前述热力学分析的可靠性,同时显示了从TiCl4水溶液制备尖晶石Li4Ti5O12的技术可行性。
3 结论
1) 运用双参数模型和同系线性规律对Li2Ti3O7、Li4Ti5O12和Li4TiO4的标准生成吉布斯自由能进行估算,并绘制出298.15K时,不同离子浓度的Ti-H2O和Li-Ti-H2O系的φ—pH图。在101 325 Pa的H2和O2下,Ti-H2O系主要以TiO2的形式存在,TiO2+和Ti3+只在强酸性条件下存在。引入Li+后,随着pH值的升高,化合物的稳定区域依次为TiO2、Li4Ti5O12、Li2TiO3和Li4TiO4。
2) 在计算条件下,Li4Ti5O12在水溶液中存在较大的热力学稳定区域,且在水合条件下得到的稳定区域(对离子浓度0.1 mol?L-1, pH在4.1~13.7之间)比无水条件下得到的稳定区域(pH在11.5~13.4之间)要大得多,吞噬大部分水合TiO2的稳定区以及一部分水合Ti2O3的稳定区。实验结果证实这一热力学分析的正确性和可靠性,在90℃的碱性LiOH溶液中,TiCl4可以水解合成尖晶石Li4Ti5O12。
REFERENCES
[1] GUREFI A, CHAREST P, KINOSHITA K, PERRIER M, ZAGHIB K. Nano electronically conductive titanium-spinel as lithium-ion storage negative[J]. Journal of Power Source, 2004, 126(1/2): 163-168.
[2] KIM D H, AHN Y S, KIM J. Polyol-mediated synthesis of Li4Ti5O12 nanoparticle and its electrochemical properties[J]. Electrochemistry Communications, 2005, 7(12): 1340-1344.
[3] OHZUKU T, UEDA A., YAMAMOTO N. Zero-Strain Insertion Material of Li[Li1/3Ti5/3]O4 for Rechargeable Lithium Cells[J]. J Electrochem Soc, 1995, 142(5): 1431-1435.
[4] AMATUCCI G G. Nanostructure lithium titanate electrode for high cycle rate rechargeable electrochemical cell[P]. US 7211350, 2002.
[5] 熊利芝, 何则强, 尹周澜, 陈启元. Li4Ti5O12/石墨复合材料的湿法制备与表征[J]. 中国有色金属学报, 2008, 18(1): 306-309.
XIONG Li-zhi, HE Ze-qiang, YIN Zhou-lan, CHEN Qi-yuan. Wet method preparation and characterization of Li4Ti5O12/graphite composite[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(1): 306-309.
[6] 李运姣, 邱文顺, 习小明, 陈盼盼. TiCl4 水溶液强水解合成Li4Ti5O12的研究[J]. 矿冶工程, 2009, 29(3): 78-82.
LI Yun-jiao, QIU Wen-shun, XI Xiao-ming, CHEN Pan-pan. Study on synthesis of Li4Ti5O12 by forced hydrolysis of TiCl4[J]. Mining and Metallurgical Engineering, 2009, 29(3): 78-82.
[7] 陈盼盼, 李运姣, 习小明, 邱文顺. 酸性条件下TiCl4水解法原位合成尖晶石Li4Ti5O12[J]. 金属材料与冶金工程, 2008, 6: 7-13.
CHEN Pan-pan, LI Yun-jiao, XI Xiao-ming, QIU Wen-shun. In-situ Synthesis of spinel Li4Ti5Ol2 by hydrolysis of TiCl4[J]. Metal Materials and Metallurgy Engineering, 2008, 36(6): 7-13.
[8] KELSALL G H, ROBBINS D J. Thermodynamics of Ti-H2O-F(-Fe) systems at 298K[J]. J Electroanal Chem, 1990, 283: 135-157.
[9] DEAN J A. 兰氏化学手册[M]. 尚久方, 译. 北京: 科学出版社, 1991: 1467-1532.
DEAD J A. Lange’s handbook of chemistry[M].SHANG Jiu-fang, transl. Beijing: Science Press, 1991: 1467-1532.
[10] 郭培民, 赵 沛. 双参数模型估算复合氧化物的标准生成焓[J]. 钢铁研究学报, 2007, 19(5): 25-29.
GUO Pei-min, ZHAO Pei. Estimation of standard enthalpies of formation of complex oxide using two-parameter model[J]. Journal of Iron and Steel Research, 2009, 19(5): 25-29.
[11] 郭培民, 赵 沛, 李正邦. 矿物炼钢[M]. 北京: 化学工业出版社, 2007: 14-37.
GUO Pei-min, ZHAO Pei, LI Zheng-bang. Mineral steel-refining[M]. Beijing: Chemical Industry Press, 2007: 14-37.
[12] 温元凯, 邵 俊. 离子极化导论[M]. 合肥: 安徽教育出版社, 1985: 247-280.
WEN Yuan-kai, SHAO Jun. The theory of ionic polarization[M]. Anhui: Anhui Education Press, 1985: 247-280.
[13] BARIN I. 纯物质热化学数据手册[M]. 程乃良, 牛四通, 译. 北京: 科学出版社, 2003: 1-1885.
BARIN I. Thermochemical data of pure substances[M]. CHEN Nai-liang, NIU Si-tong, transl. Beijing: Science Press, 2003: 1-1885.
[14] COMBA P, MERBACH A. The titanyl question revisited[J]. Inorg Chem, 1987, 26(8): 1315-1323.
[15] MILAZZO G, CAROLI S. Table of standard electrode potentials[M]. American: John Wiley and Sons Ltd, 1978: 174-179.
[16] KRTIL P, DINA F. Li insertion into Li-Ti-O spinels: voltammetric and electrochemical impedance spectroscopy study[J]. J Electrochemical Society A, 2001, 148(9): 1045-1048.
[17] LI Y J, GEORGE P D. Precipitation of nanosized titanium dioxide from aqueous titanium(Ⅳ) chloride solutions by neutralization with MgO[J]. Hydrometallurgy, 2008, 90(1): 26-33.
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(50774103)
收稿日期:2009-11-23;修订日期:2010-04-22
通信作者:李运姣,教授,博士;电话:0731-88830476;E-mail:yunjiaoli6601@hotmail.com,believe1000000@yahoo.com.cn