
Screening and characterization of Acidiphilium sp. PJH and its role in bioleaching
PENG Juan-hua(彭娟花), ZHANG Rui-yong(张瑞永), ZHANG Qian(张 倩),
ZHANG Li-min(张立民), ZHOU Hong-bo(周洪波)
Key Laboratory of Biometallurgy of Ministry of Education, School of Minerals Processing and Bioengineering,
Central South University, Changsha 410083, China
Received 20 September 2008; accepted 5 November 2008
Abstract: A Gram-negative, facultative autotrophic bacterium strain PJH was isolated from the acidic mine drainages sampled in Dexing Mine, Jiangxi Province, China. The isolate grew in a pH range of 1.0-6.0, with pH and temperature optima of 3.5 and 40 ℃, respectively. Spectroscopic experiments show that strain PJH can synthesize polyhydroxybutyrate (PHB). A phylogenetic analysis based on 16S rRNA sequences shows that the isolate belongs to the genus Acidiphilium. Strain PJH has the capability of leaching chalcopyrite. Bioleaching experiments show that it can improve the leaching rate of chalcopyrite by 10.3% when mixed with L. ferriphilium YTW315 after 35 d. Strain PJH has the potential in application to bioleach chalcopyrite with other mesophilic leaching microbes.
Key words: isolation; Acidiphilium sp.; phylogenetic analysis; bioleaching; chalcopyrite
1 Introduction
Bioleaching is an economical method for the recovery of metals from minerals, especially from low grade ores, which requires moderate capital investment with low operating cost. Furthermore, bioleaching is generally more environmentally friendly than conventional metal recovery processes such as concentration and smelting[1]. The prokaryotic acidophiles involved in the biooxidation of minerals cause natural leaching of sulfide minerals via direct or indirect mechanism[2]. A number of autotrophic or mixtrophic acidophilic microorganisms capable of oxidizing ferrous iron and sulfur have been isolated from acidic water samples[3]. Acidithiobacillus and Leptospirillium species present in bioleaching environment often [4-5], were both sensitive to organic compounds which inhibited the growth of these microbes. Whereas Acidiphilium species could remove this inhibition by metabolising the organic materials, consequently enhance the growth of the autotrophs and as a result improve the leaching rate of metal sulfides[6]. The capacity of Acidiphilium species to utilize a variety of substrates and to reduce Fe(Ⅲ) indicates that they might be of ecological significance in bioleaching environment. Furthermore, Acidiphilium species are the most abundant species in many commercial bioleaching operation sites, and they are believed to play a crucial role in the bioleaching processes[7].
In the present study, a newly isolated facultative autotrophic bacteria strain PJH was characterized. And bioleaching tests indicate that the new isolate has the potential of application in industry.
2 Experimental
2.1 Bacteria origin and isolation
Water samples taken from Dexing Mine, Jiangxi Province, China, were enriched with 9K medium containing 0.01% (w/v) yeast extract at 40 ℃ and initial pH 3.0. When the liquid enrichment culture became turbid, serial dilution technique was adopted to isolate the microorganisms onto agar 9K solid medium. The agar plates incubated at 40 ℃ for 7 d and inspected daily for microbial growth. Single milky colonies were carefully picked and streak cultures on solid medium were repeated at least five times. L. ferriphilum YTW315 (the accession number of 16S rRNA gene: EU733647) used in this experiment was isolated and conserved by our laboratory.
2.2 Culture media
Unless otherwise stated, strain PJH was cultivated in basal salts of modified 9K medium and 0.01% (w/v) yeast extract. Modified 9K medium consisted of the following compounds: (NH4)2SO4 3.0 g/L, MgSO4?7H2O 0.5 g/L, K2HPO4 0.5 g/L, KCl 0.1 g/L, Ca(NO3)2 0.01 g/L. 2.0 g/L glucose used as the energy source in 9K medium was replaced by organic and inorganic substrates. 0.01% yeast extract was added as a growth factor. The pH of the medium was adjusted to 3.5 with 10% (voluem fraction) H2SO4. Cultures were incubated in rotary shakers at the indicated temperatures.
2.3 Morphological observation
General cell morphology was studied under an phase-contrast microscope (Olympus, Japan). Gram staining was performed with a Gram stain reagent kit(Haitai biotech). Fine morphological features were revealed by transmission (TEM, JEOL JEM-1230) and scanning electron microscopy (SEM, JEOL JSM-6360 LV) as described previously[8].
2.4 Biochemical and physiological characteristics
2.4.1 pH and temperature optima
The optimum temperature for growth was obtained by determining bacterial growth in liquid medium at different temperatures, i.e., 25, 30, 35, 40, 45 and 50 ℃. And optimum pH for bacterial growth was determined at pH values ranging from 1.0 to 7.0. The growth was compared by estimating the amount of biomass under different conditions.
2.4.2 Sensitivity to antibiotics and tolerance to heavy metals
Tests for sensitivity to antibiotics and tolerance to heavy metals were carried out according to the previous report[9]. Different concentrations of ampicillin, chloramphenicol, neomycin, kanamycin, streptomycin, tetracycline and erythromycin, and varying concentrations of CuSO4, BaSO4, FeSO4, Fe2(SO4)3, ZnSO4, CoSO4, MnSO4, CdSO4, PbSO4 and NiSO4 were used in tests.
2.5 Amplification, sequencing and phylogenetic analysis of 16S rRNA genes
The DNA was extracted in accordance with the manufacturer’s instructions by DNA extraction kit (Bio Basic Inc.). The 16S rRNA gene was amplified by PCR using the primers: 27F: 5′-AGAGTTTGATCCTGGCT- CAG-3′, and 1492R: 5′-TACCTTGTTACGACTT-3′ [10]. The PCR program was 94 ℃ for 4 min, followed by 30 cycles of 94 ℃ for 45 s, 55 ℃ for 45 s, and 72 ℃ for 90 s and finally 72 ℃ for 10 min. The product of PCR was purified using an E.Z.N.A? Gel Extraction Kit (Omega Bio-Tek, Inc., USA) and cloned with pBS-T PCR Products Clone Kit (Tiangen Biotech Co. Ltd. Beijing, China) according to the method described by XIA et al[11]. Sequence identification was initially estimated by the BLASTN facility of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih. gov/blast/). All available subsets of 16S rRNA gene sequences were selected, analyzed and aligned with CLUSTALX 1.8, and the final phylogenetic tree was generated by MEGA 3.0.
2.6 Bioleaching experiments
Bioleaching experiments were performed by using one of the following inocula: pure culture of strain PJH, pure culture of L. ferriphilum YTW315, and mixed culture of L. ferriphilum YTW315 and strain PJH, and sterile controls. The mineral used in the experiments contained 75% chalcopyrite and the concentration was 3% (w/v). The medium was adjusted to pH 1.8 with sulfuric acid. The inocula was obtained by centrifugation and suspended in basal salts medium, and the cell density in the culture medium was 107 /mL after inoculation. Bioleaching tests were carried out in 250 mL flasks containing 100 mL medium. Flasks were maintained at 40 ℃ and shaken at 180 r/min. The concentration of copper and iron was determined by atomic absorption spectrometry (Hatichi Z-8000) within 35 d of incubation. The lost water in the medium was supplemented with sterilized deionized water after sampling each time.
The components of chalcopyrite used in experiments are listed in Table 1.
Table 1 Component analysis of chalcopyrite used in leaching tests (mass fraction, %)

3 Results and discussion
3.1 Isolation and morphological characterization
By streak plate method, a strain of facultative autotrophic microorganism was isolated. Colonies of the organism were circular, smooth, round and pink colored (Fig.1(a)). Single colonies appeared with 3 d at 40 ℃. The cells stained Gram-negatively were rod-shaped measuring 0.5-0.7 μm in width and 1.0-1.5 μm in length (Fig.1(b)). They occurred singly or in pairs and reproduced through binary fission without forming spores. Electron microscopy of cells showed that motile cells had single polar or subpolar flagellum (indicated with arrows in Fig.1(b)).
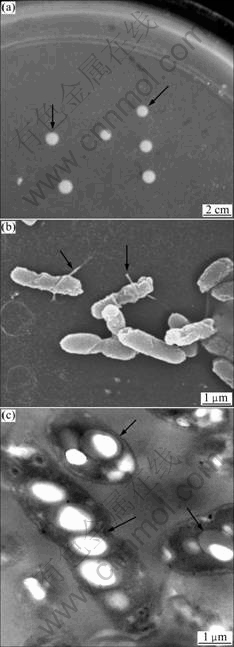
Fig.1 Photographs of colony of strain PJH (a) and electron micrographs of strain PJH ((b) SEM; (c) TEM)
Ultrathin-section electron microscopy indicated that cells of strain PJH had five or more electron-dense granules which might be PHB. The isolate obtained was designated as PJH.
3.2 Physiological and biochemical characteristics
3.2.1 PHB production
The polymer granules of isolate PJH were extracted and detected by the method described by YANG et al[12]. Spectroscopic experiments showed that the ultrasonic cell extract of strain PJH had a maximum at 244 nm (Fig.2). The FTIR spectra obtained for the samples confirmed the practically identical structure of the standard PHB and the biopolymers produced by strain PJH (Fig.3). The band found at 1 380 cm-1 is the equivalent for —CH3 groups, while the one found at 1 720 cm-1 corresponds to the stretching of the C=O bond. A series of intense bands located at 1 000-1 300 cm-1 correspond to the stretching of the C=O bond of the ester group. Also bands of minor relevance, such as those found at 3 440 cm-1, originated by terminal —OH groups or water adsorption onto the sample, are found in all spectra. All these bands are in full agreement with those observed by ROJAS et al[13]. This suggests that strain PJH has the potential of synthesizing biodegradable materials (PHB).
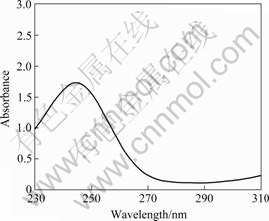
Fig.2 Absorption spectrum of sonicated cell extract from strain PJH
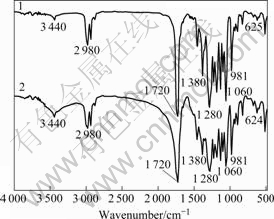
Fig.3 Fourier transform infrared spectroscopy: 1—Standard; 2—Cell extract of strain PJH
3.2.2 Optimal pH and temperature for growth
As shown in Fig.4, strain PJH grew at temperature ranging from 20 to 45 ℃, and no growth was observed at 4, 15 or 50 ℃. The optimal temperature was 40 ℃. The growth response of strain PJH to pH conditions is shown in Fig.5. Growth was observed over a pH range of 1.5- 5.0, and the optimal pH was 3.5.
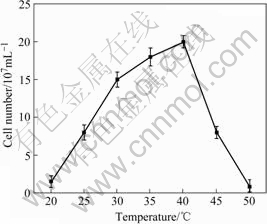
Fig.4 Effect of temperature on growth of strain PJH
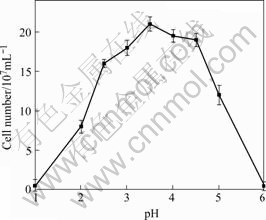
Fig.5 Effect of pH on growth of strain PJH
3.2.3 Growth on organic and inorganic substrates
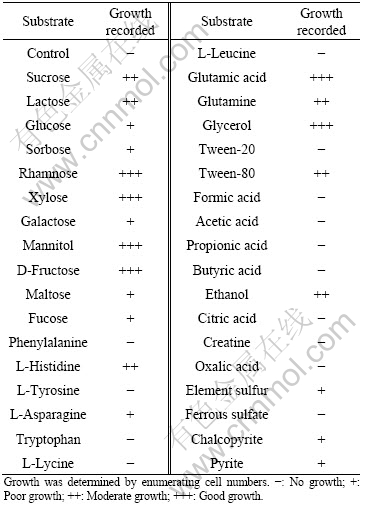
Strain PJH was not capable of growing in defined (single carbon source) inorganic or organic medium. The addition of small amounts of yeast extract to a defined medium was essential for cell growth. However, the growth is inhibited in the presence of 0.2% (w/v) yeast extract. The isolate used a wide range of substrates as carbon/energy sources, as indicated in Table 2. It can be inferred that strain PJH is facultative autotrophic bacterium.
Table 2 Growth of isolate PJH on various organic/inorganic compounds
3.2.4 Sensitivity to antibiotics
Strain PJH was sensitive to all antibiotics to some degree at the concentration tested. It had slight sensitivity to the following antibiotics: chloramphenicol (50 mg/L), neomycin (80 mg/L), kanamycin (50 mg/L), streptomycin (60 mg/L) and erythromycin (100 mg/L) that inhibited cell wall formation in microorganism. Tetracycline (5 mg/L) and ampicillin (5 mg/L) obviously inhibited the growth of isolate PJH.
3.2.5 Tolerance of heavy metals
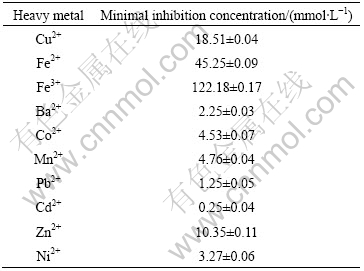
The minimal inhibition concentrations(MICs) of some heavy metals for strain PJH were determined (Table 3). The isolate tends to be more sensitive to the metals tested than other acidophiles such as Acidithiobacillus ferrooxidans[14]. The tolerance degree to ferric ion was found to be the greatest; in contrast, the growth of isolate PJH was inhibited in the presence of low concentration of cadmium ion.
Table 3 Tolerance of isolate PJH to some metals
3.2.6 Growth curve
According to the optimum growth parameters as mentioned above, the growth curve of isolate PJH at 40 ℃ and initial pH 3.5 was described. As shown in Fig.6, the growth curve followed the lag, logarithmic and stationary phases. The logarithmic phase was from 10 to 45 h. After being cultivated for about 45 h, the number of cells would reach the maximum (about 3.49×108 /mL). The generation time and the maximum specific growth rate (u max) are 7.89 h and 0.08 h-1, respectively.

Fig.6 Growth curve of strain PJH
3.3 Phylogenetic analysis
The phylogenetic position of the new isolate was evaluated by studying the 16S rRNA sequence information. A total of 1 397 nucleotides were sequenced. A neighbour-joining phylogenetic tree was constructed on the basis of the distance matrix data of the isolate PJH and several reference bacteria (Fig.7).

Fig.7 Distance matrix tree showing phylogenetic affiliations of new isolate PJH and referenced bacteria based on 16S rRNA sequences
Bootstrap values obtained with 1 000 bootstrap resamplings are given at branching points of interest. The DDBJ/EMBL/GenBank accession numbers of the 16S rRNA sequences used are shown in parentheses.
The new isolate was clustered in the genus Acidiphilium. The 16S rRNA sequences of the isolate
were most similar to the sequences of Acidiphilium multivorum, Acidiphilium cryptum and Acidiphilium organovorum with similarity of 99%. The phylogenic analysis of 16S rRNA clearly indicated that it should be classified into the genus Acidiphilium.
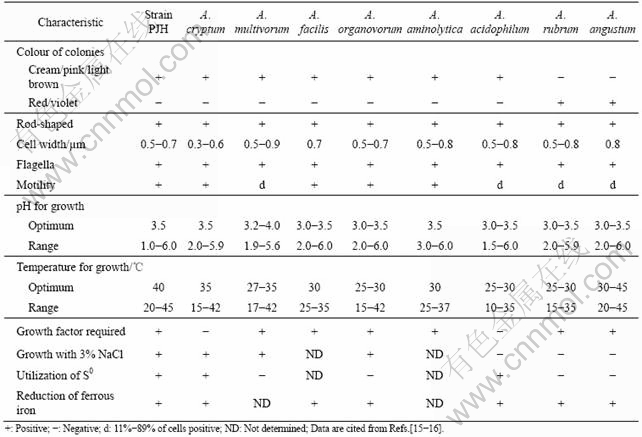
Morphological, physiological and biophysical traits of strain PJH and members of the genus Acidiphilium are listed in Table 4. With the morphological, biochemical, physiological characterizations and the analysis based on 16S rRNA gene, strain PJH should be classified into genus Acidiphilium.
Table 4 Morphological, physiological and biophysical traits of strain PJH and members of genus Acidiphilium
3.4 Effects of Acidiphilium sp. PJH on bioleaching of chalcopyrite
Comparison of chalcopyrite leaching at 40 ℃ by pure and mixed cultures of L. ferriphilum and Acidiphilium sp. PJH is shown in Fig.8. The copper released in the bioleaching experiment was much greater than that observed in the abiotic experiment (10.9% after 35 d). 16.2% of copper was released from chalcopyrite by pure culture of strain PJH after 35 d, and this result indicates that pure culture of Acidiphilium sp. PJH has limited ability of leaching chalcopyrite. Comparatively, 43.1% of copper was released from chalcopyrite by pure culture of L. ferriphilum YTW315. However, the leaching efficiency of chalcopyrite by mixed cultures of strain PJH and L. ferriphilum YTW315 could reach 53.4%, which indicates that Acidiphilium sp. PJH can improve chalcopyrite leaching efficiency of L. ferriphilum YTW315. This result is consistent with the previous work by OKIBE and Johnson[17].
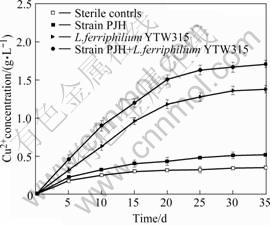
Fig.8 Copper extraction from chalcopyrite by Acidiphilium sp. PJH and L. ferriphilum YTW315
Bioleaching of chalcopyrite mainly includes reactions as follows[1]:
CuFeS2+4Fe3+→Cu2++2S0+5Fe2+ (1)
2Fe2++2H++0.5O2→2Fe3++H2O (2)
2S0+3O2+2H2O→2SO42-+4H+ (3)
There is also another possible mechanism of increasing the leaching rate of chalcopyrite directly by acid in the following reactions[18]:
CuFeS2+2H++0.5O2→Cu2++2S+Fe2++H2O (4)
3Fe3++2SO42-+6H2O+K+→KFe3(SO4)2(OH)6+6H+ (5)
The intermediary sulfur is prone to accumulate on the surface of ores and form sulfur-layer. The sulfur- layer can significantly inhibit the direct contact and reduce the efficiency of bioleaching of chalcopyrite[19]. In the pure culture of A. ferrooxidans, jarosite precipitation resulted in the decrease of soluble iron in the leaching solution, and formed a passivation layer on the mineral surface[20]. The passivation layer strongly inhibits the ferric iron reduction and subsequently decrease the copper leaching efficiency. In the leaching experiments, because strain PJH can utilize the sulfur and reduce the accumulation of jarosite precipitation[21], the leaching rates of chalcopyrite by mixed culture were significantly enhanced. Additionally, Acidiphilium sp. PJH can remove the organic materials produced by L. ferriphilum YTW315 and thus enhance the leaching efficiency of chalcopyrite. The leaching experiments indicate that Acidiphilium sp. PJH mixed with other mesophilic leaching microbes has the application potential in the bioleaching of chalcopyrite.
4 Conclusions
1) A facultative autotrophic bacteria strain PJH is isolated from Dexing Mine, Jiangxi Province, China. The optimum temperature and pH for strain PJH are 40 ℃ and 3.5, respectively. According to the investigation of the morphological, biochemical and physiological characteristics and the phylogenetic analysis based on 16S rRNA gene, PJH is identified as Acidiphilium sp..
2) Acidiphilium sp. PJH contains PHB and has the potential of synthesizing biodegradable materials.
3) Acidiphilium sp. PJH alone can leach chalcopyrite with 16.2% of copper extraction after 35 d. In the mixed culture, Acidiphilium sp. PJH can improve the chalcopyrite leaching efficiency of L .ferriphilium YTW315 by 10.3% after 35 d. As the optimum temperature is 40 ℃, Acidiphilium sp. PJH is worthy to focus further attention on the cooperative bioleaching with L. ferriphilum and other moderate mesophiles for extracting copper from chalcopyrite.
References
[1] ROHWERDER T, GEHRKE T, KINZLER K, SAND W. Bioleaching review (part A): Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation [J]. Applied Microbiology and Biotechnology, 2003, 63(3): 239-248.
[2] DOPSON M, AUSTIN C B, KOPPINEEDI P R, BOND P L. Growth in sulfidic mineral environments: Metal resistance mechanisms in acidophilic micro-organisms [J]. Microbiology, 2003, 149(88): 1959-1970.
[3] WATLING H R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review [J]. Hydrometallurgy, 2006, 84(1/2): 81-108.
[4] ROHWERDER T, GEHRKE T, KINZLER K. Bioleaching review (A): Progress in bioleaching fundamentals and mechanisms of bacterial sulfide oxidation [J]. Applied and Environmental Microbiology, 2003, 63(3): 239-248.
[5] NAOKO O, MARIEKIE G, HALLBERG B. Enumeration and characterization of acidophilic microorganisms isolated from a pilot plant stirred-tank bioleaching operation [J]. Applied and Environmental Microbiology, 2003, 69(4): 1936-1943.
[6] Johnson D B. Importance of microbial ecology in the development of new mineral technologies [J]. Hydrometallurgy, 2001, 59(2/3): 147-157.
[7] RAWLINGS D E, SILVER S. Mining with microbes [J]. BioTechnology, 1995, 13(88): 773-778.
[8] HIRAISHI A, URATA K, SATOH T. A new genus of marine budding phototrophic bacteria, Rhodobium gen. nov., which includes Rhodobium orientis sp. nov., and Rhodobium marinum comb. Nov [J]. International Journal of Systematic and Evolutionary Microbiology, 1995, 45(2): 226-234.
[9] Johnson D B, GHAURI M A, SAID M F. Isolation and characterization of an acidophilic, heterotrophic bacterium capable of oxidizing ferrous iron [J]. Applied and Environmental Microbiology, 1992, 58(5): 1423-1428.
[10] LIU Jian-she, XIE Xue-hui, XIAO Sheng-mu, WANG Xiu-mei, ZHAO Wen-jie, TIAN Zhuo-li. Isolation of Leptospirillum ferriphilum by single-layered solid medium [J]. J Cent South Univ Tech, 2007, 14(4): 467-473.
[11] XIA Jin-lan, PENG An-an, HE Huan. A new strain Acidithiobacillus albertensis BY-05 for bioleaching of metal sulfides ores [J]. Trans Nonferrous Met Soc China, 2007, 17(1): 168-175.
[12] YANG Yu, XU Ai-ling, ZHANG Yan-fei. Isolation and characteriation of a facultative autotrophic bacterial strain and its cellular polymer granules from acid mine drainage [J]. Journal of Wuhan University, 2007, 53(6): 753-758.
[13] ROJAS G B, MANOSALVA J L, LIENDO G. Caracterización a partir de la microscopía óptica de luzpolarizada, de las propiedades térmicas y la espectrocopía deltermoplástico biodegradable poli(hidroxibutirato) [J]. Rev Latinoam Metal Mater, 2000, 20: 47-53. (in French)
[14] RAWLINGS D E. Characteristics and adaptability of iron- and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates [J]. Microbial Cell Factories, 2005, 4(13): 1-5.
[15] KISHIMOTO N, KOSAKO Y, WAKAO N, TANO T. Transfer of Acidiphilium facilis and Acidiphilium aminolytica to the genus Acidocella gen. nov., and emendation of the genus Acidiphilium [J]. Systematic and Applied Microbiology, 1995, 18(1): 85-91.
[16] HIRAISHI A, NAGASHIMA KV, MATSUURA K. Phylogeny and photosynthetic features of Thiobacillus acidophilus and related acidophilic bacteria: Its transfer to the genus Acidiphilium as Acidiphilium acidophilum comb. nov [J]. International Journal of Systematic and Evolutionary Microbiology, 1998, 48(4): 1389-1398.
[17] OKIBE N, JOHNSON D B. Biooxidation of pyrite by defined mixed cultures of moderately thermophilic acidophiles in pH-controlled bioreactors: Significance of microbial Interactions [J]. Biotechnology and Bioengineering, 2004, 87(5): 574-583.
[18] SUZUKI I. Microbial leaching of metals from sulfide minerals[J]. Biotechnology Advances, 2001, 19(2): 119-132.
[19] SHI S Y, FANG Z H. Bioleaching of marmatite flotation concentrate with a moderately thermoacidophilic iron-oxidizing bacterial strain [J]. Minerals Engineering, 2005, 18(11): 1127-1129.
[20] STOTT M B, WATLING H R, FRANZMANN P D, SUTTON D. The role of iron-hydroxy precipitates in the passivation of chalcopyrite during bioleaching [J]. Minerals Engineering, 2000, 13(1/10): 1117-1127.
[21] PAIMENT A, LEDUC L G, FERRONI G D. The effect of the facultative chemolithotrophic bacterium Thiobacillus acidophilus on the leaching of low-grade Cu-Ni sulfide ore [J]. Geomicrobiology Journal, 2001, 18(2): 157-165.
Foundation item: Project(50621063) supported by the National Natural Science Foundation of China; Project(2004CB619204) supported by the National Basic Research Program of China
Corresponding authors: ZHOU Hong-bo; Tel: +86-731-8877216; E-mail: zhouhb@mail.csu.edu.cn
(Edited by PENG Chao-qun)