Effects of additives on nickel electrowinning from sulfate system
来源期刊:中国有色金属学报(英文版)2010年增刊第1期
论文作者:卢静 阳启华 张昭
文章页码:97 - 101
Key words:nickel electrowinning; sulfate system; boric acid; dodecyl sodium sulfate; pitting; cathode polarization
Abstract: In order to eliminate the pitting and improve the surface morphology of cathode nickel, the influence of additives of boric acid and dodecyl sodium sulfate (SDS) on the process of nickel electrowinning from sulfate system was studied by cathode polarization tests and nickel electrodeposition experiments. The experimental results show that the addition of boric acid can increase the cathode polarization while SDS can decrease the cathode polarization. Both boric acid and SDS are useful to eliminate the pitting on nickel deposits and improve the morphology of surface. Good deposit morphology with rare pitting and high current efficiency is favored by adding 10 g/L boric acid and 40 mg/L SDS in the electrolyte under the condition of nickel ion concentration of 80 g/L, sodium ion concentration of 10 g/L, pH of 3, current density of 220 A/m2 and temperature of 70 ℃.

LU Jing(卢 静), YANG Qi-hua(阳启华), ZHANG Zhao(张 昭)
School of Chemical Engineering, Sichuan University, Chengdu 610065, China
Received 6 July 2009; accepted 30 December 2009
____________________________________________________________________________________________
Abstract: In order to eliminate the pitting and improve the surface morphology of cathode nickel, the influence of additives of boric acid and dodecyl sodium sulfate (SDS) on the process of nickel electrowinning from sulfate system was studied by cathode polarization tests and nickel electrodeposition experiments. The experimental results show that the addition of boric acid can increase the cathode polarization while SDS can decrease the cathode polarization. Both boric acid and SDS are useful to eliminate the pitting on nickel deposits and improve the morphology of surface. Good deposit morphology with rare pitting and high current efficiency is favored by adding 10 g/L boric acid and 40 mg/L SDS in the electrolyte under the condition of nickel ion concentration of 80 g/L, sodium ion concentration of 10 g/L, pH of 3, current density of 220 A/m2 and temperature of 70 ℃.
Key words: nickel electrowinning; sulfate system; boric acid; dodecyl sodium sulfate; pitting; cathode polarization
____________________________________________________________________________________________
1 Introduction
Metal nickel can be produced from a variety of electrolytes, including chloride, sulfate systems or a mixed electrolyte of chloride and sulfate. The latter is often used for electrorefining matte from traditional matte-smelting operations, while the former two are acid systems used in hydrometallurgical processing and electrowinning[1]. Many researches have been done on nickel electrodeposition from chloride and chloride/sulfate systems[2-4]. Even though the existence of Cl- in chloride and chloride/sulfate systems can improve the activity of nickel matte electrode, increase the current efficiency and strengthen the process of nickel electrodeposition, Cl2 gas produced from the anode contaminates the environment and degrades the quality of the nickel deposit with high internal stress[5]. By comparison, nickel electrowinning from sulfate system has a friendly environment and the quality of nickel deposit can be improved with a lower internal stress. Because of these reasons, nickel electrowinning from sulfate system has a good development in recent years[6].
Usually, the deposition of nickel from sulfate electrolyte depends on pH more seriously than that from chloride system. The reduction of hydrogen ion not only decreases the cathode current efficiency, but also degrades the morphology of nickel surface with pitting generated from evolution and staying of hydrogen gas. There are two reasons. On one hand, hydrogen has a lower overpotential on nickel so that hydrogen ions tend to form hydrogen gas easily in nickel electrodeposition [7]. On the other hand, the sulfate electrolyte has a bad wetting property on cathode; and hydrogen gas can stay on the surface of cathode and is hard to depart from the surface of cathode[8]. In order to prevent pitting from forming on nickel deposit, the overpotential of hydrogen on nickel should be enhanced to reduce the generation of hydrogen gas and the wetting property of the electrolyte on nickel cathode should also be improved to allow little hydrogen gas to stay on the nickel surface. This can be resolved by adding some additives in the process of nickel electrodeposition to reduce the pitting on the surface of the nickel deposit. In recent years, additives have been studied more in zinc and copper electrowinning[9-10], while many researches about nickel electrowinning in sulfate system are mainly limited to technical studies, and the use of additives in sulfate system has been barely concerned[11-12]. The surface morphology of cathode nickel is an important standard measure of the nickel product[6]. In this work, the influence of additives boric acid and dodecyl sodium sulfate (SDS) on the process of nickel electrowinning from sulfate system was studied by cathode polarization tests and nickel electrodeposition experiments. Also, the mechanism of additives on improving the surface morphology was discussed.
2 Experimental
2.1 Solution preparation
All solutions were prepared by dissolving in distilled water with analytically pure regents except that the nickel hexahydrate (NiSO4?6H2O) was at the first industrial grade (HG/T2824—1997). The basic compositions of the electrolyte were NiSO4 and Na2SO4.
2.2 Cathode polarization test
The experiments were conducted in a glass cell with a three-electrode system, a Ni working electrode sheet (1 cm×1 cm), a Pt counter electrode (1.8 cm×1.8 cm) of 99.99% purity and a saturated calomel reference electrode (SCE). The surface of the working electrode was polished before each experiment with 400 and then 1200 grade silicon carbide paper to a mirror finish and then was rinsed with alcohol followed by pure water. The polarization curves were tested with a CHI602C model for scanning the potential at a rate of 1 mV/s and were recorded by a computer. All potential values in this work were vs SCE. The liquid junction potential and ohmic drop were compensated by salt bridge connecting the reference electrode and the working electrode. During the tests, the electrolytes were stirred at 20 r/min.
2.3 Electrodeposition experiment
The nickel deposit was prepared by electrodeposition experiment. It was conducted in a rectangular vessel of 8 cm×8 cm×20 cm. The electrolyte of 3 L was continuously circulated in a closed flow circuit through the vessel with a flow rate of 80 mL/min. The acidity of the electrolyte was regulated with NaOH and H2SO4 solution, and the pH was measured by the pH detector. Cathode blank was made of a pure titanium sheet of 8 cm long, 2.5 cm wide and 1 mm thick. The Pb/Ag alloy was used as anode with the dimensions of 10 cm long, 5 cm wide and 4 mm thick. The deposits were examined visually after electrodeposition for 7.5 h. The current efficiency was calculated by comparing the actual mass of the deposit to the theoretical mass discharged assuming that the nickel metal was the only cathode product.
3 Results and discussion
3.1 Effect of boric acid
Fig.1 shows the polarization curves with different boric acid concentrations under the condition of ρ(Ni2+)=120 g/L, ρ(Na+)=15 g/L, θ=65 ℃, pH=3, Fig.1(b) is the partial enlargement of Fig.1(a).
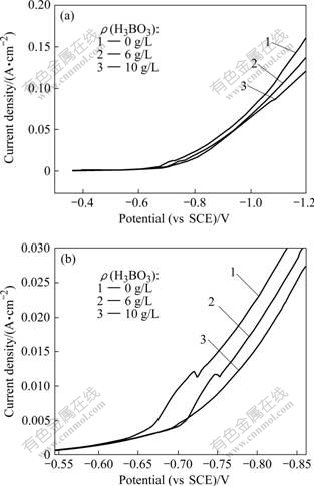
Fig.1 Effect of concentration of boric acid on polarization curves of nickel reduction under conditions of ρ(Ni2+)=120 g/L, ρ(Na+)=15 g/L, θ=65 ℃ and pH=3: (a) Polarization curves; (b) Partial enlargement of polarization curves
As seen in Fig.1(a), the polarization curves shifted right with the increase of boric acid concentration in the electrolyte. Therefore, the addition of boric acid in the electrolyte can increase the cathode polarization. Fig.1(b) shows the change of current density when the potential changed from -0.55 V to -0.85 V. In the absence of boric acid (Curve 1), Ni2+ began to deposit at around -0.665 V obviously. The nickel deposition potentials shifted negatively to -0.702 V (Curve 2 and 3) with boric acid of 6 g/L and 10 g/L in the electrolyte, respectively. The results indicate that the higher the boric acid concentration in electrolyte is, the more negative the nickel deposition potential is. The reason is that the boric acid can inhibit the deposition of Ni2+ obviously. In the absence of boric acid, when the potential shifted negatively to about -0.72 V, the increased slope of polarizations was caused by the discharge of H+. On Curve 1, a “sag” was formed because the current density slightly declined and then upturned significantly when the potential ranged from -0.719 V to -0.720 V. Curve 2 had the same change when the potential was around -0.746 V but this “sag” was smoother than that of Curve 1. With increasing the boric acid concentration up to 10 g/L, the “sag” almost disappeared. The cause might be that in the absence of boric acid in the electrolyte, the discharge of H+ near the cathode resulted in less H+ near the cathode. The pH value increased so as to form Ni(OH)2[13] that was absorbed on the surface of cathode, inhibiting Ni2+ discharging and Ni crystal growing in its formal way. However, in the presence of boric acid, boric acid and Ni(OH)2 could form the negatively-charged 2H3BO3?Ni(OH)2 colloid ion that was difficult to be absorbed by the cathode. So, the cathode polarization curves became smoother as boric acid concentration increased in the electrolyte.
After the potential of -0.726 V on Curve 1 and -0.759 V on Curve 2, Ni2+ began to deposit normally without the formation of Ni(OH)2. The evolution potential of H+ on Curve 3 was obviously lower than that on Curve 1 or 2 even though its specific figure was hard to confirm. This indicates that the existence of boric acid in the electrolyte inhibited the discharge of H+. Boric acid could increase the overpotential of hydrogen so that the evolution of H2 was inhibited. The role of boric acid in the electrolyte acted primarily as a buffer that maintained pH of the double layer changing in a small range[14-15]. But, it is known that boric acid cannot buffer the solution by a simple proton dissociation mechanism because pKa=9.2 for boric acid while pH of the electrolyte was about 3. Therefore, the nickel borate might be formed to promote boric acid to release H+. In a word, the function of boric acid was that it was absorbed on the surface of cathode in the form of nickel borate as well as a pH buffer in the electrolyte[16]. That was why the addition of boric acid in the electrolyte makes the deposition potentials of both Ni2+ and H+ more negative.
Fig.2 shows the cathode polarization curves of different boric acid concentrations in the electrolyte when Ni2+ concentration was 80 g/L and other conditions were the same as those shown in Fig.1. The “sag” in Fig.2 was less evident than that in Fig.1, indicating the influence of boric acid was related to the concentration of Ni2+. The higher the Ni2+ concentration, the larger the influence of boric acid on H+ discharge. Therefore, a proper boric acid concentration should be considered in the nickel practical electrowinning.
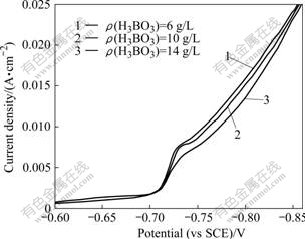
Fig.2 Effect of H3BO3 concentration on polarization curves of nickel reduction under conditions of ρ(Ni2+)=80 g/L, ρ(Na+)= 15 g/L, θ=65 ℃ and pH=3
The nickel electrodeposition experiments were done for 7.5 h, without boric acid and with boric acid of 6 g/L, respectively, under the conditions of θ=65-70 ℃, ρ(Na+)=10 g/L, ρ(Ni2+)=80 g/L and J=220 A/m2. The pH value of the circulated electrolytes was detected every half an hour during the process of the experiments. Fig.3 shows the changes of pH value in the process.
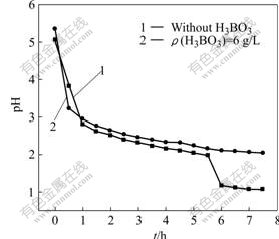
Fig.3 pH of electrolyte during process of nickel electrodeposition
As shown in Fig.3, the pH of electrolyte without boric acid changed from 5.1 to 1.1 after electrodepositing for 7.5 h, While the pH of electrolyte with boric acid changed from 5.2 to 2.0. The latter has a smaller changing range. This suggests the addition of boric acid indeed buffered the pH of the electrolyte solution. The surface of Sample 1 was visually dark and not flat at all with some pitting. Compared with Sample 1, Sample 2 was smoother and brighter, but the amount of the pitting on the surface did not obviously decrease. Therefore, boric acid had some good effects on improving the surface morphology of nickel deposit.
Furthermore, the pH of electrolyte in the electrodeposition process was controlled in the range of 3-4 when boric acid concentration was 6, 10 and 14 g/L, respectively. The results show that with the increase of boric acid concentration in the electrolyte, the surface of nickel became smoother and flatter, and the brightness of surface was improved a lot. When boric acid was added, the current efficiency also increased to about 97% higher than the current efficiency of 90% under the condition without boric acid in the electrolyte. The existence of boric acid inhibited the formation of H2 so that the current efficiency increased. When the boric acid concentration was 10 g/L and pH was about 3, a deposit with good quality and a relative high current efficiency was obtained.
3.2 Effect of SDS
Fig.4 shows the polarization curves with different SDS concentrations under the condition of ρ(Ni2+)=120 g/L, ρ(Na+)=15 g/L, θ=65 ℃ and ρ(H3BO3)=10 g/L. Fig.4(b) shows the partial enlargement of Fig.4(a).
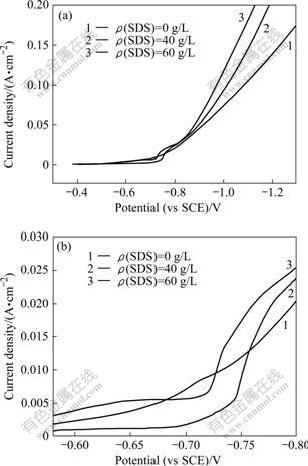
Fig.4 Effect of SDS concentration on polarization curves of nickel reduction under conditions of ρ(Ni2+)=120 g/L, ρ(H3BO3)=10 g/L, ρ(Na+)=15 g/L and θ=65 ℃: (a) Polarization curves; (b) Partial enlargement of polarization curves
SDS, an anionic surfactant, can reduce the surface tension of solution, so that the hydrogen gas generating from cathode was easy to leave from the surface of cathode. Small amount of SDS in the electrolyte can decrease the surface tension and improve the wetting ability of the electrolyte obviously, which favors the elimination of pitting. According to Ref.[8], when the concentration of SDS reached to mg/L in the electrolyte, the surface tension of the electrolyte tends to be the minimum.
As seen in Fig.4(a), when the potential was more negative than -0.9 V, the polarization curves shifted left with the increase of SDS concentration in the electrolyte. Therefore, the addition of SDS in the electrolyte could decrease the cathode polarization in this stage. The potential range between -0.6 V and -0.8 V in Fig.4(a) was enlarged as shown in Fig.4(b). In the absence of SDS in the electrolyte (Curve 1), Ni2+ began to discharge around -0.7 V and the cathode current density increased with the rise of potential gradually. In the presence of SDS of 40 mg/L and 60 mg/L, nickel deposition potentials changed to -0.725 V and -0.750 V, respectively(Curve 2 and 3), then the cathode current densities increased with larger slopes than those in absence of SDS and soon exceeded the cathode current density without SDS. This shows that the addition of SDS in electrolyte obviously inhibited the deposition of Ni2+ in the initial stage of electrodeposition. The reason is that SDS could be absorbed on the surface of cathode, so that Ni2+ had to overcome the energy barrier to go through the space cavities of adsorption layer in order to deposit on the surface of cathode. So, the process of Ni2+ passing through the absorption layer became the controlling step of electrodeposition, and the SDS exhibited a strong inhibitory effect[7]. The cathode current density started to increase sharply when the overpotential increased to the desorption potential of SDS. In a word, the existence of SDS in the electrolyte could reduce the cathode potential and promote the reduction of Ni2+; and the cell voltage could also declined in the practical electrodeposition.
The nickel electrodeposition experiments were conducted for 7.5 h under the conditions of θ=65-70 ℃, ρ(Na+)=10 g/L, ρ(Ni2+)= 80 g/L, ρ(H3BO3)=10 g/L, pH=3 and J=220 A/m2. The results show that after adding 20 mg/L SDS in the electrolyte, the amount of pitting on the surface of nickel deposit was less than that without SDS added. When SDS reached 40 mg/L, the pitting barely existed on the surface, but further addition of SDS did not have obvious effect on the surface of nickel deposit. Moreover, it is noticed that the addition of SDS did not degrade the current efficiency of electrodeposition.
4 Conclusions
1) Boric acid can increase cathode polarization. In the process of electrodeposition, boric acid absorbed on the surface of cathode results in enhancing the deposition potential of Ni2+. Boric acid and Ni2+ can form nickel borate that can increase the overpotential of hydrogen and decrease the evolution of H2. Nickel deposit with flat and smooth surface and relatively high current efficiency can be achieved when boric acid is used.
2) In the initial stage of polarization, the Ni deposition is inhibited by SDS absorbed on the cathode; after desorption, SDS in the electrolyte promotes the reduction of Ni2+ and reduces the cathode polarization because of the possible improvement of electrolyte wetting ability. In addition, SDS can also eliminate the pitting on the surface and improve the morphology of nickel deposit.
3) The electrodeposition experiments show that good deposit morphology with rare pitting and high current efficiency is favored by adding 10 g/L boric acid and 40 mg/L SDS in electrolyte under the conditions of nickel ion concentration of 80 g/L, sodium ion concentration of 10 g/L, pH of 3, current density of 220 A/m2 and temperature of 70 ℃.
References
[1] HOLM M, O’KEEFE T J. Electrolyte parameter effects in the electrowinning of nickel from sulfate electrolytes[J]. Minerals Engineering, 2000, 13(2): 193-204.
[2] GUO Xue-yi, HUANG Kai, LIU Zhi-hong, ZHANG Duo-mo, CHEN Hui-guang. Electrochemical behavior of nickel cathode reduction in chloride solution under different conditions[J]. Nonferrous Metals, 2000, 52(1): 55-58. (in Chinese)
[3] GAO Tian-xing, LI Shi-xiong, LIU Ai-xin. Role of additive in nickel electrolysis cathodic process and its on-line control[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(10): 1806-1811. (in Chinese)
[4] HE Huan-hua, CAI Qiao-fang. Metallurgy of nickel and cobalt in China[M]. Beijing: Metallurgical Industry Publishing House, 2000: 547. (in Chinese)
[5] YE Xiao-xiong. Talks about chloride ions in nickel electroplating[J]. Surface Technology, 1984(1): 14-17. (in Chinese)
[6] HUANG Qi-xing, WANG Li-chuan, ZHU Ding-yuan. Metallurgy of nickel[M]. Beijing: Science and Technology of China Press, 1990: 307-326. (in Chinese)
[7] ZHOU Shao-min. Mental electrodeposition-theory and research methods[M]. Shanghai: Shanghai Science and Technology Publishing House, 1982: 32-35. (in Chinese)
[8] SHU Yu-de, ZHAO Rui-rong, CHEN Bai-zhen, FU Qing-yuan, JIANG Han-ying. Study of preventing the pitting from growing on nickel cathode[J]. Nonferrous Metals, 1983(3): 47-50. (in Chinese)
[9] TRIPATHY B C, DAS S C, MISRA V N. Effect of antimony(III) on the electrocrystallisation of zinc from sulphate solution containing SLS[J]. Hydrometallurgy, 2003, 69: 81-88.
[10] XUE Juan-qin, WU Qian, WANG Zhao-qi, YI She-feng. Function of additives in electrolytic preparation of copper powder[J]. Hydrometallurgy, 2006, 82: 154-156.
[11] WU Tao, WANG Yong-jun. Study of factors affecting the nickel electrowinning using insoluble anode[J]. Xinjiang Nonferrous Metals, 2003(S2): 35-37. (in Chinese)
[12] CHEN Jian-bing. Discussion about main factors affecting the physical quality of nickel deposit[J]. Xinjiang Nonferrous Metals, 2004(S1): 24-25. (in Chinese)
[13] ORHAN G, ARSLAN C, BOMBACH H, STELTER M. Nickel recovery from the rinse waters of plating baths[J]. Hydrometallurgy, 2002, 65: 1-8.
[14] MA Zhe-lang. Study of the functions of boric acid in nickel electroplating[J]. Shanghai Electroplating, 1995(3): 6. (in Chinese)
[15] LUPI C, PASQUALI M, DELL’ERA A. Studies concerning nickel electrowinning from acidic and alkaline electrolytes[J]. Minerals Engineering, 2006, 19: 1246-1250.
[16] YIN K M, LIN B T. Effects of boric acid on the electrodeposition of iron, nickel and iron-nickel[J]. Surface and Coatings Technology, 1996, 78: 205-210.
_______________________
Corresponding author: ZHANG Zhao; Tel: +86-28-85405322; E-mail: zzhang@scu.edu.cn

