氨浸出混合铜矿石的表征及动力学
来源期刊:中国有色金属学报(英文版)2014年第5期
论文作者:A. A. BABA M. K. GHOSH S. R. PRADHAN D. S. RAO A. BARAL F. A. ADEKOLA
文章页码:1587 - 1595
关键词:氨浸出;黄铜矿;菱铁矿;浸出动力学
Key words:ammonia leaching; chalcopyrite; siderite; leaching kinetics
摘 要:研究了含8.8%Cu和36.1%Fe的混合铜矿石的氨浸出动力学。矿物学表征表明,该矿石的含铁成分以菱铁矿为主,硫化矿以黄铜矿为主。研究了工艺参数,如搅拌速度、反应温度、氨浓度、矿石粒径、氧分压对氨浸出过程的影响。在标准的浸出条件下,即粒径125~212 μm、反应温度120 °C、NH3浓度1.29 mol/L、氧分压202 kPa, 在2.5 h内Cu的浸出率达到83%。在使用较高浓度的氨和较小粒径的矿石时,Cu的浸出率能够达到95%。动力学研究结果表明,浸出过程为表面反应控制,估算出的活化能为(37.6±1.9) kJ/mol,氧分压与氨浓度的反应级数分别为0.2 和1。
Abstract: Ammonia leaching kinetics of a complex Cu-ore assaying 8.8% Cu and 36.1% Fe was examined. Mineralogical characterization indicated that the major phase of the ore was siderite with chalcopyrite as the major sulfide mineral. The effects of parameters such as agitation, temperature, NH3 concentration, particle size and oxygen partial pressure (pO2) were investigated. Under the standard leaching conditions of 125-212 μm particle size, 120 °C, 1.29 mol/L NH3 and 202 kPa of pO2, about 83% Cu could be selectively extracted in 2.5 h. However, when using higher NH3 concentration and lower particle size, more than 95% extraction was achieved. The leaching process was found to be surface reaction controlling. The estimated activation energy was (37.6±1.9) kJ/mol and empirical orders of reaction with respect to pO2 and [NH3] were about 0.2 and 1, respectively.
Trans. Nonferrous Met. Soc. China 24(2014) 1587-1595
A. A. BABA1, M. K. GHOSH2, S. R. PRADHAN2, D. S. RAO2, A. BARAL2, F. A. ADEKOLA1
1. Department of Industrial Chemistry, University of Ilorin, P.M.B. 1515, Ilorin-240003, Nigeria;
2. CSIR-Institute of Minerals and Materials Technology, Bhubaneswar-751013, India
Received 6 September 2013; accepted 4 November 2013
Abstract: Ammonia leaching kinetics of a complex Cu-ore assaying 8.8% Cu and 36.1% Fe was examined. Mineralogical characterization indicated that the major phase of the ore was siderite with chalcopyrite as the major sulfide mineral. The effects of parameters such as agitation, temperature, NH3 concentration, particle size and oxygen partial pressure (pO2) were investigated. Under the standard leaching conditions of 125-212 μm particle size, 120 °C, 1.29 mol/L NH3 and 202 kPa of pO2, about 83% Cu could be selectively extracted in 2.5 h. However, when using higher NH3 concentration and lower particle size, more than 95% extraction was achieved. The leaching process was found to be surface reaction controlling. The estimated activation energy was (37.6±1.9) kJ/mol and empirical orders of reaction with respect to pO2 and [NH3] were about 0.2 and 1, respectively.
Key words: ammonia leaching; chalcopyrite; siderite; leaching kinetics
1 Introduction
Chalcopyrite (CuFeS2) has been the chief copper sulfide mineral studied for copper extraction from sulfide ores. This is mainly due to two reasons: 1) it is the most abundant source of sulfide copper in the earth’s crust; and 2) it is one of the most refractory sulfide copper minerals [1]. At present, about 70% of the world’s copper production is carried out from chalcopyrite ore through flotation followed by the pyrometallurgical route [2,3]. However, due to environmental regulations and the requirement of high-grade ores for the pyrometallurgical processing route, the hydrometallurgical methods are becoming more popular.
In recent years, much attention has been focused on the leaching of copper sulfide ores/concentrates in sulfate medium [1,4-6]. In acidic sulfate media, sulfide sulfur transforms to elemental sulfur under oxidizing conditions as can be seen from Eh-pH stability diagram [7] unless extreme high temperature and oxygen pressure are imposed [8]. Formation of elemental sulfur may impede the process kinetics by forming passivating layer on the unreacted particles. Other drawback is the contamination of leaching liquor with iron due to simultaneous dissolution and stability of copper and iron ions.
On the contrary, chalcopyrite leaching in aqueous ammonia leads to the formation of SO42- instead of S0 due to high pH of ammoniacal media. In general, the major advantage of ammonia leaching process lies in its selectivity towards copper since ammonia forms soluble ammine complex with Cu ions and iron is completely rejected as iron oxides. Aqueous ammonia as a leaching agent is fairly non-corrosive and leaching reactions occur in relatively mild conditions [9]. The best known application of direct reaction of ammonia and sulfide minerals is the process pioneered by the Sheritt-Gordon Co. for treating nickel sulfide concentrates [10]. The applications of ammonia leaching process for different sulfide minerals were widely investigated [11-14].
REILLY and SCOTT [9] investigated the leaching kinetics of chalcopyrite in NH3 medium and proposed an electrochemical surface reaction model with cathodic reduction of oxygen on the solid surface as the rate determining step. The activation energy for the reaction was estimated to be 74.1 kJ/mol. BECKSTEAD and MILLER [15] proposed that the catalytic electrochemical surface reaction by cupric ions was the rate-controlling factor during oxidizing ammonia leaching of chalcopyrite concentrate. The estimated activation energy of 41.84 kJ/mol further supported the surface chemical reaction control mechanism.
Using statistical experimental design, BELL et al [16] demonstrated that temperature and ammonium salt concentration as well as the interaction between these two factors greatly influences the in situ leaching of chalcopyrite in ammoniacal solutions. Particularly, the dissociation of NH4OH into H2O and NH3 was found to be a crucial step since ammonia is known to be an active contributor to the Cu leaching reaction.
Besides copper sulphide, NH3/NH4+ leaching was used for other copper minerals such malachite, low-grade mixed Cu ore, tenorite [17-19].
The reported studies on chalcopyrite leaching in ammonia solutions were based on either high-grade chalcopyrite (26.5% Cu) concentrate [9] or nearly pure (97% CuFeS2) concentrate [15]. Moreover, previous studies on copper recovery by ammonia leaching were carried out with sulphide concentrates or oxide ores but this route was never tested for a complex Cu ore which has neither sulfide nor oxide as the major matrix. Hence in the present study, such a complex Cu ore was selected and after mineralogical characterization, ammonia leaching kinetics was studied. Main influencing factors such as temperature, ammonia concentration, particle size, oxygen partial pressure (pO2) were examined and the rate controlling step was determined. Ore sample and typical leach residues were characterized for better understanding of the process.
2 Experimental
The ore sample from Kokona District, Nasarawa State, Nigeria was obtained from the Department of Geology and Mineral Sciences, University of Ilorin, Nigeria. The bulk ore samples were ground and sieved into different narrow size fractions: 75-90, 90-125, 125-212 and 212-425 μm. Experiments were mostly performed with the 125-212 μm fraction, unless otherwise stated. The elemental analysis of the ore (125-212 μm fraction) is given in Table 1. Copper and iron contents of the various size fractions are given in Table 2 which indicates within the size fractions studied Cu and Fe contents do not vary widely. Analytic grade NH3 and distilled water were used in the preparation of the leaching media.
Table 1 Chemical composition of ore (125-212 μm size fraction)

Table 2 Cu and Fe contents of ore at various size fractions

The mineralogical phase identification of the ore sample was carried out using Philips X-ray diffractometer (PW 3050/60 X’pert-Pro) with Mo Kα1 radiation generated at 30 mA and 40 kV. X-ray diffraction pattern of the ground ore is shown in Fig. 1. XRD pattern confirms the presence of siderite (FeCO3) as a dominant iron bearing phase and chalcopyrite as the main copper- bearing phase.
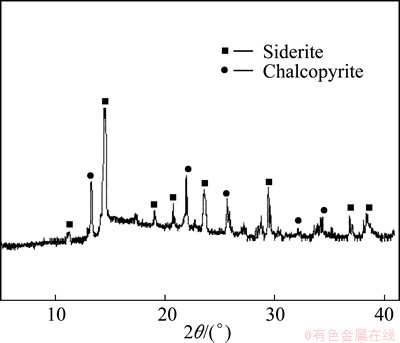
Fig. 1 X-ray diffraction pattern of Cu-ore sample
Leaching experiments were carried out in PARR  2 L capacity stainless steel autoclave. For each experiment, 500 mL of aqueous ammonia solution of predetermined molarity and 10 g ore were mixed in the reactor vessel making 20 g/L slurry density (S/L ratio). Reactor contents were initially heated with mild agitation during heating stage and on attaining the set temperature, oxygen was introduced and full agitation was placed. The reaction time was counted from this point. Samples were collected at an interval of 0.5 h. When total pressure dropped, gas inlet valve was opened and oxygen was introduced to maintain the total pressure constant. Samples were filtered and analyzed for copper by EDTA titration using Fast Sulphon Black F indicator. Some of the selected leached residues were collected after filtration, thoroughly washed with dilute NH3- (NH4)2SO4 solution and oven dried at about 80 °C prior to characterization.
2 L capacity stainless steel autoclave. For each experiment, 500 mL of aqueous ammonia solution of predetermined molarity and 10 g ore were mixed in the reactor vessel making 20 g/L slurry density (S/L ratio). Reactor contents were initially heated with mild agitation during heating stage and on attaining the set temperature, oxygen was introduced and full agitation was placed. The reaction time was counted from this point. Samples were collected at an interval of 0.5 h. When total pressure dropped, gas inlet valve was opened and oxygen was introduced to maintain the total pressure constant. Samples were filtered and analyzed for copper by EDTA titration using Fast Sulphon Black F indicator. Some of the selected leached residues were collected after filtration, thoroughly washed with dilute NH3- (NH4)2SO4 solution and oven dried at about 80 °C prior to characterization.
Fraction of copper extraction (α) was calculated using the following expression:
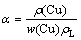
where ρ(Cu) is Cu concentration in leach liquor (g/L), w(Cu) is mass fraction of Cu in the ore and ρL is solid loading or solid-liquid ratio (g/L)
3 Results and discussion
3.1 Material characterization
The optical microscopy of a polished bulk sample was carried out in Leitz optical microscope under reflected light. In the photomicrograph (Fig. 2), chalcopyrite (C) and siderite (S) phases are observed as separate grains.
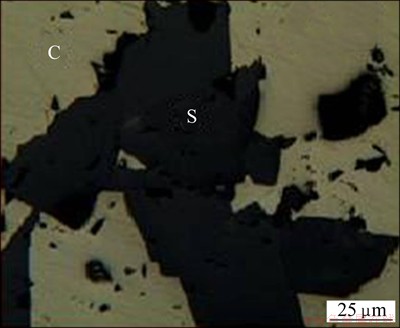
Fig. 2 Photomicrograph of polished bulk sample (S—Siderite; C—Chalcopyrite)
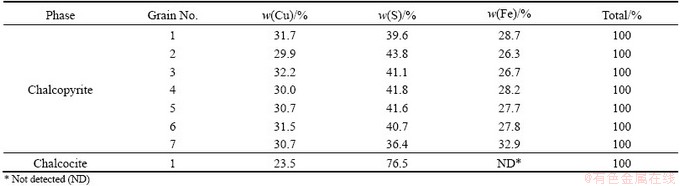
Quantitative estimation of elemental concentration of the chalcopyrite along with other copper sulfide phases in the polished section was carried out using ZEISS SUPRA-55 VP field emission scanning electron microscope (FESEM). The sample was subjected to elemental mapping by FESEM (Fig. 3). The point analysis of some of the grains was also carried out (Table 3). The FESEM images of the polished section of a bulk sample in combination with point analysis indicate that chalcopyrite contains 29.9%-32.2% Cu; 36.4%-43.8% S and 26.3%-32.9% Fe. Rarely, chalcocite was also recorded in the sample. Microscopic as well as FESEM studies further indicate that the samples are fresh.

Fig. 3 Elemental mapping of chalcopyrite for its Cu, S and Fe components
Table 3 Quantitative chemical analysis of copper sulfide phases under FESEM
3.2 Leaching reactions
Chalcopyrite oxidation reaction can be represented by the following equation [20]:
2CuFeS2+8NH3 (aq)+8.5O2(aq)+(n+2)H2O=2Cu(NH3)42++Fe2O3·nH2O+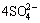 +4H+ (1)
+4H+ (1)
H+ generated by the above reaction is neutralized by ammonia as follows:
NH3+H+=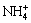 (2)
(2)
Overall reaction for the leaching can be written as follows:
2CuFeS2+12NH3+8.5O2+(n+2)H2O=2Cu(NH3)4SO4+Fe2O3·nH2O+2(NH4)2SO4 (3)
Generation of 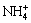 ion as per reaction (2) decreases the solution pH which is initially very high (above 11) by buffering action as given in Eq. (4) and helps to maintain pH in the stable region of copper ammine complex.
ion as per reaction (2) decreases the solution pH which is initially very high (above 11) by buffering action as given in Eq. (4) and helps to maintain pH in the stable region of copper ammine complex.
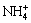 +OH-→NH4OH→NH3+H2O (4)
+OH-→NH4OH→NH3+H2O (4)
The siderite phase (FeCO3) does not take part in the leaching reaction and remains mostly unaltered.
The main parameters in the present investigation are degree of agitation, concentration of ammonia, partial pressure of oxygen, temperature and particle sizes. Standard leaching conditions are (unless otherwise stated): 120 °C, 125-212 μm, pO2=203 kPa, 1.29 mol/L NH3 and solid-liquid ratio 20 g/L. The results observed are indicated and discussed below.
3.3 Influence of agitation
The influence of agitation on the Cu extraction from the ore was studied in the range of 150- 650 r/min at 120 °C. It was observed that the stirring speed had a significant effect on the copper dissolution up to about 450 r/min. Above 550 r/min, however, agitation had no further effect on the dissolution rate. Therefore, the stirring speed was maintained at 600 r/min for further studies and considered to be appropriate to eliminate the effect of this variable.
3.4 Influence of ammonia concentration
The effect of ammonia concentration on the extent of copper extraction was studied in the range of 0.65-1.94 mol/L. Figure 4 shows the dissolution data as a function of ammonia concentration at various time of leaching.

Fig. 4 Effect of NH3 concentration on Cu extraction (120 °C, pO2=203 kPa, 125-212 mm particle size)
From Fig. 4, it can be seen that by increasing total ammonia concentration from 0.65 to 1.94 mol/L, copper extraction increases from 47% to 95% in 2.5 h leaching time. The reddish brown leach residue obtained at 1.94 mol/L ammonia (after 95% Cu extraction) indicates that iron component of chalcopyrite phase had been primarily oxidized to hematite. This observation is supported by XRD patterns. The initial pH values were 11.45 (0.65 mol/L), 11.65 (1.29 mol/L), 11.7 (1.67 mol/L) and 11.8 (1.94 mol/L). Although initial pH values were very high, with the progress of oxidation reaction pH decreased and the corresponding end pH values were 10.1, 10.45, 10.33 and 10.25 respectively and within the stable region of ammine complex. The decrease in pH was due to the generation of 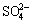 and H+ through oxidation reaction and simultaneous neutralization of H+ ion as explained in Section 3.2. The decrease in pH is related to the initial ammonia concentration and amount of S to
and H+ through oxidation reaction and simultaneous neutralization of H+ ion as explained in Section 3.2. The decrease in pH is related to the initial ammonia concentration and amount of S to 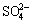 oxidation reaction.
oxidation reaction.
3.5 Influence of leaching temperature
The effect of leaching temperature on chalcopyrite dissolution was studied in the range of 90-130 °C under standard conditions of 1.29 mol/L NH3, 125-212 μm size and 203 kPa oxygen pressure. As shown in Fig. 5, increasing the leaching temperature significantly increases the leaching rate. Copper extraction after 2.5 h increased from 45% to 92% by increasing the leaching temperature from 90 °C to 130 °C.
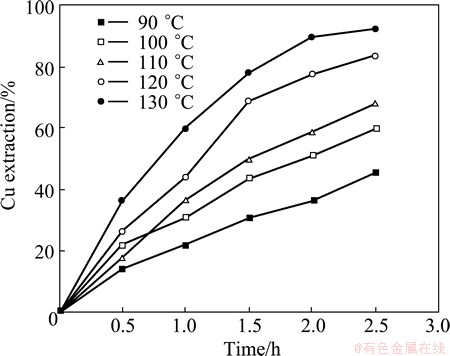
Fig. 5 Effect of temperature on Cu extraction (1.29 mol/L NH3, pO2=203 kPa, 125-212 mm particle size)
3.6 Influence of oxygen partial pressure
The effect of oxygen partial pressure on the rate of copper dissolution was examined by varying pO2 in the range of 50.6-304 kPa in 1.29 ml/L ammonia solution at 120 °C. The results are presented in Fig. 6. From Fig. 6, it is observed that without introduction of oxygen in the system copper dissolution was only about 20% in 1.5 h which remained constant up to 2.5 h. This extraction might be due to already present oxygen inside reactor from the start. By maintaining oxygen partial pressure to only 50.6 kPa, it was possible to achieve 72% Cu extraction and further increase in partial pressure to 203 kPa increased the extraction to 83%. However, increasing oxygen partial pressure beyond 203 kPa has no effect on improving the copper extraction. These results are in agreement with the observation made by BECKSTEAD and MILLER [15] who concluded that due to surface saturation by oxygen, leaching is not affected by oxygen partial pressure after a certain pressure. Dissolved oxygen adsorbs on the ore particle surface before reacting with it. In the present investigation, a low slurry density of 20 g/L may result in the surface saturation after about 203 kPa oxygen partial pressure, hence, the rate of reaction becomes insensitive to oxygen partial pressure above 203 kPa.

Fig. 6 Effect of O2 partial pressure (pO2) on Cu extraction (1.29 mol/L NH3, 120 °C, 125-212 μm particles)
3.7 Influence of particle size
To determine the effect of particle size on the rate of copper dissolution, experiments were performed using four different size fractions in the range of 75-425 μm. The results of the investigation are illustrated in Fig. 7. As seen from Fig. 7, the rate of copper dissolution increases sharply as the size of the ore particle decreases. Nearly 100% copper dissolution was achieved with 75-90 μm and 90-125 μm particle sizes. Conversely, only 51% Cu extraction was obtained with 212-425 μm size. The reason for this could be due to the increase in the interfacial area of reaction as the solid particles become smaller [21,22].
3.8 Characterization of leach residues
Residues from several leaching experiments at various levels of copper extraction were examined using XRD and SEM. XRD pattern of a typical leach residue with >90% copper recovery is shown in Fig. 8. It is observed that all the chalcopyrite peaks disappeared and hematite phase appeared as a result of chalcopyrite oxidation in alkaline media. As expected, the siderite peaks remains nearly unaltered.

Fig. 7 Effect of particle size on Cu extraction (1.29 mol/L NH3, 120 °C, pO2=203 kPa)
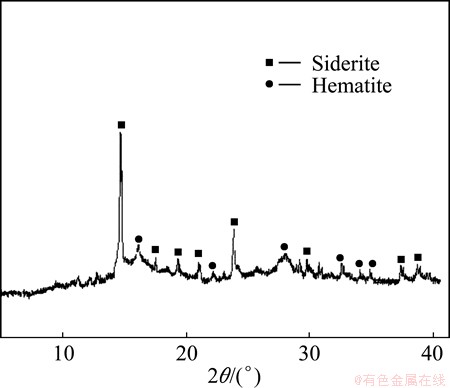
Fig. 8 XRD pattern of a typical leach residue (Cu extraction of 94%)
The morphologies of the ground ore and typical leached residues were examined by JEOL JSM-6510 scanning electron microscope. SEM image of the ore particles (Fig. 9(a)) clearly shows the morphological features of carbonate minerals. The grains show perfectly three sets of cleavage planes. In this case, it is siderite as revealed by XRD. Depending upon the level of copper extraction, hematite phase abundance in the leached residue is visible. Figure 9(b) shows small amount of hematite particles on the siderite surface for copper recovery of 22%. When the copper recovery increases to 63% (Fig. 9(c)), hematite particles are visible as separated grains besides on the surface of the siderite grains. In the case of residue with 95% copper extraction, siderite grains are nearly covered with hematite particles (Fig. 9(d)).

Fig. 9 SEM images of original ore (a), 22% Cu leached residue (b), 63% Cu leached residue (c) and 95% Cu leached residue (d)
3.9 Kinetic analysis
One of the major objectives of this study is the basic understanding of the mechanism of copper dissolution kinetics from the chalcopyrite containing complex Cu ore in ammonia solution.
Leaching is a fluid-solid heterogeneous reaction and can be represented as follows:
Afluid+bBsolid→products (5)
For the above reaction system, the following main steps are considered to occur in succession during the reaction:
1) Diffusion of fluid reactants from bulk liquid to fluid film;
2) Diffusion of reactants across the fluid film to the particle surface;
3) Diffusion of reactants across the product layer to the unreacted core;
4) Reaction on the unreacted core surface between fluid reactant and solid.
Each of the above steps offers a resistance to the overall reaction. The step with the largest resistance i.e. the slowest step becomes the rate controlling. Steps 1 and 2 are dependent on the hydrodynamics or mixing effects inside the reactor and can be enhanced by increasing the speed of rotation of the impeller or by improving the reactor design to promote better mixing. It can be assumed that the bulk diffusion is not rate controlling when the mixing is high enough to maintain all the particles in suspension. In the case of a chalcopyrite leaching, insoluble layer of iron oxides may form a diffusion barrier for the reactants to reach the unreacted core. Diffusion across the product layer is mainly dependent on the thickness and porosity of the layer. For a particle reacting under shrinking core mode, the integrated rate equations for three different rate controlling mechanisms can be written as follows [23,24].
1) Film diffusion:
α=kf t (6)
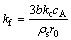 (7)
(7)
2) Product layer diffusion:
 (8)
(8)
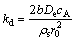 (9)
(9)
3) Surface reaction:
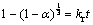 (10)
(10)
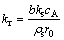 (11)
(11)
To determine the rate controlling mechanism and kinetic parameters, the experimental conversion data were analyzed on the basis of shrinking core models. From the shape of time vs conversion plots (Figs. 4-7), it shows that film diffusion is not a rate-controlling step in the present investigation. Hence, the experimental time vs conversion data were tested with the model Eqs. (8) and (10). The best fit plots with coefficient of determination (R2) more than 0.97 were obtained with the surface reaction controlling mechanism, i.e. model Eq. (10).
Application of Eq. (10) to the experimental data at different temperatures yielded linear relationship, which suggests that the dissolution rate of chalcopyrite ore is controlled by the surface chemical reaction (Fig. 10).

Fig. 10 Plots of 1-(1-α)1/3 vs time at various temperatures (Data correspond to Fig. 5)
Arrhenius equation kr=Aexp[-Ea/(RT)] was used to estimate the activation energy for the process. Apparent reaction rate constants calculated from the slopes of Fig. 10 were used in the Arrhenius plot, i.e. ln kr vs 1/T plot (Fig. 11). The estimated activation energy from the slope was (37.6±1.9) kJ/mol. The obtained activation energy value further supports surface reaction controlling model because diffusion controlling reactions generally have activation energies in the range of 4.2-12.5 kJ/mol [24]. Surface reaction controlled leaching with activation energy lower than 40 kJ/mol has been reported in several studies [25,26]. Low value of apparent activation energy can be associated with a reaction mechanism involving adsorption of reactants followed by chemical reaction itself [26]. In the present study, dissolved O2 may first be adsorbed on the ore surface followed by oxidation reaction of chalcopyrite.
Also, applying Eq. (10) to the kinetic data in Fig. 6 at different pO2, chemical reaction rate constants were calculated from the slopes. In order to determine the reaction order with respect to oxygen partial pressures, ln kr vs ln pO2 plot was made as shown in Fig. 12. In this figure, two slopes exist. Empirical order with respect to oxygen pressure (50.6-203 kPa) is 0.22±0.02. However, at higher oxygen partial pressure (>203 kPa), the rate becomes independent of oxygen pressure variation and order approaches zero.
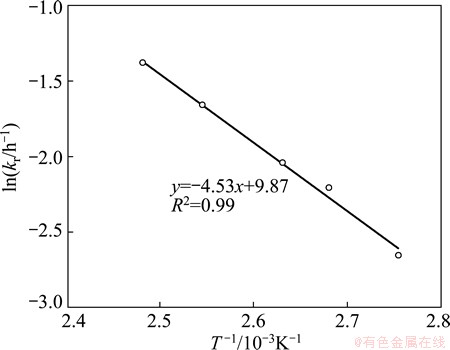
Fig. 11 Arrhenius plot for Cu ore leaching (Conditions: 1.29 mol/L NH3, pO2=203 kPa, 125-212 mm particles)
The surface chemical reaction model fitted well with the dissolution data at various ammonia concentrations. Apparent rate constants at various NH3 concentrations were determined from 1-(1-α)1/3 vs t plots. From the plot of ln kr vs ln [NH3], reaction order with respect to ammonia concentration is found to be 1.04±0.08 (Fig. 13).

Fig. 12 Plot of ln kr vs ln pO2 to estimate reaction order

Fig. 13 ln kr vs ln [NH3] plot to estimate reaction order
In order to calculate the average particle radius, geometric average was calculated as follows:
 (12)
(12)
The apparent rate constant (kr) values calculated from 1-(1-α)1/3 vs time plots for different particle sizes were plotted against inverse of initial particle radii (Fig. 14). The linear dependence of the rate constant on the inverse of particle radius and passing through the origin further supports that the surface chemical reaction is the rate controlling step here.
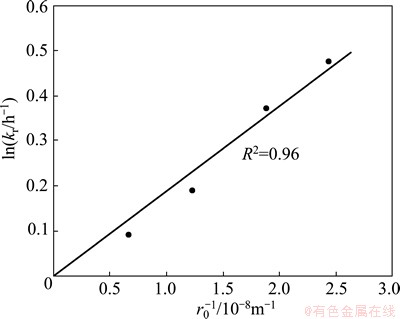
Fig. 14 Apparent rate constant vs inverse of particle radius plot
4 Conclusions
Mineralogical characterization of the copper ore sample reveals that siderite is the major phase and chalcopyrite is the main sulfide mineral. Ammoniacal leaching using O2 gas under pressure is a feasible treatment route for this ore for selective dissolution of copper. Copper leaching rate is strongly influenced by temperature, NH3 concentration and particle size whereas oxygen partial pressure has moderate influence up to about 203 kPa. Under standard leaching conditions of 1.29 mol/L NH3, 120 °C, 125-212 μm particle size and 203 kPa O2 partial pressure, it is possible to achieve 83% copper recovery in 2.5 h. However, under higher ammonia concentration and lower particle sizes >95%, Cu extraction is feasible. The leaching process is surface reaction controlling one and precipitation of hematite does not alter the reaction mechanism. Empirical orders of reaction with respect to O2 partial pressure and NH3 concentration are about 0.2 and 1, respectively. The linear plot of rate constant vs inverse of particle radius passing through the origin coupled with activation energy value of (37.6±1.9) kJ/mol further supports the surface reaction rate controlling mechanism.
Nomenclature
A
Pre-exponential factor in Arrhenius equation;
b
Stoichiometric coefficient in Eq. (5);
cA
Concentration of fluid reactant (mol/m3);
De
Effective diffusivity (m2/s);
Ea
Activation energy (J/mol);
f1, f2
Upper and lower sizes in a particular size fraction, respectively;
kc
Liquid-solid mass transfer coefficient (m/s);
kd
Apparent rate constant for product layer diffusion (s-1);
kr
Apparent rate constant for surface chemical reaction (s-1);
ks
Intrinsic reaction rate constant;
R
Mole gas constant (8.3145 J/(mol·K));
r0
Initial particle radius (m);
t
Time (h or s);
T
Temperature (K);
α
Fraction of extraction;
ρs
Density of solid
Acknowledgements
Authors are thankful to Prof. B. K. MISHRA (Director, IMMT) for his kind permission to publish this work. Authors are also grateful to Dr. R. B. BALE, Geology and Mineral Sciences, University of Ilorin, Nigeria for providing the ore samples. One of the authors (A. A BABA) wishes to thank the Academy of Sciences for the Developing World, Trieste, Italy for the award of CSIR-TWAS Fellowship and also to the Director, IMMT for the placement to carry out the research work.
References
[1] Padilla R, Vega D, Ruiz M C. Pressure leaching of sulfidized chalcopyrite in sulfuric acid–oxygen media [J]. Hydrometallurgy, 2007, 86: 80-88.
[2] oleini S M J, Jafarian M, Abdollahy M, Aghazadeh V. Galvanic leaching of chalcopyrite in atmospheric pressure and sulphate media: Kinetics and surface studies [J]. Industrial & Engineering Chemistry Research, 2010, 49: 5997-6002.
[3] Cordoba E M, Munoz J A, Blazquez M L, Gonzalez F, Ballester A. Leaching of chalcopyrite with ferric ion. Part I: General Aspects [J]. Hydrometallurgy, 2008, 93: 81-87.
[4] Ruiz M C, Montes K S, Padilla R. Chalcopyrite leaching in sulfate-chloride media at ambient pressure [J]. Hydrometallurgy, 2011, 109: 37-42.
[5] Padilla R, Zambrano P, Ruiz M C. Leaching of sulfidized chalcopyrite with H2SO4-NaCl-O2 [J]. Metallurgical and Materials Transactions B, 2003, 34: 153-159.
[6] Lu Z Y, Jeffery M I, Lawson F. The effect of chloride ions on the dissolution of chalcopyrite in acidic solutions [J]. Hydrometallurgy, 2000, 56: 189-202.
[7] Habashi F A. Textbook of hydrometallurgy [M]. Quebec, Canada: Métallurgie Extractive Québec, Enr, 1993: 268.
[8] HACKL R P, DREISINGER D B, PETERS E, KING J A. Passivation of chalcopyrite during oxidative leaching in sulfate media [J]. Hydrometallurgy, 1995, 39: 25-48.
[9] Reilly I G, Scott D S. The leaching of a chalcopyrite concentrate in ammonia [J]. Canadian Journal of Chemical Engineering, 1977, 55: 527-533.
[10] Forward F A. Ammonia pressure leach process for recovering nickel, copper, and cobalt from Sherrit Gordon nickel sulphide concentrate [J]. Canadian Mining and Metallurgical Bulletin, 1953, 499: 677-684.
[11] Alymore M G. Treatment of a refractory gold–copper sulphide concentrate by copper ammoniacal thiosulphate leaching [J]. Minerals Engineering, 2001, 14(6): 615-637.
[12] Feng D, van Deventer J S J. Leaching behaviour of sulphides in ammoniacal thiosulphate systems [J]. Hydrometallurgy, 2002, 63: 189-200.
[13] Ghosh M K, Das R P, Biswas A K. Oxidative ammonia leaching of sphalerite: Part I: Non-catalytic kinetics [J]. International Journal of Mineral Processing, 2002, 66: 241-254.
[14] Ghosh M K, Das R P, Biswas A K. Oxidative ammonia leaching of sphalerite: Part II: Cu(II)-catalyzed kinetics [J]. International Journal of Mineral Processing, 2003, 70: 221-234.
[15] Beckstead L W, Miller J D. Ammonia oxidation leaching of chalcopyrite [C]//AIChE Symposium Series: Fundamental Aspects of Hydrometallurgical Processes. New York: AIChE, 1978, 173: 28-40.
[16] Bell S L, Welch G D, Bennett P G. Development of ammoniacal lixiviants for the in-situ leaching of chalcopyrite [J]. Hydrometallurgy, 1995, 39(1-3): 11-23.
[17] Bingol D, Canbazoglu M, Aydogan S. Dissolution kinetics of malachite in ammonia/ammonium carbonate leaching [J]. Hydrometallurgy, 2005, 76: 55-62.
[18] LIU Wei, TANG Mo-tang, TANG Chao-bo, HE Jing, YANG Sheng-hai, YANG Jian-guang. Dissolution kinetics of low grade complex copper ore in ammonia-ammonium chloride solution [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 910-917.
[19] Ekmekyapar A, Aktas E, Kunkul A, Demirkiran N. Investigation of leaching kinetics of copper from malachite ore in ammonium nitrate solutions [J]. Metallurgical and Materials Transactions B, 2012, 43: 764-773.
[20] Tozawa K, Umetsu Y, Sato K. On chemistry of ammonia leaching of copper concentrates [M]//YANNOPOULOS J C, AGARWAL J C. Extractive Metallurgy of Copper. New York: AIME, 1976: 706-721.
[21] Baba A A, Adekola F A. Hydrometallurgical processing of a Nigerian sphalerite in hydrochloric acid: Characterization and dissolution kinetics [J]. Hydrometallurgy, 2010, 101(1-2): 69-75.
[22] Ucar G. Kinetics of sphalerite dissolution by sodium chloride in hydrochloric acid [J]. Hydrometallurgy, 2009, 95: 39-43.
[23] Levenspiel O. Chemical reaction engineering [M]. 2nd ed. New York: Wiley, 1972: 361-371.
[24] Habashi F. Principles of extractive metallurgy: Vol.1 [M]. New York: Gordon and Breach Science Publishers, 1969:143.
[25] SOUZA A D, PINA P S, LEAO V A, SILVA C A, SIQUEIRA P F. The leaching kinetics of a zinc sulphide concentrate in acid ferric sulphate [J]. Hydrometallurgy, 2007, 89: 72-81.
[26] ADEBAYO A O, IPINMOROTI K O, AJAYI O O. Leaching of sphalerite with hydrogen peroxide and nitric acid solutions [J]. Journal of Minerals & Materials Characterization & Engineering, 2006, 5(2): 167-177.
A. A. BABA1, M. K. GHOSH2, S. R. PRADHAN2, D. S. RAO2, A. BARAL2, F. A. ADEKOLA1
1. Department of Industrial Chemistry, University of Ilorin, P.M.B. 1515, Ilorin–240003, Nigeria;
2. CSIR-Institute of Minerals and Materials Technology, Bhubaneswar-751013, India
摘 要:研究了含8.8%Cu和36.1%Fe的混合铜矿石的氨浸出动力学。矿物学表征表明,该矿石的含铁成分以菱铁矿为主,硫化矿以黄铜矿为主。研究了工艺参数,如搅拌速度、反应温度、氨浓度、矿石粒径、氧分压对氨浸出过程的影响。在标准的浸出条件下,即粒径125~212 μm、反应温度120 °C、NH3浓度1.29 mol/L、氧分压202 kPa, 在2.5 h内Cu的浸出率达到83%。在使用较高浓度的氨和较小粒径的矿石时,Cu的浸出率能够达到95%。动力学研究结果表明,浸出过程为表面反应控制,估算出的活化能为(37.6±1.9) kJ/mol,氧分压与氨浓度的反应级数分别为0.2 和1。
关键词:氨浸出;黄铜矿;菱铁矿;浸出动力学
(Edited by Hua YANG)
Corresponding author: M. K. GHOSH; Tel: +91-674-2379374; E-mail: mkg.immt@gmail.com
DOI: 10.1016/S1003-6326(14)63229-5

