
Leaching behavior of heavy metals with hydrochloric acid from fly ash generated in municipal waste incineration plants
HUANG Kai1, Katsutoshi INOUE2, Hiroyuki HARADA2, Hidetaka KAWAKITA2, Keisuke OHTO2
1. Department of Nonferrous Metallurgy, University of Science and Technology Beijing, Beijing 100083, China;
2. Department of Applied Chemistry, Saga University, Honjo 1, Saga 840-8502, Japan
Received 30 November 2010; accepted 18 April 2011
Abstract:The extraction behavior of heavy metals from municipal waste incineration (MWI) fly ash was investigated systematically. The extraction process includes two steps, namely, fly ash was firstly washed with water, and then subjected to hydrochloric acid leaching. The main parameters for water washing process were tested, and under the optimal conditions, about 86% Na, 70% K and 12% Ca were removed from fly ash, respectively. Hydrochloric acid was used for the extraction of valuable elements from the water-washed fly ash, and the optimal extraction was achieved for each heavy metal as follows: 86% for Pb, 98% for Zn, 82% for Fe, 96% for Cd, 62% for Cu, 80% for Al, respectively. And the main compositions of the finally obtained solid residue are Ca2PbO4, CaSi2O5, Pb5SiO7, Ca3Al2Si3O12 and SiO2.
Key words: municipal waste incineration; fly ash; heavy metals; leaching; hydrochloric acid
1 Introduction
In Japan, nearly all of the huge amount of municipal waste generated in daily life is treated by incineration at municipal incineration plants, which produces a large amount of incineration residue, bottom and fly ashes, as well as waste gas. Among them, the fly ash contains high level of chloride compounds of calcium, sodium and potassium as the major components, while it also contains considerably hazardous substances, e.g., toxic heavy metals such as Pb, Zn, Cu, Cd, As and toxic organic materials such as dioxin which are concentrated and accumulated in the fly ash as the minor components [1]. In practice, dioxins can be detoxified into carbon dioxide by vitrification treatment at high temperature, but heavy metals can not be decomposed. Therefore, the incineration fly ashes of municipal wastes are regulated in Japan to be treated as a hazardous waste to prevent leakage of heavy metals before landfilling disposal by effective detoxification processes.
Usually, the fly ashes are treated by means of one of the following four methods [2-5], cement solidification, chemical stabilization, melting treatment, and acid extraction. Among these treatment methods, lixiviant extraction method seems more attractive from the viewpoint of recovery and recycling of metals. And accompanied by the remarkable rise in the price of base metals in recent years, it is now appreciated as a better way all over the world, with the great potential to make some special contribution to this issue for their mild and efficient reaction characteristics. And usually the leaching is the first important operation for the whole treatment process.
In practice, leaching methods, such as hydrothermal [6-7], subcritical water treatment [8], fungal bioleaching [9-13], were seldom considered for the practical process due to their too strict operation conditions and high cost or too long operation time, so the process performed at ambient temperature should be preferentially considered, and the selection of the suitable lixiviant is essentially significant. Many lixiviants can be chosen for the extraction of heavy metals from the fly ash as reported in literatures, such as inorganic mineral acids like sulfuric acid, hydrochloric acid, and nitric acid [1], organic acids like citric acid [13-16], oxalic acid [16], acetic acid [17], tartaric acid [18], or chelating reagents like nitrilotriacetic acid (NTA) [7, 19], ethylendiaminetetraacetate (EDTA), and diethylenetriaminepentaacetate (DTPA) [19]. And in some cases, alkaline solutions like ammonium and sodium hydroxides [1, 20] have been also investigated for this purpose. Among the above lixiviants, synthetic chelating agents such as NTA, EDTA and DTPA exhibit good leaching efficiency. However, they are not biodegradable and also very difficult to recover the metals from their leach liquor due to their strong chelating affinity with the metals. Alkaline leaching using ammonium or sodium hydroxide solutions has the advantages that only Pb and Zn, amphoteric metals, are dissolved in alkaline solution while other impurities remain in the solid residue. However, alkaline leaching process has to be conducted together with subsequent leaching using other acidic lixiviants for further extracting other heavy metals such as Cu and Cd, which makes the whole process complex and inconvenient, resulting in the increase in the practical operation cost. Consequently, acids are more advantageous as the lixiviants. Organic acids such as citric acid, oxalic acid, acetic acid and tartaric acid are biodegradable and environmentally benign, and also exhibit good leaching behavior, but the recovery of heavy metals from these leach liquor is not necessarily easy similar to the cases of chelating agents. Although sulfuric and hydrochloric acids generally appear to be the most suitable lixiviants due to their low cost, lead in fly ash can not be extracted because of the formation of water insoluble species, PbSO4 or PbCl2. But, taking account of the complexation of chloride anions with lead ion, e.g., Pb2+ + 4Cl– = PbCl42–, hydrochloric acid appears to be one of the most potential candidates for the effective lixiviant. Since a quite limited number of research works of leaching with hydrochloric acid have been reported to date [13-16], we conducted a fundamental study for systematic investigation on the application of hydrochloric acid to the leaching process of heavy metal from fly ash in present work.
2 Experimental
2.1 Source and analysis of MWI fly ash
Fly ash sample was kindly donated by Taiheiyo Cement Corp., Japan. Metal constituents were analyzed by using a Shimadzu model ICP8100 ICP/AES spectrometer after aqua regia digestion as follows (mass fraction, %): Na 4.57, K 1.21, Ca 28.88, Zn 5.81, Pb 2.21, Fe 0.15, Al 0.20, and non-metallic elements such as O, Cl, S, P consisting of the rest of the composition were measured by using a Shimadzu model EDX-800HS energy-dispersive X-ray fluorescence spectrometer. The phase compositions of the fly ash samples were tested by using a Rigaku model Rint-1000 X-ray diffractometer.
2.2 Water washing
A portion of the fly ash was pre-washed with distilled water. 10 g sample of dried fly ash was mixed together with distilled water at varying liquid/solid ratios of (5-50):1 (in mL:g) in a beaker and stirred at room temperature of around 25 °C. The sampling of the suspension was taken at different time intervals up to 16 h. After vacuum filtration and drying completely in the oven at 75 °C overnight, metallic elements in the leach liquor and the residual insoluble slag were analyzed by using the ICP/AES spectrometer and X-ray diffractometer, respectively.
2.3 Leaching tests
The water-washed fly ash samples were subjected to acid treatments. Typically, 1.00 g of above pre-washed fly ash was mixed with 50 mL de-ionized water with its pH keeping constant at a certain value adjusted by concentrated HCl or NaOH. After magnetic stirring for 1 h, the suspension was filtered and the clear solution was collected for analysis of the heavy metal concentration by ICP/AES and the residual solid was tested by X-ray diffractometer. The leaching time varied from 5 to 250 min, and the temperatures varied from 30 to 50 °C. The pH values were kept constant from 1 to 6 and the liquid/solid ratios of (12-75):1(mL:g) were performed.
3 Results and discussion
3.1 Effect of washing time, liquid/solid ratio and washing frequency
Figure 1 presents the XRD results of the fly ash sample. It can be found that it mainly contains Ca(OH)2, NaCl, KCl, and ZnO, which is identical with the chemical analytical results obtained by ICP/AES and X-ray fluorescence spectrometer. Since during the incineration of municipal waste a large amount of calcium is added to remove acid gases such as SOx, NOx, and hydrogen chloride from the effluent gases, the fly ash contains lot of calcium and, consequently, is alkaline. It also contains quite large amount of sodium and potassium chlorides which are both volatile at high temperature and enriched in the fly ash. Taking account of the large quantities of water soluble compositions such as NaCl and KCl, the most economic approach is a simple washing of the fly ash with water to separate water soluble elements before acid leaching.
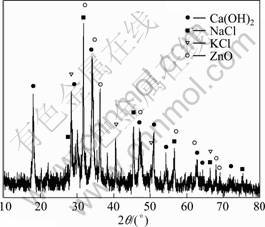
Fig. 1 XRD pattern of fly ash sample
Figure 2 present the relationship between washing time and removal ratio of some elements in water after washing at varying liquid/solid ratios. It is seen that the extraction of metal elements, such as Na, K, Ca, from fly ash rapidly reached a maximum value within 5-10 min; longer washing time resulted in a decrease in the amount of metal ions extracted. Most of Na and K (70%-90%) and only small part of Ca (less than 20%) can be removed after contacting with water. Although the higher liquid/solid ratios are favorable for the extraction of metal ions, a liquid/solid ratio of 10:1 is recommended, taking the extraction efficiency and water conservation into consideration.
Figure 3 presents the effect of water washing times on the extraction of each element in fly ash. It is seen that the water washing times has a quite high efficiency for soluble elements removal from fly ash. For example, after washing three times with water, the concentrations of sodium and potassium ions in the wash water fell sharply to 155 mg/L and 30 mg/L, respectively, which are not more than 4% of that in the first water wash. Therefore, in order to conserve water, two times of water washing at a liquid/solid ratio of 10:1 for a stirring time of 5 min at room temperature is recommended.
Figure 4 shows the XRD pattern of the fly ash after water washing under the above recommended experimental conditions. It can be found that after simple water washing, most of the soluble compositions such as NaCl and KCl will be removed quite effectively because their XRD peaks disappear, and the main compositions are Ca(OH)2, ZnO, and PbO with other unknown ones that are difficult to be identified. Anyway, water washing has been verified quite necessary to remove the water soluble compositions from the fly ash, and it will help to reduce the consumption of chemical reagents in the following acid leaching process.
3.2 Effect of pH on extraction of heavy metals in hydrochloric acid
The effect of pH value on heavy metals extraction from the fly ash was examined at a liquid/solid ratio of 50?1, contact time of 60 min at room temperature. It is found from Fig. 5 that the extraction rate for all the metals investigated generally decreased with increasing pH value, while for different metals the trends are also quite different. For example, the lead extraction rates after 60 min of leaching at pH=1 and 3.5 were found to be 89% and 11%, respectively, which is strongly dependent on the solution pH value. While for other metals, the good water solubility of their metal compounds makes them not so strongly dependent on pH. For example, 97% and 60% Zn, 62% and 45% Cu, 83% and 0 Fe, 81% and 35% Al were extracted at pH=1 and 3.5, respectively. So higher concentration of acid is favorable for the extraction of metals from the fly ash. This suggests that the leaching can be regarded as some kind of ions exchanging process, that is, metal elements will be replaced by H+ and dissolved into the solution from the fly ash particles. Considering the leaching efficiency, pH = 1.0 is recommended.
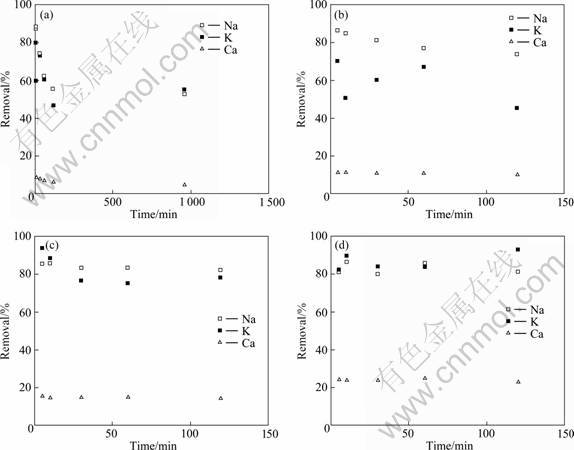
Fig. 2 Effect of washing time on removal ratio of soluble alkaline materials from fly ash under different liquid/solid ratios: (a) L/S=5?1; (b) L/S=10?1; (c) L/S=20?1; (d) L/S=50?1
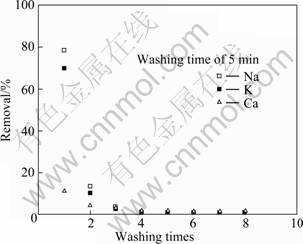
Fig. 3 Effect of washing times on removal ratio of soluble alkaline materials from fly ash(L/S=10?1)
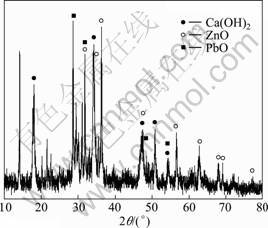
Fig. 4 XRD pattern of fly ash after water washing
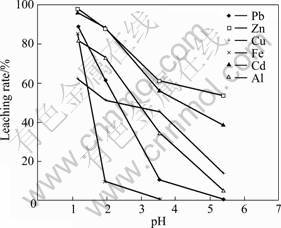
Fig. 5 Effect of pH on extraction rate of heavy metals from water-washed fly ash in hydrochloric acid
3.3 Effect of contact time on extraction of heavy metals in hydrochloric acid
The effect of leaching time on heavy metals extraction from the fly ash was examined at liquid/solid ratio of 50:1, pH=1.0 at room temperature. It is found from Fig. 6 that the maximum metal extraction rate is obtained at 20 min, and further leaching is not necessary. Actually, 5 min is also acceptable for effective extraction since the leaching rate for each metal is only very small different when comparing to values for the case of 20 min. For example, under the conditions of Fig. 6, 85% Pb, 93% Zn, 63% Cu and 87% Fe could be extracted into the leachate at a period of 5 min leaching, and 84% Pb, 93% Zn, 61% Cu and 88% Fe at a period of 20 min leaching. For sure the complete extraction, 15 min is chosen for the following leaching experiments in this study.
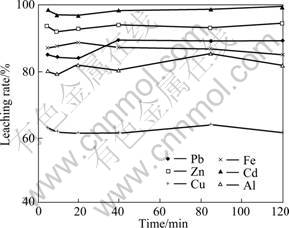
Fig. 6 Effect of leaching time on extraction rate of heavy metals form water-washed fly ash in hydrochloric acid (pH=1.0)
3.4 Effect of liquid/solid ratio on extraction of heavy metals in hydrochloric acid
The effect of liquid/solid ratios on heavy metals extraction from the fly ash was examined at contact time of 15 min, and pH=1.0 and room temperature. It is found from Fig. 7 that the extraction rate for all the metals attains their maximum values at liquid/solid ratio of 12:1 and no further drastic increase with increasing liquid/solid ratios, except for Fe, only reaches equilibrium at liquid/solid ratio of 30:1. For example, the lead extraction rates after 15 min of leaching at liquid/solid ratio of 12:1 and 50:1 were found to be 85% and 87%, respectively. For different metals in the fly ash, the effect of the liquid/solid ratios on their extraction rate was correspondingly different. For example, 96% and 99% Zn, 49% and 87% Fe, 58% and 63% Cu were extracted at liquid/solid ratio of 12:1 and 50:1, respectively. Considering the leaching efficiency, liquid/solid ratio of 30?1 is recommended.
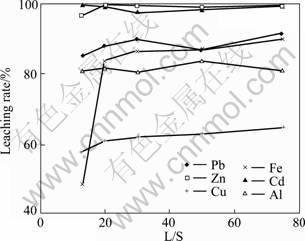
Fig. 7 Effect of liquid/solid ratio on extraction rate of heavy metals form water-washed fly ash in hydrochloric acid (pH=1.0)
3.5 Effect of leaching temperature on extraction of heavy metals in hydrochloric acid
The effect of temperature on heavy metals extraction from the fly ash was examined at a liquid/solid ratio of 50?1, contact time of 15 min, and pH=1.0. It is found from Fig. 8 that temperature shows very slight effect on the extraction rate, suggesting that the leaching reaction for the metals is temperature-independent. Considering the leaching efficiency, room temperature is suitable.
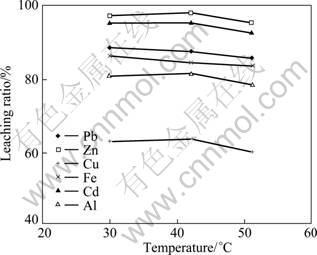
Fig. 8 Effect of temperature on extraction rate of heavy metals form water-washed fly ash in hydrochloric acid (pH=1.0)
Figure 9 presents the XRD result of the fly ash after acid leaching under the above recommended experimental conditions. It can be found that after acid leaching, most of the compositions such as Ca(OH)2, ZnO, PbO in the water washed sample are dissolved into the acid solution, and only sparingly soluble compositions such as Ca2PbO4, CaSi2O5, Pb5SiO7, SiO2, Ca3Al2Si3O12 are left in the residual solid. Because in the original fly ash sample, these compounds are minor constituents, they do not appear in the X-ray diffraction patterns. But after water washing and hydrochloric acid leaching, the main constituents such as NaCl, KCl, ZnO, Ca(OH)2 and PbO, are removed effectively, and the insoluble residue becomes the main phases consisting of the complex oxides, in which almost all the toxic metals have been extracted,except that a small portion of lead is still contained in the residue which is relatively stable and may not cause serious pollution in the landfill disposal.
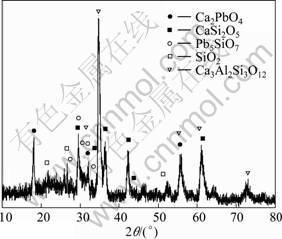
Fig. 9 XRD pattern of fly ash after hydrochloric acid leaching
4 Conclusions
1) The extraction process of heavy metals from the fly ash in the hydrochloric acid consists of two steps, i.e. pre-washing with water and leaching in hydrochloric acid. It was confirmed that this process is quite effective to extract the valuable metals from the fly ash.
2) It was verified that by simple water washing most of the NaCl and KCl can be removed from the fly ash effectively, and almost 86% Na, 70% K, 12% Ca will be extracted into the water from fly ash under the investigated optimal conditions, and the main compositions of the washed fly ash are Ca(OH)2, ZnO, PbO and some other complex phases are difficult to be identified. The optimal pre-washing parameters were determined as follows: washing time of 5-10 min, liquid/solid of 10:1, washing frequency of twice.
3) Hydrochloric acid is quite useful for the extraction of metals from the fly ash, and the optimal metal extraction can be achieved by hydrochloric acid under the following conditions: pH=1.0, liquid/solid ratio of 20, contact time of 5 min and room temperature. After acid leaching, the produced final solid residue mainly consists of Ca-Pb-Si-Al-O compounds, such as Ca2PbO4, CaSi2O5, Pb5SiO7, Ca3Al2Si3O12, SiO2.
Acknowledgments
We are indebted to Taiheiyo Cement Corp., Japan for providing the fly ash sample.
References
[1] NAGIB S, INOUE K. Recovery of lead and zinc from fly ash generated from municipal incineration plants by means of acid and/or alkaline leaching [J]. Hydrometallurgy, 2000, 56(3): 269-292.
[2] HONG K J, TOKUNAGA S, ISHIGAMI Y, KAJIGUCHI T. Extraction of heavy metals from MSW incinerator fly ash using saponins [J]. Chemosphere, 2000, 14(3): 345-352.
[3] KATSUURA H, INOUE T, HIRAOKA M, SAKAI S. Full-scale plant study on fly ash treatment by the acid extraction process [J]. Waste Management, 1996, 16(5-6): 491-499.
[4] IZUMIKAWA C. Metal recovery from fly ash generated from vitrification process for MSW ash [J]. Waste Management, 1996, 16(5-6): 501-507.
[5] NABESHIMA Y. Summary of research on waste minimization studies by Japan waste research foundation (JWRF) [J]. Waste Management, 1996, 16(5-6): 407-415.
[6] ZHANG F, ITOH H. Extraction of metals from municipal solid waste incinerator fly ash by hydrothermal process [J]. Journal of Hazardous Materials, 2006, 136(3): 663-670.
[7] GUERRRO A, FERNANDEZ E, MACIAS A, GONI S. Hydrothermal treatment of fly ash from municipal solid waste incineration [J]. Waste Management Series, 2000, 1: 178-185.
[8] ZHANG F, ITOH H. A novel process utilizing subcritical water and nitrilotriacetic acid to extract hazardous elements from MSW incinerator fly ash [J]. Science of Total Environment, 2006, 369(1-3): 273-279.
[9] ISHIGAKI T, NAKANISHI A, TATEDA M, IKE M, FUJITA M. Bioleaching of metal from municipal waste incineration fly ash using a mixed culture of sulfur-oxidizing and iron-oxidizing bacteria [J]. Chemosphere, 2005, 60(8): 1087-1094.
[10] PAUL M, SANDSTROM A, PAUL J. Prospects for cleaning ash in the acidic effluent from bioleaching of sulfidic concentrates [J]. Journal of Hazardous Materials, 2004, 106(1): 39-54.
[11] SEIDEL A, ZIMMELS Y, ARMON R. Mechanism of bioleaching of coal fly ash by thiobacillus thiooxidans [J]. Chemical Engineering Journal, 2001, 83(2): 123-130.
[12] KREBS W, BROMBACHER C, BOSSHARD P P, BACHOFEN R, BRANDL H. Microbial recovery of metals from solids [J]. FEMS Microbiology Reviews, 1997, 20(3-4): 605-617.
[13] WU H, TING Y. Metal extraction from municipal solid waste (MSW) incinerator fly ash?Chemical leaching and fungal bioleaching [J]. Enzyme Microbial Technology, 2006, 38(6): 839-847.
[14] KINOSHITA T, YAMAGUCHI K, AKITA S, NII S, KAWAIZUMI F, TAKAHASHI K. Hydrometallurgical recovery of zinc from ashes of automobile tire wastes [J]. Chemosphere, 2005, 59(8): 1105-1111.
[15] SAITO C, OKADA H, TITUS M J, YOSHIOKA T, MIZOGUCHI T. Leaching of heavy metals from fly ash generated from gasification and melting furnace for municipal solid wastes by organic acids [J]. Japanese Society of Waste Management Expert, 2007, 18(3): 157-166. (in Japanese)
[16] ETTLER V, VRTISKOVA R, MIHALJEVIC M, SEBEK O, GRYGAR T, DRAHOTA P. Cadmium, lead and zinc leaching from smelter fly ash in simple organic acids ? Simulators of rhizospheric soil solutions [J]. Journal of Hazardous Materials, 2009, 170(2-3): 1264-1268.
[17] FUOCO R, CECCARINI A, TASSONE P, WEI Y, BRONGO A, FRANCESCONI S. Innovative stabilization/solidification processes of fly ash from an incinerator plant of urban solid waste [J]. Microchemistry Journal, 2005, 79(1-2): 29-35.
[18] SMICHOWSKI P, MARRERO J. Comparative study to evaluate the effect of different acids on the determination of germanium by hydride generation-inductively coupled plasma atomic emission spectrometry [J]. Analytica Chimica Acta, 1998, 376(3): 283-291.
[19] HONG K J, TOKUNAGA S, KAJIUCHI T. Extraction of heavy metals from MSW incinerator fly ashes by chelating agents [J]. Journal of Hazardous Materials, 2000, 75(1): 57-73.
[20] WANG J, BAN H, TENG X J, WANG H, LADWIG K. Impact of pH and ammonia on the leaching of Cu(II) and Cd(II) from coal fly ash [J]. Chemosphere, 2006, 64(1): 1892-1898.
城市垃圾焚烧飞灰中的重金属在盐酸中的浸出行为
黄 凯1, 井上胜利2, 原田浩幸2, 川喜田英孝2, 大渡启介2
1. 北京科技大学 有色冶金系,北京 100083;
2. 佐贺大学 应用化学系,佐贺市本庄街1号,840-8502,日本
摘 要:系统研究城市生活垃圾焚烧飞灰中的重金属湿法冶金浸出行为,主要包括两个过程:水洗和酸浸。研究发现,在最佳条件下,水洗可以提取出飞灰中86% Na、70% K和12% Ca,水洗飞灰在盐酸浸出时约有86% Pb、98% Zn、82% Fe、96% Cd、62% Cu和80% Al可被一次性提取出来。浸渣的主要成分为Ca2PbO4、CaSi2O5、Pb5SiO7、Ca3Al2Si3O12、SiO2。
关键词:城市生活垃圾;飞灰;重金属;浸出;盐酸介质
(Edited by YUAN Sai-qian)
Corresponding author: Katsutoshi INOUE; Tel: +81-952-28-8671; Fax: +81-952-28-8591; E-mail: inoue@elechem.chem.saga-u.ac.jp
DOI: 10.1016/S1003-6326(11)60876-5