Quantitative analysis of homogenization treatment of INCONEL718 superalloy
来源期刊:中国有色金属学报(英文版)2011年第5期
论文作者:缪竹骏 单爱党 吴元彪 卢俊 徐文亮 宋洪伟
文章页码:1009 - 1017
关键词:INCONEL718;高温合金;均匀化处理;Laves相;偏析
Key words:INCONEL718; superalloy; homogenization; Laves phase; segregation
摘 要:
利用定量分析手段建立INCONEL718高温合金的均匀化模型。采用金相法确定d406 mm铸锭的初熔温度,结果表明其初熔温度为1 170~1 180 °C。采用两阶段均匀化热处理消除枝晶显微偏析。为了定量预测Laves相的溶解过程,提出一个和时间、温度相关的均匀化模型。在铸态组织的所有偏析元素中,Nb和Ti为偏析程度最大的正偏析元素。然后,分别计算在1 140 °C时各合金元素的扩散系数,并与另外两种常见的含铌高温合金进行比较。
Abstract:
Quantitative analysis was employed to establish reasonable and practical homogenization model of INCONEL718 superalloy. Metallographic method was applied to determining the incipient melting temperature. The result shows that the incipient melting temperature of d406 mm INCONEL718 ingot is situated between 1 170 °C and 1 180 °C. In order to predict the elimination process of Laves phase in quantity, a time and temperature dependent homogenization model was proposed. Among all the elements in the as-cast microstructure, Nb and Ti are the most positive segregated elements. The diffusion coefficients of alloying elements at 1 140 °C were obtained by fitting the linear relationship between ln δ (δ: residual segregation index) and time. The calculation results of diffusion coefficients were compared with other two commercial Nb-bearing superalloys.

MIAO Zhu-jun1, SHAN Ai-dang1, WU Yuan-biao1,
LU Jun2, XU Wen-liang2, SONG Hong-wei2
1. School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China;
2. Baosteel Research Institute, Baoshan Iron & Steel Co., Ltd., Shanghai 201900, China
Received 28 June 2010; accepted 4 January 2011
Abstract: Quantitative analysis was employed to establish reasonable and practical homogenization model of INCONEL718 superalloy. Metallographic method was applied to determining the incipient melting temperature. The result shows that the incipient melting temperature of d406 mm INCONEL718 ingot is situated between 1 170 °C and 1 180 °C. In order to predict the elimination process of Laves phase in quantity, a time and temperature dependent homogenization model was proposed. Among all the elements in the as-cast microstructure, Nb and Ti are the most positive segregated elements. The diffusion coefficients of alloying elements at 1 140 °C were obtained by fitting the linear relationship between ln δ (δ: residual segregation index) and time. The calculation results of diffusion coefficients were compared with other two commercial Nb-bearing superalloys.
Key words: INCONEL718; superalloy; homogenization; Laves phase; segregation
1 Introduction
Ni-based superalloys are important structural materials used in aero-engine and land-based power generation[1-2]. In order to meet the critical demands for high temperature applications, some refractory and strengthened elements such as Nb and Mo are alloyed in superalloy chemistry[3]. However, due to their large atomic sizes and segregation behaviors, severe micro-segregation and undesirable topologically close-packed (TCP) phases will form in many as-cast Ni-based superalloys[4-5]. For example, the addition of 5.3% Nb in INCONEL718 superalloy results in the existence of Laves phase, which has detrimental effects on hot working ability[6-7]. Thus, homogenization treatment at high temperatures plays an essential role in obtaining uniform structures free from harmful phases.
While, in the previous studies, parameters for homogenization treatments were always obtained by trial and error. HAN and ZHANG[8] investigated the segregation of Nb and Al in GH783 alloy and found that the eutectic microstructure could be eliminated completely after homogenization for 30 h. PAN et al[9] studied the microstructure evolution during homogenization in as-cast ЭП742 alloy and proposed a two-step homogenization schedule to relieve micro-segregation. Despite recent progress, little work has been carried out to establish quantitative models on homogenization treatment. Especially, with the advancement of large ingots, homogenization design has become a significant issue. Firstly, also most importantly, homogenization must be completely effective in removing the TCP phases to obtain a uniform distribution of alloying elements. Secondly, the industrial homogenization time should be as short as possible for the cost saving. Owing to the above two reasons, establishment of homogenization models will be both scientific and technical significance, which will provide an economical tool for industrial homogenization designs.
The present study is focused on INCONEL718 superalloy since it is accounting for more than 50% of commercial superalloy productions in the world [10]. In addition, the investigation of homogenization of INCONEL718 superalloy is meaningful as the guidance for other commercial superalloys. Therefore, quantitative analysis was employed to establish reasonable and practical homogenization model dependent of two parameters (time and temperature), by which homogeneous structure could be achieved.
2 Experimental
d406 mm IN718 ingot with overall compositions listed in Table 1 was produced by doubled melting technique (VIM+VAR), which is shown in Fig.1(a). Figure 1(b) illustrates the typical dendrite structures (secondary dendrite arm spacing: 70 μm) and white blocky Laves phase is observed in the center of inter-dendritic region.
Table 1 Chemical compositions of IN718 superalloy (mass fraction, %)
![]()
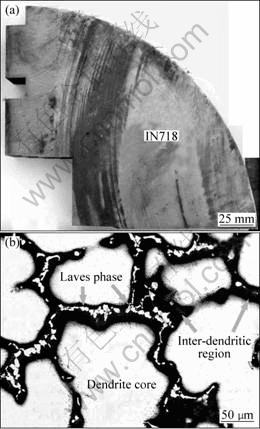
Fig.1 IN718 superalloy used in this study (a) and as-cast dendrite structure (b)
Small samples (20 mm×5 mm×5 mm) were cut from the center of ingot for homogenization treatment. The parameter of homogenization was generated according to the incipient melting temperature determined by the metallographic method. After homogenization treatment for 3, 5, 10, 20, 30, 40 and 60 h respectively, samples were quenched in water for examining the microstructure evolution. The metallographic samples were prepared by mechanical polishing and electro-etching with a solution containing 1 part hydrochloric acid, 3 parts nitric acid and 5 parts glycerin at a voltage of 6 V for 10 s. Afterwards, the optical microscopy (Leica DM6000) and SEM (Hitachi S-3400) with energy dispersive spectrometry (EDS) examinations were carried out to characterize the microstructure of the samples. Here, metallographic analysis software (Mias 2000) was used to calculate the volume fraction of Laves phase during homogenization. The differential scanning calorimetry (DSC, Netzsch STA 449C) examination was performed to verify the existence of Laves phase and the heating rate was controlled at 10 °C/min from 1 100 °C to 1 400 °C.
3 Results and analysis
3.1 Incipient melting temperature (IMT)
Metallographic method was applied to determining the IMT in d406 mm ingot. It was reported that the IMT of d423 mm INCONEL718 ingot was approximately situated between 1 163 and 1 175 °C[11]. In reference with this result, the samples were soaked at 1 150, 1 160, 1 170, 1 180 and 1 190 °C for 30 min and then quenched in water. Figure 2 shows the microstructure and Laves phase morphology evolution through different heating processes. From Figs.2(a)-(c), the content of Laves phase exhibits a decreasing tendency with the increase of soaking temperatures while no obvious change is found concerning to the appearance of Laves phase. When the soaking temperature was raised to 1 180 °C, incipient melting appeared in some areas of Laves phase. When 1 190 °C was reached, γ/Laves eutectic was observed, as shown in Fig.3. This phenomenon indicates that all of the Laves phase has been melted when soaked at 1 190 °C and the existence of γ/Laves eutectic was the result of water quenching. From the above analysis, it can be inferred that the IMT of d406 mm INCONEL718 ingot is situated between 1 170 °C and 1 180 °C and then the subsequent homogenization temperature should be lower than 1 170 °C.
3.2 Time and temperature dependent homogenization model for elimination of Laves phase
Previously, it has been revealed that the homogenization temperature should be lower than 1 170 °C. Here, 1 140 °C is chosen as the experimental temperature and there are two main reasons to be addressed. First, real heating temperature in industry will be fluctuated around the setting temperature because of the heating furnace itself and interference from outside environment. So, it is greatly important to investigate homogenization process at a bit lower temperature in order to have an overall command of homogenization technique, which will be valuable to industry producing. Second, homogenization information at 1 140 °C can be utilized to establish quantitative model in the present study for prediction at higher temperatures. Based on the two reasons above, 60 h homogenization at 1 140 °C is applied and quantitative analysis of the Laves phase is conducted to evaluate the homogenization effects.
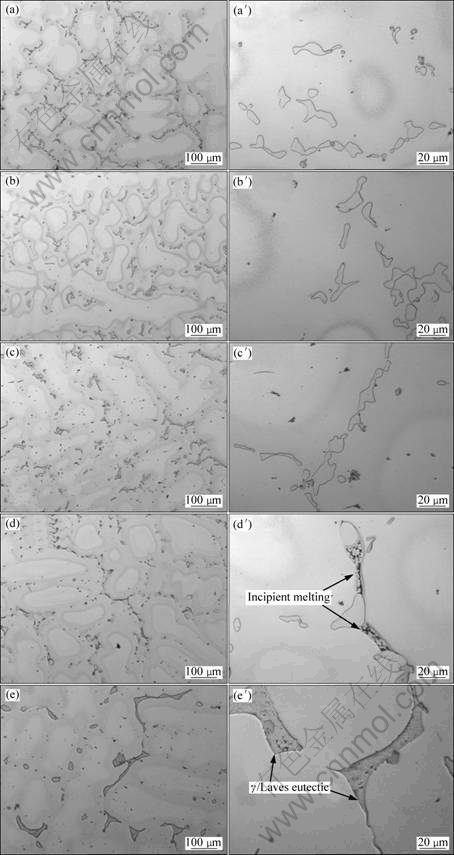
Fig.2 Microstructures of as-cast alloy after soaking at 1 150 °C (a, a′), 1 160 °C (b, b′), 1 170 °C (c, c′), 1 180 °C (d, d′) and 1 190 °C (e, e′) for 30 min and then quenched in water
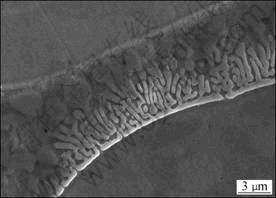
Fig.3 γ/Laves eutectic microstructure after soaking at 1 190 °C for 30 min and then quenched in water
Figure 4 shows the microstructure evolution from 3 h to 40 h. With the proceeding of homogenization, both dendrite structure and Laves phase gradually disappeared. When the homogenization time reached 30 h, a little Laves phase still existed. But no Laves phase except for MC carbide was observed after 40 h, as shown in Figs.4(e, e′). DSC results in Fig.5 also demonstrate that the Laves phase has been completely eliminated after 40 h, which is consistent with the metallographic observation.
After quantitative metallographic analysis, the Laves phase volume fraction (φ) of as-cast alloy is 3.2% and variation of that during homogenization treatment is illustrated in Fig.6(a). Apparently, this curve obeys an exponential relationship, which is given by
![]() (1)
(1)
where t is the homogenization time; KT is the elimination parameter as a function of homogenization temperature. The value of KT at 1 140 °C is 0.222 after non-linear fitting.
Here, another variable (Remaining Laves phase fraction, R) is defined to quantify the elimination process of Laves phase, as shown in Fig.6(b). It is easy to understand that the value of R is 100% before homogenization and 0 after 40 h homogenization at 1 140 °C. Thus, R is related to the homogenization time according to
![]() (2)
(2)
In the previous research, LIANG et al[11] proposed an equation for predicting time to eliminate Laves phase completely in d423 mm IN718 ingot, which can be rationally applied in this case:
![]() (3)
(3)
where τ is the time of eliminating Laves phase completely; T is the homogenization temperature; the coefficient B is decided by the segregation degree of ingots. In this study, the time of eliminating Laves phase completely at 1 140 °C is 40 h, so B is 2.33×1019. According to above results, the time of eliminating Laves phase completely at different homogenization temperatures is displayed in Fig.7.
In order to predict the Laves phase elimination process at high temperatures, it is assumed that the Laves phase elimination process is over when the Laves phase volume fraction is 0.01%. Afterwards, Eq.(2) and Eq.(3) are used to deduce the following equation:
![]() (4)
(4)
Based on Eq.(4), the elimination process of Laves phase could be predicted in quantity at a fixed temperature during homogenization treatment. Figure 8 shows the relationship between the remaining Laves phase fraction and homogenization time at 1 140, 1 150, 1 160 and 1 170 °C, respectively.
3.3 Micro-segregation evolution during homogeni- zation
In addition to the elimination of Laves phase, elimination of micro-segregation is also an important issue for homogenization treatment. Here, the element segregation coefficient (K) can be determined by K=cmax/cmin, where cmax and cmin are the maximum and minimum concentrations in dendrite core or inter-dendritic region, respectively. The positive segregated elements are strongly enriched in the inter-dendritic region while negative segregated elements vice versa.
According to EDS results, Nb, Ti and Mo are positive segregated elements in as-cast structure, where KNb=3.68, KTi=2.31, KMo=1.42. And Cr and Fe are negative segregated elements, where KCr=1.28, KFe=1.38. After 10 h homogenization, the K value is becoming smaller, and KNb=1.24, KTi=1.13, KMo=1.05, KCr=1.02, KFe=1.05, as shown in Fig.9. Apparently, the entire segregated elements distributed more uniform in the progress of homogenization treatment.
In order to study the diffusion kinetics, it is assumed that the alloying elements are distributed between dendrites as following equation[12]:
![]() (5)
(5)
where c(x) is the element concentration at x position; ![]() is the average concentration; Δc0 is the difference between the maximum or minimum concentration and average concentration; D is the diffusion coefficient; L is the secondary dendrite arm spacing; t is the homogenization time.
is the average concentration; Δc0 is the difference between the maximum or minimum concentration and average concentration; D is the diffusion coefficient; L is the secondary dendrite arm spacing; t is the homogenization time.

Fig.4 Microstructure evolution of alloy during homogenization at 1 140 °C: (a, a′) 3 h; (b, b′) 5 h; (c, c′) 10 h; (d, d′) 30 h; (e, e′) 40 h
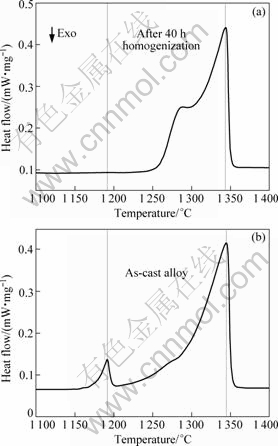
Fig.5 DSC curves when heated at 10 °C/min from 1 100 °C to 1 400 °C
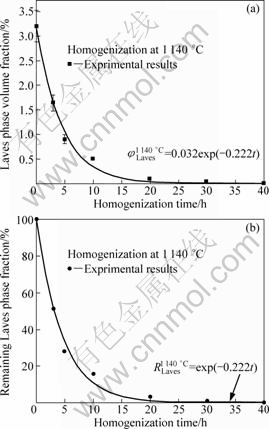
Fig.6 Homogenization kinetics at 1 140 °C for elimination of Laves phase: (a) Laves phase volume fraction; (b) Remaining Laves phase fraction in homogenization process
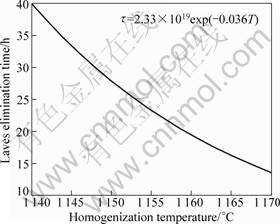
Fig.7 Time of eliminating Laves phase completely as function of homogenization temperature
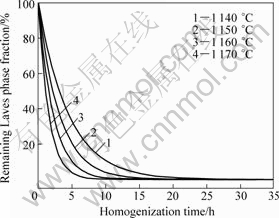
Fig.8 Relationship between remaining Laves phase fraction and homogenization time at various temperatures
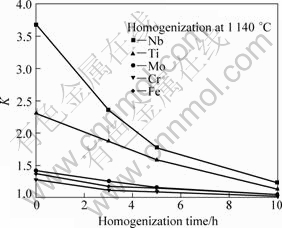
Fig.9 Change of K values when homogenized at 1 140 °C
And residual segregation index δ is defined as follows[13]:
![]() (6)
(6)
where ![]() and
and ![]() are the maximum and minimum concentrations after t homogenization, respectively.
are the maximum and minimum concentrations after t homogenization, respectively.
![]() (7)
(7)
![]() (8)
(8)
where ![]() and
and ![]() are the maximum and minimum concentrations in as-cast microstructure, respectively.
are the maximum and minimum concentrations in as-cast microstructure, respectively.
![]() (9)
(9)
![]() (10)
(10)
Table 2 shows the change of residual segregation index (δ) during 10 h homogenization at 1 140 °C.
In conjunction with Eq.(6) to Eq.(10), a more simplified equation for residual segregation index (δ) can be obtained:
![]() (11)
(11)
Then, diffusion coefficient (D) can be calculated by fitting the linear relationship between lnδ and t, which is shown in Fig.10. As a result, the calculated diffusion coefficients of Nb, Ti, Mo, Cr and Fe at 1 140 °C are listed in Table 3. As indicated previously, Nb is the most segregated element for INCONEL718 superalloy. However, the diffusion coefficient of Nb is relatively smaller compared with other elements. These two factors are contributed to the difficulties in homogenization treatment of INCONEL718 superalloy.
Table 2 Residual segregation index (δ) during 10 h homogenization at 1 140 °C
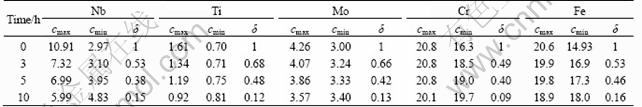
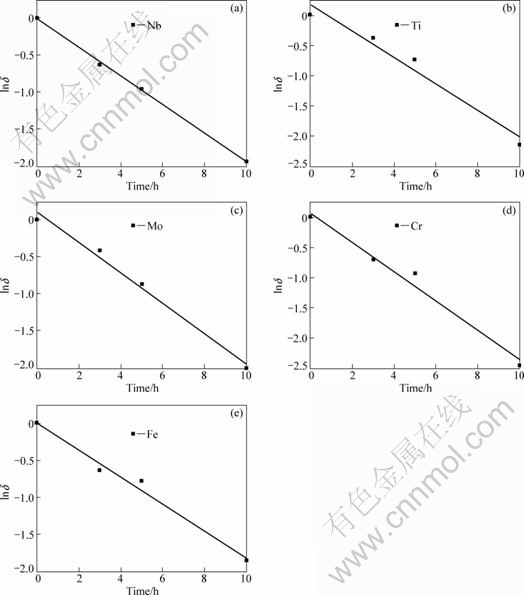
Fig.10 Linear relationship between ln δ and t for Nb (a), Ti (b), Mo (c), Cr (d) and Fe (e) at 1 140 °C
Table 3 Diffusion coefficients (10-11cm2/s) of elements at 1 140 °C

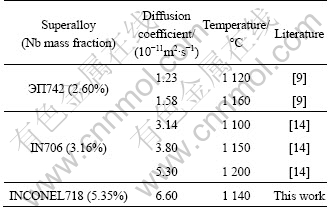
Recently, a few studies[9,14] involved calculating diffusion coefficient of Nb in different series of Nb-bearing superalloys, which are summarized in Table 4. In view of the chemical composition, ЭП742 is a γ′-Ni3(Al, Ti, Nb) strengthened superalloy and the nominal content of Nb is 2.60%. IN706 superalloy is strengthened by both γ′-Ni3(Al, Ti, Nb) and γ″-Ni3Nb, so the Nb content is a little higher than that in ЭП742. INCONEL718 is a predominantly γ″-Ni3Nb strengthened superalloy and the Nb content is the highest among three alloys. From Table 4, it is reasonable to conclude that with the increase of Nb content, diffusion coefficient of Nb also increases accordingly but in the same magnitude.
Table 4 Diffusion coefficients of Nb for different series superalloys
4 Conclusions
1) Incipient melting temperature of d406 mm INCONEL718 ingot is situated between 1 170 °C and 1 180 °C.
2) The Laves phase elimination model is proposed according to the study results and the remaining Laves phase fraction (R) can be derived as a function of homogenization temperature and time: RLaves= exp![]() .
.
3) Among all the alloying elements in as-cast microstructure, Nb and Ti are the most positive segregated elements, where KNb=3.68 and KTi=2.31. The diffusion coefficients of alloying elements at 1 140 °C are calculated by fitting the linear relationship between lnδ and homogenizing time.
4) Compared with other Nb-bearing superalloys (ЭП742 and IN706), the diffusion coefficient of Nb in INCONEL718 is higher when homogenized at the same temperature level.
Acknowledgments
The authors would like to thank Dr. W. R. SUN and Dr. L. X. YU from Institute of Metal Research, Chinese Academy of Sciences for alloy preparation and useful discussions.
References
[1] FURRER D, FECHT H. Ni-based superalloys for turbine discs [J]. JOM, 1999, 51(1): 14-16.
[2] SCHWANT R C, THAMOO S V, ANDERSON A F, ADASCZIK C B, BOND B J, JACKMAN L A, UGINET J F. Large 718 forgings for land based turbines [C]//LORIA E A. Superalloys 718, 625, 706 and Various Derivatives. Warrendale, PA: TMS, 1997: 141-152.
[3] REED R C. The superalloys fundamentals and applications [M]. Cambridge: Cambridge University Press, 2006.
[4] JABLONSKI P, COWEN C. Homogenizing a nickel-based superalloy: Thermodynamic and kinetic simulation and experimental results [J]. Metallurgical and Materials Transactions B, 2009, 40(2): 182-186.
[5] RADHAKRISHNA C, PRASADRAO K. The formation and control of Laves phase in superalloy 718 welds [J]. Journal of Materials Science, 1997, 32(8): 1977-1984.
[6] CHANG K, LAI H, HWANG J. Existence of Laves phase in Nb-hardened superalloys [C]//LORIA E A. Superalloys 718, 625, 706 and Various Derivatives. Warrendale, PA: TMS, 1994: 683-694.
[7] FORBES JONES R M, JACKMAN L A. The structural evolution of superalloy ingots during hot working [J]. JOM, 1999, 51(1): 27-31.
[8] HAN G W, ZHANG Y Y. Segregation of niobium and aluminum in GH783 alloy ingots [J]. Materials Science and Engineering A, 2005, 412 (1-2): 198-203.
[9] PAN Xiao-lin, SUN Wen-ru, YANG Shu-lin, LI Zhen, GUO Shou-ren, YANG Hong-cai, HU Zhung-qi. Microstructure transformation during homogenization treatment in as-cast GH742 alloy [J]. Chinese Journal of Materials Research, 2008, 22 (6): 651-656. (in Chinese)
[10] DECKER R F. The evolution of wrought age-hardenable superalloys [J]. JOM, 2006, 58 (9): 32-36.
[11] LIANG X, ZHANG R, YANG Y, HAN Y, ZHOU D, FANG L, XIE X. An investigation of the homogenization and deformation of alloy 718 ingots [C]//LORIA E A. Superalloys 718, 625, 706 and Various Derivatives. Warrendale, PA: TMS-AIME, 1994: 947-956.
[12] SUN Zhen-yan, LIU Chun-ming. Diffusion and phase transformation in alloys [M]. Shenyang: Northeastern University Press, 2002. (in Chinese)
[13] SEMIATIN S L, KRAMB R C, TURNER R E, ZHANG F, ANTONY M M. Analysis of the homogenization of a nickel-base superalloy [J]. Scripta Materialia, 2004, 51(6): 491-495.
[14] LONG Zheng-dong, MA Pei-li, ZHONG Zeng-yong. Homogenizing treatments of IN706 alloy [J]. Journal of Iron and Steel Research, 1997, 9(1): 21-24. (in Chinese)
缪竹骏1,单爱党1,吴元彪1,卢 俊2,徐文亮2,宋洪伟2
1. 上海交通大学 材料科学与工程学院, 上海 200240;
2. 宝山钢铁股份有限公司 宝钢研究院, 上海 201900
摘 要:利用定量分析手段建立INCONEL718高温合金的均匀化模型。采用金相法确定d406 mm铸锭的初熔温度,结果表明其初熔温度为1 170~1 180 °C。采用两阶段均匀化热处理消除枝晶显微偏析。为了定量预测Laves相的溶解过程,提出一个和时间、温度相关的均匀化模型。在铸态组织的所有偏析元素中,Nb和Ti为偏析程度最大的正偏析元素。然后,分别计算在1 140 °C时各合金元素的扩散系数,并与另外两种常见的含铌高温合金进行比较。
关键词:INCONEL718;高温合金;均匀化处理;Laves相;偏析
(Edited by YANG Hua)
Foundation item: Project (08dj1400402) supported by the Major Program for the Fundamental Research of Shanghai Committee of Science and Technology, China
Corresponding author: SHAN Ai-dang; Tel: +86-21-54747441; Fax: +86-21-54748974; E-mail: adshan@sjtu.edu.cn
DOI: 10.1016/S1003-6326(11)60814-5

