
Photoconductivity of novel poly(N-vinyl)-3-[p-nitrophenylazo]carbazole/CdS-nanoparticle polymer composite
DING Li-yun (丁莉芸)1, JIANG De-sheng(姜德生)1, HUANG Jun (黄 俊)1,2, DAI Lei (戴 磊)1,
LIU Cheng(刘 诚)1, WANG Jun-tao (王军涛)1, LI Bin (李 斌)1
1. Key Laboratory of Fiber Optic Sensing Technology and Information Processing, Ministry of Education, Wuhan University of Technology, Wuhan 430070, China;
2. Key Laboratory of Nonferrous Materials and Processing Technology, Guilin University of Technology, Ministry of Education, Guilin 541004, China
Received 10 April 2006; accepted 25 April 2006
Abstract: The photoconductive characteristic of the inorganic/organic hybridized polymer system is reported, in which a novel bi-functional photorefractive (PR) poly(N-vinyl)-3-[p-nitrophenylazo]carbazole (PVNPAK) serves as a polymeric charge-transporting and second-order nonliner optical matrix and quantum dots composed of surface passivated cadmium sulfide serve as a charge-generation sensitizer. The hybrid PVNPAK/CdS-nanoparticles polymer composites with different mass ratio of CdS to PVNPAK were prepared. The generation of photocurrent on illumination and photoconductive properties of the PVNPAK/CdS-nanoparticles polymer composites were studied. The results show that the addition of CdS nanoparticle as a photosensitizer can enhance the photoconductivity of the PVNPAK significantly because of the properties of the high quantum efficiency of photosensitization and high charge transport to conducting polymer.
Key words: photoconductivity; inorganic/organic hybridization; CdS nanoparticle; poly(N-vinyl)-3-[p-nitrophenylazo]carbazole
1 Introduction
Photorefractive (PR) materials have attracted a great amount of attention due to their potential applications in optical information memory and processing such as high-density optical data storage, optical imaging processing, phase conjugation, dynamic holography and optical computing. Since PR effect was firstly discovered in organic polymer in 1991[1], PR polymers have been extensively studied due to their large optical nonlinearities, low dielectric constants, ease of preparation and low cost. In recent years, bi-functional PR polymers [2-4], which possess both photoconductivity and EO effect, were proposed due to their high stability and easy synthesis. However, the key parameter affecting the PR effect in polymeric materials is the electric field dependent, necessitating the application of a relatively large dc field, E. Several approaches have been proposed to reduce E, including the substitution of the traditional organic photosensitizers (e.g. TNF or C60) with inorganic nanocrystals. Semiconductor nanoparticles are a potential useful class of polymer photosensitizers in many applications that require materials with photoconductive and PR properties. Photoconductivity enhancement of polymer has been demonstrated when CdS and other semiconductor nanoparticles were finely dispersed in the polymer matrix[5-7]. Nanocomposites containing inorganic quantum dots dispersed in the polymer matrix exhibit increased photogeneration efficiency, broad spectrally tunable photoresponse and enhanced carrier mobility.
In this study, we report a novel bi-functional PR poly(N-vinyl)-3-[p-nitrophenylazo]carbazole(PVNPAK) synthesized by a post azo coupling reaction, in which there is an azo derivative as the EO chromophore and carbazolyl groups as photoconduc-tive moiety. And the CdS nanoparticle was prepared by the reverse micelle method to serve as a photosensitizer. The generation of photocurrent on illumination and photoconductive properties of the PVNPAK/CdS-nanoparticles polymer composites were studied. A significant enhancement of photoconductivity was observed in nanocomposite as compared with pure PVNPAK because of the properties of the high quantum efficiency of photosensitization and high charge transport to conducting polymer.
2 Experimental
2.1 Materials
All chemicals, poly(N-vinylcarbazole) (PVK) (secondary standard, ACROS), p-thiocresol (Aldrich), sodium bis(2-ethylhexyl)sulfosuccinate (ACROS), nitrobenzene, p-nitroaniline, sodium nitrous acid, n-heptane, methanol, cadmium acetate dehydrate, sodium sulfide and tetrahydrofuran were bought commercially and used without further purification unless otherwise noted.
A novel bi-functional PR poly(N-vinyl)-3-[p-
nitrophenylazo]carbazole (PVNPAK) synthesized by a post azo coupling reaction. Detailed synthesis procedure can be found elsewhere[8]. The chemical structure of PVNPAK is shown in Fig.1.
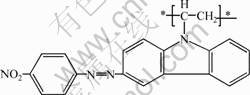
Fig. 1 Chemical structure of PVNPAK
2.2 Synthesis of CdS nanoparticles
The CdS nanoparticles were prepared using the reverse micelle method. The size of the nanoparticles could be controlled by the surfactant-to-water ratio W0, as well as the reaction time. Here, the continuous oil phase consisted of n-heptane and sodium bis(2-ethylhexyl)sulfosuccinate (AOT) served as the surfactant. Aqueous solutions of cadmium acetate dihydrate and sodium sulfide were prepared by dissolving the respective salts in deionized water. A surfactant-to-water ratio of W0=1 was used to provide sensitivity at 632.8 nm. The surface of the CdS nanoparticles was treated with p-thiocresol. This surface treatment provides good chemical stability and simultaneously ensures good solubility in the common solvents used to mix the different components necessary to produce PR polymers. After mechanical stirring the reaction mixture for 24 h, the precipitated surface capped CdS was collected and washed with methanol yielding a free flowing powder.
2.3 Samples for photoconductivity measurement
A different amount of the CdS nanoparticles was added into each of the three PVNPAK solutions under magnetic stirring, resulting in three solutions labeled as PVNPAK/1CdS, PVNPAK/2CdS and PVNPAK/5CdS. The one-digit numbers in the sample labels indicate the mass ratios (×100) of the CdS nanoparticles to PVNPAK. PVNPAK/CdS nanocomposites were filtered through a 0.2 μm pore size membrane. Thin films (1-2 μm) were fabricated via conventional spin-coating techniques on a glass substrate coated with indium-tin oxide (ITO). Then another ITO-glass was placed on the top to form a sandwich device structure.
Photoconductivity measurements were made using a simple DC photocurrent technique at room temperature in air. Here a voltage was applied to the sample and the current through the sample was measured after a short delay of approximately 10 s, during which traps in the material are filled and a steady state has been reached when it was illuminated by a He-Ne laser with a wavelength of 632.8 nm.
2.4 Characterization
UV-Vis absorption spectra of the samples were collected on SHIMADZU UV-2450 spectrophotometer. The samples for transmission electron microscope (TEM) were prepared by placing a drop of solution of sample on a copper grid, and the image was obtained using H-8100
TEM operating at 75 kV. Photoluminescence spectra of the samples were collected on HITACHI F-4500 fluorescence spectrophotometer.
3 Results and discussion
3.1 CdS nanoparticles characterization
The absorption spectra of CdS nanoparticles were collected at room temperature as shown in Fig. 2. The absorption edge of the CdS clusters (λedge=483 nm) is blue-shifted from that of bulk CdS (λedge=515 nm) due to the quantum-confinement effect, which results the size of semiconductor decreases and the band gap of it increases.
To confirm that the blue shift observed in this sample was accompanied by the expected reduction in average size, size of CdS nanoparticle was determined through the transmission electron microscope (TEM) as shown in Fig.3. It can be seen the size of CdS clusters is about 20nm (including 3-4 CdS nanoparticles with the
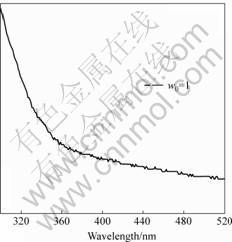
Fig.2 UV-Vis spectroscopy of CdS nanoparticles
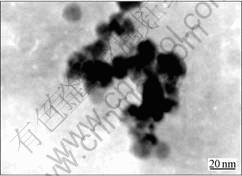
Fig.3 TEM image of CdS nanoparticles prepared with W0=1
spherical shape).
3.2 Optical properties of poly(N-vinyl)- 3-[p-nitrophenylazo]carbazole/CdS nanocompo-sites
Fig.4 shows the UV-Vis absorption spectra of the PVNPAK and the PVNPAK/CdS nanocomposites. These spectra are simple the sum of the absorption spectra of the constituent parts of the composites, with no evidence of any additional absorption peaks in the spectral range measured (200-800 nm). These results indicate that there is negligible ground-state charge-transfer between the polymer and the nanoparticles.
Fig.5 shows the photoluminescence (PL) spectra of pure PVNPAK and PVNPAK/CdS-nanoparticle composite excited at a wavelength of 280 nm. It can be seen that the photoluminescence spectra did not change when CdS nanoparticles were doped in PVNPAK, but the intensities of the photoluminescence were reduced compared with that of the pure PVNPAK solution with the same concentration. The PL appears to be from the transition in the carbazole moieties of PVNPAK, and the band gap emission from CdS was not observer. It is
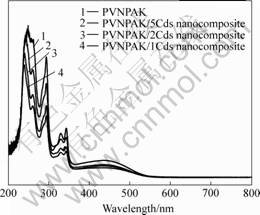
Fig.4 UV-Vis absorption spectra of PVNPAK and PVNPAK/CdS nanocomposites
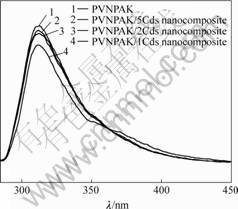
Fig.5 Photoluminescence spectra of pure PVNPAK and PVNPAK/CdS nanocomposite
because amines (e.g. carbazole moiety in this system) are effective complexing agents with the surfaces of CdS clusters[8], the quenching of the luminescence of PVNPAK in the PVNPAK/CdS nanocomposites and the absence of band-gap luminescence from CdS result from the charge carrier transport between PVNPAK and CdS nanoparticles.
3.3 Photoconductivity of nanocomposites
The photogeneration of charge carriers and their subsequent migration by diffusion or drift in an electric field play a key role in determining the magnitude and speed of the PR response, so photoconductivity of PVNPAK/CdS nanocomposites is the important factor for PR effect. Since only a small amount of nanoclusters are required (typically a few mass fraction) to photosensitize the matrix, the nanoclusters are isolated from each other and are responsible for the charge generation while the polymer is responsible for the charge transport[9]. Here, only the photoconductivity of PVNPAK/1CdS nanocomposite was studied. Fig.6 shows the current-voltage curve of pure PVNPAK and PVNPAK/1CdS nanocomposite films under illumination at excitation wavelengths of 632.8 nm. It shows that the photocurrent is increased nearly exponentially with the electric field under the illumination when CdS nanoparticles were hybridized in PVNPAK matrix.
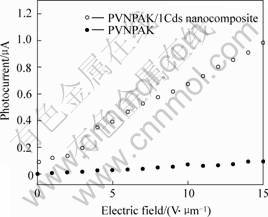
Fig.6 Current-voltage curves of pure PVNPAK and PVNPAK/1CdS nanocomposite film under illumination at excitation wavelength of 632.8 nm
Photoconductivity is a convolution of photoinduced charge generation and charge transport, so the photoconductive composites obtained by doping of a host polymer with semiconductor nanoparticles exhibit an increase of the photoconductivity not only due to sensitization of photocharge generation but the presence of nanoparticles may also lead to distinct increase of the effective mobility of charge carriers [10]. We determined the photocharge generation quantum efficiency (F) of PVNPAK and PVNPAK/1CdS nanocomposite, which can be evaluated from the equation:
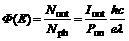
where Nout is the number of charge carriers generated per unit volume; Nph is the number of photons absorbed per unit volume in the sample; Iout is the measured photocurrent; Pon is the power of he incident light; h is Planck’s constant; c is the speed of light; e is the fundamental unit charge and λ is the wavelength of the incident radiation. In Fig.7 the photocharge generation quantum efficiency as a function of applied electric field at 632.8 nm for pure PVNPAK and PVNPAK/1CdS nanocomposite are presented. It can be seen that the PVNPAK/1CdS-nanoparticle composite exhibits the larger photocharge generation quantum efficiency for the entire range of electric field than that of the PVNPAK film. Experimental data such as that presented in Fig.7 is often analyzed via the Onsager charge recombination model [11,12].
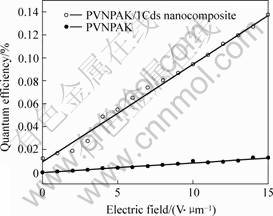
Fig. 7 Photocharge generation quantum efficiency as function of applied electric field at 632.8 nm for PVNPAK and PVNPAK/1CdS nanocomposite
References
[1] DUCHARME S, SCOTT J C, TWIEG R J, MOERNER W E. Observation of the photorefractive effect in a polymer [J]. Phys Rev Lett, 1991, 66 (14): 1846-1849.
[2] SCHAERLAEKENS M, HENDRICKX E, HAMEURLAINE A, DEHAEN W, PERSOONS A. Photorefractive properties of bifunctional N-arylated carbazole derivatives in a carbazole polymer host matrix [J]. Chem Phys, 1999, 1: 43-52.
[3] DIDUCH K, W?BBENHORST M, KUCHARSKI S. Photocurrent generation of bi-functional carbazole containing polymers [J]. Synthetic Metals, 2003, 139: 515-520.
[4] CHEN Y W, GONG Q H, WANG F, ZHANG B, CHEN Z J. Synthesis and characterization of photorefractive materials based on polymers containing photoconductors and nonlinear chromophores [J]. Mater Lett, 2003, 57: 4372-4377.
[5] WINIARZ J G, ZHANG L M, LAL M, FRIEND C S, PRASAD P N. Photogeneration, charge transport, and photoconductivity of a novel PVK/CdS-nanocrystal polymer composite [J]. Chem Phys, 1999, 245: 417-428.
[6] GREENHAM N C, PENG X G, ALIVISATOS A P. Charge separation and transport in conjugated-polymer/ semiconductor-
nanocrystal composites studied by photoluminescence quenching and photoconductivity [J]. Phys Rev B, 1996, 54 (24): 17628-17637.
[7] WANG Y, HERRON N. Semiconductor nanocrystals in carrier-transporting polymers: Charge generation and charge transport [J]. J Luminescence, 1996, 70: 48-59.
[8] WANG S H, YANG S H, YANG C L, LI Z Q, WANG J N, GE W K. Poly(N-vinylcarbazole) (PVK) photoconductivity enhancement induced by doping with CdS nanocrystals through chemical hybridization [J]. J Phys Chem, 2000, 104 (50): 11853-11858.
[9] WANG Y, HERRON N. Photoconductivity of CdS nanocluster-doped polymers [J]. Chem Phys Lett, 1992, 200:71-75.
[10] CHOUDHURY K R, SAMOC M, PATRA A, PRASAD P N. Charge carrier transport in poly(N-vinylcarbazole):CdS quantum dot hybrid nanocomposite [J]. J Phys Chem B, 2004, 108: 1556-1562.
[11] ONSAGER L. Debiations from Ohm’s law in weak electrolytes [J]. J Chem Phys, 1934, 2: 599-615.
[12] MOERNER W E, Silence S M. Polymeric photorefractive materials [J]. Chem Rev, 1994, 94 (1): 127-155.
(Edited by CHEN Ai-hua)
Foundation item: Project(60537050) supported by the National Nature Science Foundation of China; Project supported by the Opening Fund of Key Laboratory of Nonferrous Materials and Processing Technology.
Corresponding author: Jiang De-Sheng; Tel: +86-27-87651850-8005; Fax: +86 -27-87876032; E-mail: fosrcwut@public.wh.hb.cn